Abstract
Coal-smoke emissions affected photosynthesis, N-metabolism and growth characteristics of Triumpfetta rhomboidea, as observed at pre-flowering, flowering and post-flowering stages of plant growth. The net photosynthetic rate and stomatal conductance decreased, whereas intercellular CO2 concentration increased under pollution stress. The amounts of photosynthetic pigments in leaves were consistently less, up to 35% for chlorophylls and 84% for carotenoids. Nitrate level was raised while NR activity and protein contents in leaves declined at the polluted site at each growth stage. Sugar content was always lower at the polluted site in roots and stem but sizably higher in leaves, thus showing a failure of the process of photosynthate translocation. The sulphur level in roots, stem and leaves increased consistently. The leaf area was conspicuously reduced, leading to a significant loss in the total photosynthetic surface, despite an increase in the stem length and the number of leaves in the stressed population.
Abbreviations
| Chl | = |
chlorophyll |
| DMSO | = |
dimethyl sulfoxide |
| EC | = |
electrical conductivity |
| gs | = |
stomatal conductance |
| NADH | = |
nicotinamide adenine dinucleotide (reduced) |
| NEDD | = |
nephthyl ethylene diamine dihydrochloride |
| NR | = |
nitrate reductase |
| PN | = |
net photosynthetic rate |
| TCA | = |
trichloroacetic acid |
Introduction
Environmental pollution may originate on a scale as small as a backyard fire or as large as the burning of thousands of tonnes of coal each day in coal-fired power-generating plants. The smoke emitted from thermal power plants comprises of SO2, CO, NOx, HF, fly ash and other waste products (Iqbal et al. Citation2000a ,Citationb). Fly ash consists of dehydrated and dissociated clay mineral impurities along with small amounts of unburnt coal, the major constituents being SiO2, Al2O3, FeO3, CaO, CaSO4, and trace elements, some of which (like As, Cd, Hg, Mo, Pb and Se) may be highly toxic. Fly ash decreases pH and increases EC of the soil and affects the availability of some major plant nutrients like N, P and K (Khan et al. Citation1996).
A degraded environment affects vegetation cover in a variety of ways (Iqbal et al. Citation2000a ,Citationb ,Citation2005). Symptoms of morphological injury usually appear when plants are exposed to higher concentration of pollutants (Rao and Rao Citation1989). Low levels of SO2 do not produce any obvious morphological injury (Lone et al. Citation1995; Williams and Banerjee Citation1995), but may affect photosynthetic rate and stomatal behavior and finally the plant yield (Dhir et al. Citation2001; Agrawal Citation2003). NOx may sometimes have positive effects on plant growth and yield but in combination with SO2, as they normally are, N oxides prove toxic to plants (Bell and Treshow Citation2002). NO2, despite being effectively reduced to ammonia by nitrite reductase, often disturbs the metabolic processes and carbon partitioning in plants (Schmidt et al. Citation2004). Rising CO2 concentration in the atmosphere generally stimulates plant growth but some species remain relatively unaffected (Eamus and Jarvis Citation1989; Krupa and Kickert Citation1989). Increased CO2 causes shifts in the partitioning of assimilated carbon among the different plant parts (Rogers et al. Citation1994) and so does the SO2-containing coal smoke (Iqbal et al.Citationin press).
The present study, undertaken as part of a project to understand the responses of different plant types to emissions from thermal power plants, explores the effect of coal-smoke pollution on photosynthetic, biochemical and growth characteristics of Triumfetta rhomboidea Jacq. syn T. bartramia, of the family Tiliaceae, at different stages (pre-flowering, flowering and post-flowering stages) of plant growth. This herb or under-shrub (75–150 cm tall), native to old-world tropics, is found throughout the tropical region. With stellate pubescence, the winged stem bears simple, alternate, variously shaped (ovate, rhomboid to somewhat trilobed) leaves with serrate margins. Small yellow flowers in axillary cymes form clusters at nodes. Fruits, sub-globose burs (3–4 mm in diameter) are spiny, with smooth hooked bristles. The inner bark of stem yields a soft and glossy fiber (average length 2.027 mm, diameter 0.016 mm), which is extracted on a commercial scale (500–800 kg ha−1) in certain parts of West Africa (Brown Citation1920–21; Burkill Citation1935). In India, it grows as a weed with very little heterogeneity, compared with cultivated plants, thus showing a considerable stability and purity.
Materials and methods
Site description
The study was conducted on plants growing at the polluted site (around the Kasimpur Thermal Power Plant [KTPP] in the Aligarh district of Uttar Pradesh, India) and at a non-polluted site (the Aligarh Muslim University campus, located 16 km away from the KTPP), which was considered as control. The Aligarh district lies in a fertile agricultural area of the Ganga-Jamuna doab, between 77°29′ E and 78°38′ E longitude. The Kasimpur town is located at an altitude of about 187 m above sea level. KTPP is one of the major thermal power plants of the Uttar Pradesh State and consists of three power stations with a capacity of 90 MW, 210 MW and 230 MW generation of electricity. The complex runs on bituminous coal that has 2.92% moisture, 22.20% ash, 31.86% volatile matters including 0.49% sulphur, 5.61% hydrogen, 5.24% nitrogen, 20.23% oxygen and 42.45% fixed carbon on average. About 4194 metric tons of coal per day are burnt, giving rise to emissions of approx. 0.0168 ppm h−1 SO2, 0.300 ppm h−1 NOx and 6.854 ppm h−1 CO2 (). Both of the study sites have a similar loam and clayey-loam type of soil, with a high pH (8–8.5) and poor porosity. The area experiences a dry tropical monsoon climate.
Table 1. The average amounts of major gases released from the Kasimpur Thermal Power Plant Complex in different seasons during a year. Measurements were made at a height of 8 m in the stack.
Sampling of plant material
A sample of five plants was collected at each of the three ontogenetic stages (pre-flowering, flowering and post-flowering stages) of plant growth from each of the polluted and the non-polluted locations. The collection was made during two consecutive years.
Leaf characteristics
Fully and green opened leaves of individual plants were counted. The average total area of leaves of an individual plant was estimated in cm2 by measuring individual leaves on a Licor-3000A leaf area meter (LICOR, Lincoln, USA).
Functional parameters of leaf, like photosynthetic rate (PN), stomatal conductance (gs) and intercellular CO2 were measured by clamping the leaf in situ in the leaf chamber of a portable Li-COR 6400 Photosynthesis System (LI-COR, Lincoln, USA). These measurements were taken on cloud-free days between 09:00 and 11:00 h, from five leaves of each of the five plants at each of the two collection sites.
Photosynthetic pigments
The chl a, chl b and carotenoids contents of leaves were estimated by the method of Hiscox and Israelstam (Citation1979). The absorbance of the reaction mixture was recorded at 480, 510, 645 and 663nm using a Beckman DU640B spectrophotometer (Beckman, Fullerton, USA). Calculations were done by using the formulae of MacLachlan and Zalik (Citation1963) and Duxbury and Yentsch (Citation1956).
NR activity
In vivo NR activity was assayed in fresh leaf samples, following the method of Klepper et al. (Citation1971). Optical density was measured at 540 nm on a Beckman DU640 spectrophotometer.
Nitrate level
The nitrate level was estimated in the normal and the polluted fresh leaf samples, following the standard methods as described by Grover et al. (Citation1978). Optical density was measured at 540 nm on a Beckman DU640 spectrophotometer.
Total soluble protein
Concentrations of soluble proteins in leaves were determined by the method of Bradford (Citation1976), and recording the optical density of reaction mixture at 595 nm with the help of a Beckman DU640 spectrophotometer.
Soluble sugar
The soluble sugar content of roots, stem and leaves was determined by the method of Dey and Harborne (Citation1990). Optical density of reaction mixture was measured at 485 nm on a Beckman DU640 spectrophotometer.
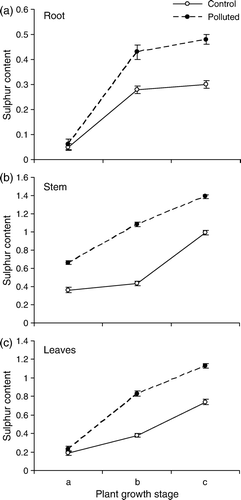
Sulphur content
The sulphur content of roots, stem and leaves was determined by the method of Chesnin and Yein (Citation1950), using the Beckman's DU640 spectrophotometer.
Plant size and biomass accumulation
The length of plant axis from ground level to the upper-most growing tip of the main axis was considered as the shoot length, whereas length of tap root from base to tip as the root length. The measurements were taken in cm. In order to find out dry weights of roots, leaves and shoots, the samples were oven-dried separately at 70°C for 48 h and the dry weight per plant (in g) was determined on a sensitive electronic balance (Sartorious, Germany).
Statistical analysis
The Student's t-test was applied to the data obtained, in order to confirm whether the differences observed between the polluted and the non-polluted plant material were statistically significant.
Results
Photosynthetic parameters
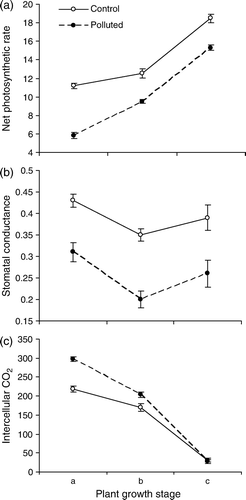
The net photosynthetic rate (PN) in leaves of T. rhomboidea increased constantly with increasing plant age. In the polluted conditions, the PN was relatively low at all the three growth stages examined, the difference from the control being significant in early two stages and non-significant in the post-flowering phase. However, the variation pattern with reference to age remained unchanged. Difference from the control was the maximum in pre-flowering stage (). Stomatal conductance (gs) was highest in pre-flowering stage and lowest in the flowering stage at both the sites, its magnitude being significantly low in the polluted conditions at each stage. The maximum variation, however, was recorded during the flowering phase. Intercellular CO2, on the other hand, was consistently on a decline with the growing plant age, and showed higher accumulations under polluted conditions; the difference from the control was the maximum in the pre-flowering stage ().
Figure 1. The net photosynthetic rate (µmol CO2 m−2 s−1), stomatal conductance (mmol m-2 s−1) and intercellular carbon dioxide (ppm) in the leaves of T. rhomboidea plants growing at the polluted as well as non-polluted sites, as observed at (a) pre-flowering, (b) flowering and (c) post-flowering stages of plant growth. Values (Mean±SE) are based on 50 readings.
Quantitative estimation of chl a, chl b and carotenoids in leaves collected from the two environmental regimes showed a consistent decline with increasing age of the plant, their contents being invariably lower at the polluted site than at the non-polluted one. For each pigment, the loss was maximum and highly significant (p≤0.01) at the post-flowering stage in the stressed population ().
Table 2. Photosynthetic pigments, protein contents, NR activity and nitrate level in T. rhomboidea plants growing at the polluted and the non-polluted sites, as observed at different stages of plant growth. The values (Mean±SE) are based on 30 independent readings.
Chemical/biochemical cell contents
The amount of total soluble proteins in leaf tissues decreased gradually with the increasing age of the plant, and kept relatively lower at the polluted site than at the non-polluted one. The maximum variation (33%) was recorded in the post-flowering stage ().
The NR activity, decreasing with increase in the plant age, was always lower in the polluted conditions, the difference from the control being significant up to the flowering stage only. The maximum difference (38%) figured at the pre-flowering stage ().
Nitrate level in the leaves also showed a declining trend with the growing plant age. The nitrate content was, however, always higher under stressed conditions, the gain being significant in the pre-flowering stage and non-significant in the subsequent stages. The maximum gain (96%) over the control figured at the pre-flowering stage ().
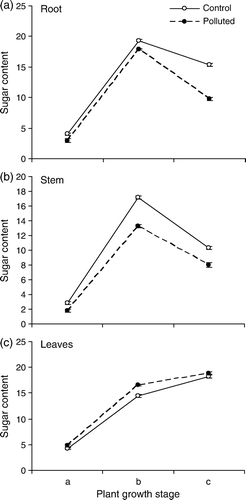
presents a comparative situation of concentration of the soluble sugar in roots, stem and leaves. The amount of sugar in root and stem was lowest in the pre-flowering and highest in the flowering stage of both populations. It was, however, consistently and significantly low at the polluted site. The maximum variation from the control was recorded at the flowering stage for stem and at the post-flowering stage for roots. In leaves, the sugar content showed a rising trend with increasing plant age both at the polluted and the non-polluted sites. In the stressed population, sugar level was significantly higher than in the control at each of the three growth stages, the highest difference occurring at the flowering stage ().
Figure 2. Sugar content (mg g−1 fw) in different parts of T. rhomboidea plants growing at the polluted as well as non-polluted sites, as observed at (a) pre-flowering, (b) flowering and (c) post-flowering stages of plant growth. Values (Mean±SE) are based on 30 readings.
Sulphur content of roots, stem and leaves displayed a rising order in the three consecutive collections. At the polluted site, sulphur level was higher in all the three organs at each sampling stage. The maximum difference from the control was recorded at the post-flowering stage for roots and at the flowering stage for stem and leaves ().
Growth characteristics
The length of root and shoot tended to increase with the increasing age of the plant. At the polluted site, both roots and shoots were longer than at the control site. However, the gain for roots was statistically non-significant in each of the collections, whereas in the stem, it became statistically significant at the flowering stage onwards. Root length was slightly larger under the pollution stress. The maximum difference from the control was noticed at the pre-flowering stage for root length, and at the flowering stage for shoot length (). The total plant height, on the whole, was greater at the polluted site, showing the maximum and highly significant gain at the flowering stage.
Table 3. Plant growth parameters of T. rhomboidea plants growing at the polluted and the non-polluted sites, as observed at different stages of plant growth. The values (Mean±SE) are based on measurements from 10 plants.
The number of leaves per plant increased with plant age as well as with pollution stress. The gain by the stressed population over the control was, however, statistically significant only in the post-flowering phase. The average single leaf area, more or less constant throughout the plant life, underwent a significant decline in the polluted condition, compared with the non-polluted environment. The decline in the leaf area overshadowed the increase in the leaf number so that the total leaf area of a plant displayed a highly significant (p≤0.01) reduction, compared with the control, at each stage of plant growth (). The maximum (36%) variation from the control appeared at the pre-flowering stage ().
The root and stem dry weights increased with age of the plant. At the polluted site, dry weight of roots was slightly less than in the control, from flowering stage onwards, but these changes were statistically non-significant. The stem dry weight, on the contrary, indicated a consistent increase under the pollution stress, though it was significant only from flowering stage onwards. Leaf dry weight, increasing with the age of the plant, was consistently low under the pollution stress, showing a significant difference from the control ().
Discussion
Photosynthesis is one of the foremost processes to be affected by coal-smoke pollution (Husen et al. Citation1999; Iqbal et al. Citation2005). High concentrations of SO2 suppress photosynthesis as in Sorghum bicolor (Joshi et al. Citation1993) and Calendula officinalis (Wali et al. Citation2007). The decrease in phospho-enolpyruvate carboxylase (PEPC) activity and concentration as a result of hydrolysis and mobilization from leaves could inhibit the rate of photosynthesis in the C4 sorghum (Joshi et al. Citation1993). A decrease in photosynthetic rate may either by a decrease in stomatal conductance (Reich Citation1987), by an oxidative damage to the biochemical processes of light harvesting and carbon fixation (Pell et al. Citation1994), or by damage to membranes and other cellular components (Matyssek et al. Citation1991; Low et al. Citation2007). An increase in the atmospheric CO2 shifts the activity of ribulose-l 5 bisphosphate carboxylase and oxygenase (Rubisco) in favor of carboxylation (Stitt Citation1991), depresses stomatal conductance (Field et al. Citation1995) and increases the intercellular CO2 concentration (Farage et al. Citation1991). In the present investigation, the photosynthesis rate was reduced by nearly 48%, 24% and 17% at pre-flowering, flowering and post-flowering stages, respectively. This decline in photosynthesis led to a massive accumulation of intercellular CO2, nearly 27% more than in the control, in the pre-flowering stage. This might have affected the stomatal conductance causing it to decline maximally in the flowering stage.
In the present investigation, the degree of damage to chl a, chl b and carotenoids was almost uniform. The damage was invariably the maximum in the post-flowering stage. Reduction in chlorophyll could be due to the production of superoxide radicals as a result of reaction of sulphite with chlorophyll under illumination. It has been suggested that chl a is degraded to phaeophytin by SO2 by replacing Mg2 + ions from chl molecules, but chl b degradation leads to the formation of chlorophyllide b as SO2 removes the phytol group of the chl b molecules (Williams and Banerjee Citation1995; Nighat et al. Citation2000). Chloroplast damage by coal-dust pollution may cause a reduction in chl concentration in the polluted leaves (Pandey et al. Citation1991). Exposure to pollutants may inhibit chl biosynthesis, as does the N deficiency (Iqbal et al. Citation2000b ,Citation2005).
The decrease in NR activity at the polluted site, as observed in each growth stage of T. rhomboidea, could be due to several reasons such as reduced supply of NADH (Dugger et al. Citation1965), or shortage of NO3 at the site of enzyme synthesis due to water stress (Burzynski and Jacob Citation1983). NR activity shows a negative correlation with nitrate concentration in T. rhomboidea leaves. The enhancement of nitrate leveler was more in pre-flowering stage than in the subsequent stages. Higher level of leaf nitrate content was found in fast-growing pioneer species such as Populus deltoides because of higher uptake of nitrate from the soil, but the NR activity was reduced, which might be allosteric inhibition of increased nitrate level (Soares et al. Citation1995; Nighat et al. Citation2008).
The decrease in the soluble protein content of pollution-affected leaves could be due to either retardation of de novo synthesis of proteins or a breakdown of existing proteins (Khan et al. Citation1990). It might also be a reflection of decreased photosynthesis (Nighat et al. Citation2008). On exposure to SO2, either the protein-synthesizing enzyme is disrupted or the internal ionic media are altered (Williams and Banerjee Citation1995).
In T. rhomboidea, sugar level was significantly reduced in root and stem, but significantly increased in leaves throughout the plant life. A higher sugar concentration in leaves despite a retarted photosynthetic activity, and a reduced sugar level in stem and roots are indicative of a severe damage to the process of translocation of photosynthate from leaves to other parts of the plant axis. Accumulation of reducing sugar and the depletion of starch due to SO2 pollution have been reported in Mangifera indica and Psidium guajava (Kumar and Singh Citation1988). The reducing, non-reducing and total sugar contents of maize decreased under increasing Cd and Zn stress (Narwal and Singh Citation1993).
Since SO2 was one of the major pollutants emitted from the thermal power plants, it could well be able to alter the concentration and dynamics of sulphur in the root, stem and leaves. Therefore, a high S level in the samples from the polluted sites is understandable. The patterns of leaf sulphur concentration of a given species at various stages of plant development are a function of the uptake of both soil and atmospheric sulphur. A large portion of sulphur uptake early in the growing season could be from the soil; this might explain the presence of sulphur in the leaves of plants growing at the reference site. Assimilation of SO2 by plants and its conversion to a less toxic sulphate state are a function of oxidation-reduction potential, which depends on the resistance of individual species, chemical nature of the leaf sap and its ability to detoxify or oxidize the SO2 absorbed, and this is probably why the situation varies from plant to plant (Williams and Banerjee Citation1995).
Root growth may be stimulated by elevated CO2 levels (Rogers et al. Citation1994) and low SO2 levels (Wali et al. Citation2007). On the other hand, length and dry weight of roots were reduced in Melilotus indica exposed to coal-smoke stress (Ghouse and Khan Citation1983) and Medicago sativa exposed to O3 stress (Renaud et al. Citation1997). In the present study, coal-smoke stress stimulated root growth of Triumfetta rhomboidea so as to gain a non-significant increase in length but, interestingly, the root dry weight experienced a marginal loss.
Stems of certain plants have shown stimulated growth under elevated CO2 concentration (Rogers et al. Citation1983) or mixed air pollutants in the coal-smoke (Iqbal Citation1984). Growth enhancement under elevated CO2 is thought to be associated with the consequent availability of carbohydrate for plant growth (Reinert and Ho Citation1995). A slight increase in shoot length was observed in alfalfa (Medicago sativa) when plants were exposed to O3 during early growth stages (Renaud et al. Citation1997). Exposure to a low level of SO2 (0.5 ppm) was gainful for the growth of Calendula officinalis, especially on a sulphur-deficient soil (Wali et al. Citation2007). Stem of T. rhomboidea, in the present study, increased both in length and dry weight at the polluted site. It appears that the pattern of carbon partitioning was altered under stress and a big chunk of phtosynthate was allocated to stem growth. On the whole, T. rhomboidea gained in the overall plant height, up to 61% in flowering stage, under the pollution stress, but the dry-weight measurements went down, thus indicating that the stem axis gained in length but not in girth. It suggests that apical meristems were activated under stress but the activity of lateral meristem was inhibited. Delayed formation of cambial ring and a reduced secondary growth have been observed in Calendula officinalis exposed to SO2 stress (Wali et al. Citation2007). Delay in the seasonal reactivation of vascular cambium and/or a shortening of the active span of cambium were caused in some dicotyledonous trees growing in the vicinity of a thermal power plant using coal as fuel (Iqbal et al. Citation2000c, Rajput et al. Citation2008).
In the present study, leaves were highly sensitive to pollution load and the extent of leaf-area loss was closely related to stomatal responses to the pollution, which in turn reflected upon the overall metabolism and growth of the plant. Although the number of leaves per plant was slightly higher at the polluted site, which could possibly be a compensatory mechani sm adopted by plants under stress, the leaf size was so much reduced that the total leaf area became significantly smaller than at the control site. As a consequence, leaf dry weight was also significantly reduced under the pollution stress. Thus, although the overall size of the plant increased, mainly due to stem axis elongation brought about by stimulated shoot apical meristem, the total dry weight of the plant decreased, which was well in accordance with the reduced rate of photosynthesis.
Acknowledgements
The authors are grateful to Dr. (Ms) Anjum Arshi and Mr Zakir A. Siddiqui of Botany Department, Hamdard University, New Delhi, India, and to Dr. S.S. Hegazy of Plant Production Department, King Saud University, Riyadh, Saudi Arabia, for their assistance in preparation of this manuscript.
References
- Agrawal M. 2003 . Plant responses to atmospheric sulphur . In : ABrol YP , Ahmad A Sulphur in plants . The Netherlands : Kluwer Academic Publishers 279 294 .
- Bell , JNB and Treshow , M. 2002 . Air pollution and plant life , 2nd ed , Chichester, , UK : John Wiley and Sons .
- Bradford , MM. 1976 . A rapid and sensitive method for the quantitation of microgram quantities of protein, utilizing the principle of protein-dye binding . Anal Biochem. , 72 ( 6 ) : 248 – 254 .
- Brown , WH. 1920-21 . Minor products of Philippine forests , Vol. 3 , Manila : Bureau of Forestry .
- Burkill , IH. 1935 . A dictionary of the economic products of the Malay Peninsula, 2 Vols , London : The Crown Agents for the Colonies .
- Burzynski , M and Jacob , M. 1983 . Influence of lead on auxin-induced cell elongation . Acta Soc Bot Poll. , 52 : 231 – 239 .
- Chesnin , L and Yein , CH. 1950 . Turbidimetric determination of available sulphates . Soil Sci Ann Proc , 15 : 149 – 151 .
- Dey PM , Harborne JB. 1990 . Methods in plant biochemistry . II , Carbohydrates . London : Academic Press , 657 .
- Dhir B , Mahmooduzzafar , Siddiqi TO , Iqbal M. 2001 . Stomatal and photosynthetic responses of Cichorium intybus leaves to sulphur dioxide treatment at different stages of plant development . J Plant Biol . 44 : 97 102 .
- Dugger , M , Mudd , JB and Koukol , J. 1965 . Effect of PAN on certain photosynthetic reactions . Arch Environ Health. , 10 : 195 – 200 .
- Duxbury , AC and Yentsch , CS. 1956 . Plankton pigment nomography . J Air Pollut Cont Assoc. , 16 : 145 – 150 .
- Eamus , D and Jarvis , PG. 1989 . The direct effects of increase in the global atmospheric CO2 concentration on natural and commercial temperate trees and forests . Adv Ecol Res. , 16 : 1 – 55 .
- Farage , PK , Long , SP , Lechner , EG and Baker , NR. 1991 . The sequence of change within the photosynthetic apparatus of wheat following short term exposure to ozone . Plant Physiol. , 95 : 529 – 535 .
- Field , CB , Jackson , RB and Mooney , HA. 1995 . Stomatal responses to increased CO2: Implications from the plant to the global scale . Plant Cell Environ. , 18 : 1214 – 1225 .
- Ghouse , AKM and Khan , FA. 1983 . Growth responses of Melilotus indica to air pollutants emerging out of coal burning . Geobios , 10 : 37 – 39 .
- Grover , HL , Nair , TVR and Abrol , YP. 1978 . Nitrogen metabolism of upper three leaf blades of wheat at different soil nutrition levels. NR activity and content of various nitrogenous constituents . Plant Physiol. , 42 : 287 – 292 .
- Hiscox , JD and Israelstam , GF. 1979 . A method for the extraction of chlrophyll from leaf tissue without meceration . Can J Bot , 57 : 1332 – 1334 .
- Husen A , Ali ST , Mahmooduzzafar , Iqbal M. 1999 . Structural, functional and biochemical responses of Datura innoxia Mill. to coal-smoke pollution . Proc Acad Environ Biol . 8 : 61 72 .
- Iqbal , MZ. 1984 . Growth of some plant species at two localities with different levels of atmospheric pollution . Geobios , 11 : 97 – 100 .
- Iqbal M , Bano R , Wali B . 2005 . Plant growth responses to air pollution . In : Chaturvedi SN , Singh KP Plant biodiversity, microbial interaction and environmental biology . Jaipur : Avishkar Publishers 166 188 .
- Iqbal M , Jacob M , Mehta S , Mahmooduzzafar . 2000a . Plant responses to particulate matters suspended in the air . In : Iqbal M , Srivastava PS , Siddiqi TO Environmental hazards: Plants and people . New Delhi : CBS Publishers 80 112 .
- Iqbal M , Jura-Morawieck J , Wloch W , Mahmooduzzafar. Foliar characteristics, cambial activity and wood formation in Azadirachta indica A. Juss. as affected by coal-smoke pollution . Flora in press .
- Iqbal M , Srivastava PS , Siddiqi TO . 2000b . Anthropogenic stresses in the environment and their consequences . In : Iqbal M , Srivastava PS , Siddiqi TO Environmental hazards: Plants and people . New Delhi : CBS Publishers 1 38 .
- Iqbal M Mahmooduzzafar Abdin MZ . 2000c . Studies on anatomical, physiological and biochemical response of trees to coal-smoke pollution around a thermal power plant . Research Project 14/62/89-MAB , Ministry of Environment & Forests, Govt. of India , New Delhi
- Joshi , UN , Arora , SK and Luthra , YP. 1993 . SO2 induced changes in CO2 fixation and photosynthetic pigments in Sorghum bicolor leaves . Ann Biol. , 9 : 102 – 108 .
- Khan , AM , Pandey , V , Shukla , J , Singh , N , Yunus , M , Singh , SN and Ahmad , KJ. 1990 . Effects of thermal power plant emission on Catharanthus roseus L . Bull Environ Cont Toxicol. , 44 : 865 – 870 .
- Khan , S , Begum , T and Singh , J. 1996 . Effect of flyash on physico-chemical properties and nutrient status of soil . J Environ Health. , 38 : 41 – 46 .
- Klepper , L , Flesher , D and Hageman , RH. 1971 . Generation of reduced nicotinamide adenine dinucleotide for nitrate reduction in green leaves . Plant Physiol. , 48 : 580 – 590 .
- Krupa , SV and Kickert , RN. 1989 . The green house effect: impacts of ultraviolet-B (UV-B) radiation, carbon dioxide and ozone on vegetation . Environ Pollut. , 61 : 263 – 393 .
- Kumar , N and Singh , V. 1988 . Sensitivity of Mangifera indica and Psidium guajava plants to SO2 pollution . Natl Acad Sci Lett. , 11 : 167 – 172 .
- Lone , FA , Saheed , S and Ghouse , AKM. 1995 . “ Impact of power plant emissions on the metabolic activities of Calotropis procera ” . In Environ adaptive biology of plants , Edited by: Chawan , DD . 283 – 287 . Jodhpur : Scientific Publishiers .
- Low , M , Haberle , K-H , Warren , CR and Matyssek , R. 2007 . O3 flux-related responsiveness of photosynthesis, respiration and stomatal conductance of adult Fagus sylvatica to experimentally enhanced free-air O3 exposure . Plant Biol. , 9 : 197 – 206 .
- MacLachlan , S and Zalik , S. 1963 . Plastid structure, chlorophyll concentration and free amino acid composition of a chlorophyll mutant of barley . Can J Bot. , 41 : 1053 – 1062 .
- Matyssek , R , Gunthardt-Georg , MS , Keller , T and Scheidegger , C. 1991 . Impairment of the gas exchange and structure in birch leaves (Betula pendula) caused by low ozone concentrations . Trees , 5 : 5 – 13 .
- Narwal , RP and Singh , M. 1993 . Effect of cadmium and zinc application on quality of maize . Indian J Plant Physiol. , 36 : 170 – 173 .
- Nighat F Mahmooduzzafar Iqbal M . 2000 . Stomatal conductance, photosynthetic rate, and pigment content in Ruellia tuberosa leaves as affected by coal-smoke pollution . Biol Plant . 43 : 263 267 .
- Nighat F Mahmooduzzafar Iqbal M . 2008 . Coal-smoke pollution modifies physio-chemical characteristics of tissues during the ontogeny of Peristrophe bicalyculata . Biologia 63 : 1128 1134 .
- Pandey , DD , Sinha , CS and Tiwari , MG. 1991 . Impact of coal dust pollution on biomass, chlorophyll and grain characteristics of rice . Biol J. , 3 : 51 – 55 .
- Pell EJ , Landry LG , Eckardt NA , Glick RE . 1994 . Effects of gaseous pollutants on ribulose-biphosphate carboxylase oxygenase: Effects and implications . In : Alscher RG , Wellburn AR Plant responses to the gaseous environment: Molecular, metabolic and physiological aspects . London : Chapman and Hall 239 254 .
- Rajput , KS , Rao , KS and Kim , YS. 2008 . Cambial activity and wood anatomy in Prosopis spicigera (Mimosaceae) affected by combined air pollutants . IAWA J. , 29 : 209 – 219 .
- Rao , MN and Rao , HVN. 1989 . Air pollution , New Delhi : McGraw Hill Book Co .
- Reich , PB. 1987 . Quantifying plant response to ozone: A unifying theory . Tree Physiol. , 3 : 63 – 91 .
- Reinert , RA and Ho , MC. 1995 . Vegetation growth of soybean as affected by elevated carbon dioxide and ozone . Environ Pollut. , 89 : 89 – 96 .
- Renaud , JP , Allard , G and Mauffette , Y. 1997 . Effects of ozone on yield growth and root starch concentration of two alfalfa (Medicago sativa L.) cultivars . Environ Pollut. , 95 : 273 – 281 .
- Rogers , HH , Bingham , GE , Cure , JD , Smith , JM and Surano , KA. 1983 . Responses of selected plant species to elevated carbon dioxide in the field . J Environ Qual. , 12 : 569 – 574 .
- Rogers , HH , Runion , GB and Krupa , SV. 1994 . Plant responses to atmospheric carbon dioxide enrichment with emphasis on roots and rhizosphere . Environ Pollut. , 83 : 155 – 189 .
- Schmidt , W , Neubauer , C , Kolbowski , J , Schreiber , U and Ubach , W. 2004 . Comparison of effects of air pollutants (SO2, O3, NO2) on intact leaves by measurement of chlorophyll fluorescence and P700 absorbance changes . Photosynthesis Res. , 25 : 241 – 248 .
- Soares , DA , Ming , SY and Pearson , J. 1995 . Physiological indicators and susceptibility of plants to an acidifying atmosphere pollution . Environ Pollut. , 87 : 159 – 166 .
- Stitt , M. 1991 . Rising CO2 levels and their potential significance for carbon flow in photosynthetic cells . Plant Cell Environ. , 14 : 741 – 762 .
- Wali B , Iqbal M Mahmooduzzafar 2007 . Anatomical and functional responses of Calendula officinalis L. to SO2 stress as observed at different stages of plant development . Flora 202 : 268 280 .
- Williams , AJ and Banerjee , SK. 1995 . Effect of thermal power plant emissions on the metabolic activities of Mangifera indica and Shorea robusta . Environ Ecol. , 13 : 914 – 919 .