Abstract
Salinity is one of the major anthropogenic as well as environmental stresses that reduce plant growth. Results show that even after being adapted up to 6% sodium chloride (NaCl) concentration, all selected isolates were able to solubilize phosphate, and produce phytohormones, siderophores and 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase enzyme. NT1 was found to exhibit the highest phosphate solubilization zones (25 mm), siderophore production (1000 µg ml−1) as well as ACC deaminase production (50 µMmg−1h−1) potential under laboratory conditions. On the other hand, pot studies conducted on tomato plants under 2% NaCl stress proved that C4 and T15 were the best growth promoters. C4 showed 50% enhancement in root and shoot length as compared to NaCl added untreated plants as well as in absence of NaCl. C4 also enhanced salinity tolerance in plants with the lowest uptake of NaCl thereby reducing the salt stress on plants. C5 enhanced biomass production in tomato plants with increased uptake of the salts by plants, thereby reducing the salt concentration in the soil. The study thus shows that the selected isolates can be used for the plant growth promotion of plants under salinity stress.
Introduction
The expansion of irrigation has been one of the key strategies in achieving self sufficiency in food production. This has been essentially achieved through over use of ground water. In almost all cases, the water table, which was several meters deep prior to the introduction of irrigation, has risen up and has played a significant role in increasing salinization. One of the major complications to this process is the increase in the concentration of soluble salts in the root zone of soils, which affects the rhizospheric populations thereby affecting the plant productivity. This is more evident in the coastal agri-ecosystems due to constant ingress of seawater, mismanagement of coastal irrigation areas and faulty agricultural practices. Salinity is one of the serious environmental problems that cause osmotic stress and reduction in plant growth and crop productivity in irrigated areas of arid and semiarid regions (Cicek and Cakirlar Citation2002). Salt stress affects many aspects of plant metabolism and as a result, growth and yield are reduced. Excess of salt in soil solution may adversely affect plant growth either through osmotic inhibition of water uptake by roots or specific ion effects. Salinity increases the uptake of Na+, which eventually results into decrease in uptake of Ca2+ and K+ (Yildirim et al. Citation2006).
Accumulation of excess Na+ may cause metabolic disturbances in processes where low Na+ and high K+ or Ca+2 are required for optimum function (Marschner Citation1995). Uptake and accumulation of Cl- may disrupt photosynthetic function through the inhibition of nitrate reductase activity (Xu et al. Citation2000). Once the capacity of cells to store salts is exhausted, salts build up in the intercellular space leading to cell dehydration and death (Sheldon et al. Citation2004). At higher salinity, the expansion rate of the leaf area reduces together with a decrease in leaf production rate and in leaf size leading to the death of the plant (Suarez and Medina Citation2005).
Ethylene, a plant hormone, also known as a stress hormone, is released by the plant as a physiological response when exposed to a variety of different stresses including both edaphic and adaphic. Salinity can increase the rate of ethylene biosynthesis via elevated levels of 1-aminocyclopropane-1-carboxylic acid (ACC), which may lead to physiological changes in plant tissues. Any check on this accelerated ethylene production in plants can improve growth of plants under salt stress. Recently it has been found that bacteria containing ACC deaminase enzyme can hydrolyze the endogenous levels of ACC, an immediate precursor of ethylene, into ammonia and a-ketobutyrate resulting in reduced production of C2H4 (Hontzeas et al. Citation2004).
Many plant growth promoting rhizobacteria (PGPR) facilitate plant growth indirectly by reducing plant pathogens or directly by facilitating the uptake of nutrients from environment. It has also been observed that plants inoculated with PGPR having different PGPR traits are more resistant to the deleterious effect of stress ethylene synthesized as a consequence of stress conditions (Zahir et al. Citation2008). In addition to facilitating plant growth, PGPR can protect plants from the deleterious effects of environmental stresses including flooding, drought, salinity, heavy metals and phytopathogens (Mayak et al. Citation2004; Yildirim et al. Citation2006). PGPR tested on the growth of pepper, bean, canola and lettuce under salt stress ameliorated deleterious effects of salinity (Glick et al. Citation1997). Reports also show an enhancement of squash plant when applied directly or as a transplant under salinity stress (Yildirim et al. Citation2006). Inoculation of plant with ACC deaminase containing PGPR has also resulted in enhanced chlorophyll contents of maize as well as lettuce (Glick et al. Citation1997; Han and Lee Citation2005).
Today much of the agricultural land, as well as coastal land in Gujarat, has become saline due to faulty irrigation practices and excessive irrigation. Reckless usage of different pesticides and agrochemicals has worsened the problem further. Hence, this study was conducted to reveal the behavior of the selected PGPR under salinity stress condition and their role in enhancing growth of tomato.
Materials and methods
Isolation and identification of the cultures
Five different bacterial strains were isolated from the tomato fields, purified and checked for their cultural and morphological characteristics. The best performing strains were then identified on the basis of 16S rDNA sequencing.
Determination of bacterial NaCl tolerance level
Bacterial strains were purified and grown on a nutrient agar plate. Nutrient broth (10 ml) was supplemented with NaCl so as to give 1–8% NaCl. Each tube was then added with actively growing selected PGPR and incubated on rotary shaker (150 rpm) at 37°C. Bacterial growth was determined as OD540 to find out NaCl tolerance. All the isolates were made resistant to 100 µg ml−1 rifampicin by one step mutation. Rifampicin-resistant PGPR were grown overnight in nutrient broth without adding NaCl. These actively growing bacteria were then serially adapted to 6% NaCl concentration.
Influence of 6% NaCl on phosphate solubilization and IAA production by the selected PGPR
Phosphate solubilization by wild type and NaCl-resistant isolates was checked using tricalcium phosphate (Pikovskyaya Citation1948) as insoluble phosphate in the form of tricalcium phosphate. Spot inoculation of the isolates was done in the center of the Pikovskyaya's medium amended with 6% NaCl. These plates were then incubated at 37°C for 48–72 h. Phosphate solubilization was checked in the form of a clear halo formed around the colony representing the production of organic acids as a possible mechanism of the phosphate solubilization. Indole acetic acid (IAA) production by parent and NaCl-resistant cultures was checked in trypton yeast medium amended with 6% NaCl. Bacteria were grown in 50 ml yeast extract broth supplemented with 50 mgl−1 of L-tryptophan and incubated in the dark on an orbital shaker at 200 rpm for 72 h. IAA production was checked in supernatant using Salkowsky's reagent method (Sarwer and Kremer Citation1995).
Influence of 6% NaCl on siderophore production by PGPR
Siderophore production by the adapted cultures was studied in succinic acid medium amended with 6% NaCl. Quantitative and qualitative siderophore production was carried out using Arnow's method (Arnow Citation1937) for catecholate type of siderophores and Csaky's method (Csaky Citation1948) for hydroxamate type of siderophores.
ACC deaminase production in the presence of 6% of NaCl
The ACC production pattern of adapted cultures was studied in the presence of 2% of NaCl in ACC production medium. The method of ACC deaminase production was carried out as described by Penrose and Glick (Citation2003) except that the medium was supplemented with 2% of NaCl. Production of ACC deaminase was measured as the amount of a-ketobutyrate produced when the enzyme cleaves ACC.
Pot study in the presence of 2% NaCl concentration
A pot study was carried out in triplicates on tomato during the monsoon season using 500 g of soil in 10 cm pots filled up to 6 cm height. Seeds were surface sterilized by gently shaking with 70% ethanol (5 min), 20% sodium hypochlorite solution followed by three rinses in sterile distilled water (SDW). Thereafter seeds were soaked overnight in SDW and germinated on a sterile cotton covered Petri dish. After three days of germination, four seeds were planted in the pots filled with unsterile soil with externally added NaCl so as to get a final concentration of 2%. Thirty ml of active culture was added in each pot, which was watered with 10 ml DW everyday. After 45 days of sowing (DOS), all plants were carefully uprooted and various vegetative parameters like root length, shoot length, number of leaves, chlorophyll content, wet weight, colonization pattern etc., were studied (Nautiyal Citation1997; Kausar and Shahzad Citation2006).
NaCl uptake by plants
NaCl taken up by the plants was estimated by the method of wet washing. The oven-dried plant sample was taken and crushed in mortar. This crushed plant material (20 mg) was then added to a mixture of HNO3 and HClO4 in the ratio of 87:13. The plant was then heated to 70°C for about 4 h and then at 100°C for 3 h. This plant digest was then used for estimating absorbed Na through AAS (Shimadzu 600) (Pesqueira et al. Citation2006).
Residual NaCl in soil
The presence of salt in soil was estimated by collecting a 2 mm layer of soil from the pot. Overnight oven-dried soil (1 g) was powered in a mortar and pestle, then mixed with 5 ml of MQ water and then shaken for about 1 h at room temperature. Concentrated HNO3 was added so as to give a concentration of 1% in supernatant and centrifuged at 10,000 rpm for 10 min. The supernatant was then used for estimating Na by using AAS. The results of these experiments are presented as tolerance index (Ti) where Ti=RLs/RLc, where RLs stands for root length of plant grown in the presence of salt and RLc stands for root length of plants grown in the absence of salt. Rate of salt transfer from soil to plant (Tf=NaCl in plant/ NaCl in soil), known as transfer factor was also calculated on the basis of salt content in plant and salt content in soil (Pesqueira et al. Citation2006).
Statistical analyses
Statistical analysis of all tests was carried out using ANOVA at p<0.05 level. Analyses were also carried out using the t-test between varieties of treatments. All tests were conducted in triplicate.
Results and discussion
Isolation and identification of bacterial strains
Bacterial strains isolated from the tomato rhizosphere were found to be a gram negative type of bacteria. Three of the best performing strains were found to be from the pseudomonas group. NT1 was found to be P. fluorescens, T15 was identified as P. aeruginosa, whereas C4 was identified as P. stutzeri on the basis of 16S rDNA sequencing and comparing the sequences by BLAST (ncbi.nlm.gov.blast/Blast.cgi) database.
Influence of NaCl on growth of PGPR
Results showed that all isolates could tolerate 8% of NaCl stress after which no growth was observed revealing that the LD95 of NaCl for all PGPR was 8%. Results also showed that when 1% NaCl was added to the growth medium, it enhanced the growth of all isolates, after which a continuous decrease was observed in the growth. As the concentration increased, the growth of bacteria was found to decrease. Increase in salt concentration outside the cell membrane increases osmotic potential by creating hyper-osmotic pressure which may be one of the major reasons for reduced growth of PGPR.
Phosphate solubilization and IAA production by selected PGPR in the presence of 6% NaCl
When phosphate solubilization potential of selected PGPR at 6% NaCl adapted cultures was studied, results show that after 48 h of incubation, all isolates showed phosphate solubilization zone on tricalcium the phosphate (TCP) agar plate. This showed that even after being adapted to grow at 6% NaCl concentration, selected PGPR maintained their PSA which can be of great importance assisting the plant growth even under high salinity stress. The maximum zone of TCP solubilization after 24 and 48 h of incubation was observed in NT1 isolate (25 mm), whereas its wild-type strain showed a zone of 24 mm after 48 h of incubation in absence of 6% NaCl (). The presence of 6% NaCl reduced phytohormones production by the adapted isolates as compared to their wild-type strains. Maximum IAA production was reported by both wild-type and adapted C5 isolate, which was reported to be 86.5 and 63 µg ml−1, respectively, after 72 h of incubation ().
Figure 1. Indole acetic acid (IAA) production by selected wild type and rifampicin tagged NaCl adapted cultures in the presence of 6% NaCl concentration. Results showed significant difference at 1% level.
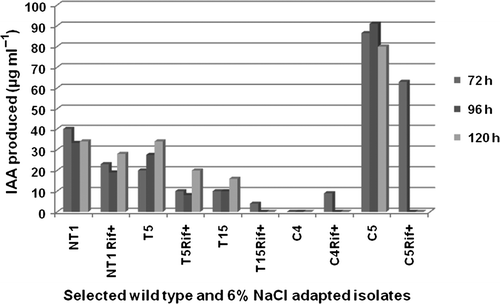
Table 1. Comparative study of phosphate solubilization* by wild type and adapted cultures.
Siderophore production at 6% NaCl concentration in different media by selected PGPR
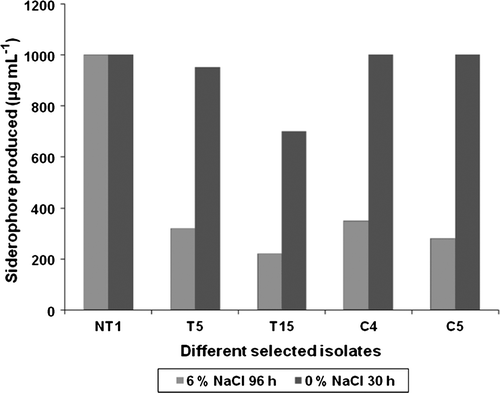
Siderophore production by 6% NaCl adapted rifampicin marked isolates in the presence of 6% NaCl concentration in liquid standard succinic acid medium showed that NT1 was the highest siderophore producer after 72 h and even 96 h of incubation (i.e. 1000 µg ml−1). Siderophore production by selected PGPR in the presence of 6% NaCl also showed that the cultures did not lose their siderophore production efficiency although the siderophore production time increased from 30 h to 72–96 h, which can be justified as the influence of NaCl on growth rate of the isolates. Reduced growth rate resulted in delayed induction of siderophore by the PGPR. Yet all isolates showed siderophore production, which was a significant achievement. The amount of siderophore produced reduced in all isolates except in NT1, which showed that despite maximum decrease in growth rate, it still maintained its potential of ferrous chelation. Isolates C4, T5, C5 and T15 showed reduced siderophore production even after 96 h of incubation, which was less than 50% as compared to their wild-type strains (). NaCl completely inhibited fluorescence in both NT1 and T15 isolates, which showed that the presence of Na+ must be a significant factor in the production of fluorescence by the isolates.
ACC deaminase production in the presence of 6% NaCl
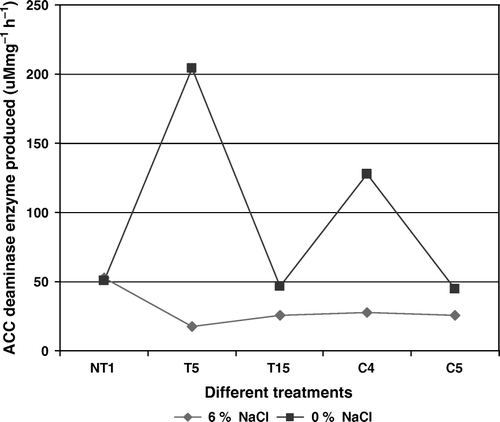
The presence of 6% NaCl in DF medium showed a reduction in ACC deaminase production by the NaCl adapted rifampicin resistant PGPR as compared to their wild-type strains, except in NT1. Maximum ACC deaminase enzyme production was reported in the wild-type strain of T5 followed by C4, which was 207 and 127.9 µMmg−1h−1. On the contrary, the salinity adapted T5 strain showed the lowest ACC deaminase production (10%) as compared to all adapted cultures. No effect of NaCl adaptation was observed on the ACC deaminase production by NT1. ACC deaminase production by NaCl adapted T15 and C5 reduced by just 10 µMmg−1h−1 (). Thus all the selected PGPR, especially NT1, were found to produce ACC deaminase in the presence of salinity of 6% NaCl, which confers on them the potential of plant growth by reducing the ethylene stress. The results of plant growth promotion by selected PGPR besides the presence of salinity can be correlated to the higher production of ACC deaminase enzyme. Similar results were also reported by Zahir et al. (Citation2008) where Pseudomonas sp. containing ACC deaminase production potential enhanced growth of Pisum sativum under drought condition, whereas Kausar and Shahzad (Citation2006) reported the effect of ACC-deaminase containing various Pseudomonas spp. to be an effective PGPR for maize against salinity stress.
Effect of salinity on growth of tomato plants
Effect of 2% NaCl on growth of tomato
The addition of 2% NaCl resulted in reduced growth of tomato plants. Primary root length in the plants grown in the presence of NaCl reduced to about 60% of the root length of the plants grown in the absence of NaCl. A similar decrease was also observed in shoot length, where shoot length of NaCl+plants was just 75% of shoot length of plants without NaCl stress (). Plants showed enhanced fresh weight as well as dry weight when grown in the presence of NaCl (). No significant decrease in the number of leaves and branches was observed, whereas a 15% reduction was observed in the number of lateral roots when grown in the presence of NaCl stress (). The tolerance index was at a minimum amongst all, representing the lowest tolerance of plants towards salinity (). An increase was observed in the chlorophyll a and b content of plants grown in the presence of salinity which was about 100% higher than that of plants grown in the absence of NaCl stress. This shows that tomato plants produced more chlorophyll during salinity stress. Reports suggest that PGPR having a potential of solubilizing phosphorus; calcium and potassium protect the plants against sodium toxicity (Grichko and Glick Citation2001; Egamberdiyeva and Hoflich Citation2003). Satti and Lopez (Citation1994) also showed that an increase in K+ concentration in tomato plants exposed to salt stress could ameliorate the deleterious effect of salt stress.
Figure 4. Influence of PGPR on root and shoot length of tomato plants in the presence of 2% NaCl. C−ve: Control plants without added stress and PGPR: C+ve: Control plants with added salinity but without PGPR. Results showed significant difference at 1% level.
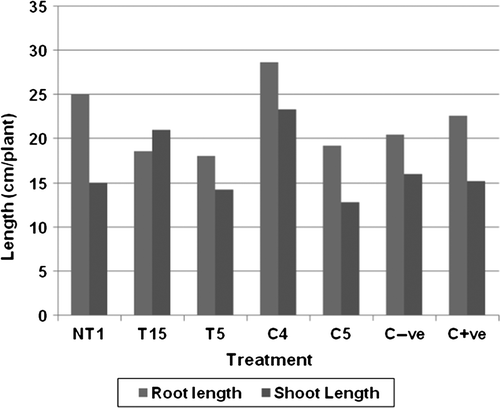
Figure 5. Influence of different PGPR treatments on fresh weight, dry weight and chlorophyll content of tomato in the presence of 2% NaCl. C−ve: Control plants without added stress and PGPR: C+ve: Control plants with added salinity but without PGPR. Results showed significant difference at 1% level.
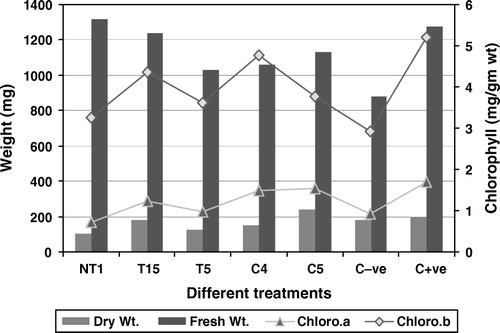
Figure 6. Influence of different PGPR treatments on tolerance index and transfer factor of tomato due to PGPR in the presence of 2% NaCl. C+ve: Control plants with added salinity but without PGPR. Results showed significant difference at 1% level.
Table 2. Influence of PGPR treatments on the number of leaves, branches and lateral root counts as well as leaf size of tomato in the presence of 2% NaCl.
Effect of PGPR treatments on toxicity of 2% NaCl on tomato
As observed, the presence of salinity reduced the plant growth but the stress was relieved in plants inoculated with the PGPR selected as compared to the plants not treated with the PGPR.
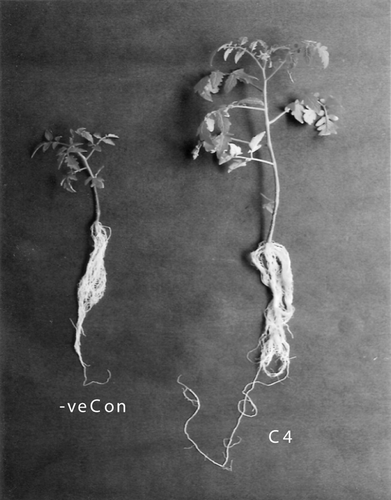
Primary root length enhancement was at a maximum in C4-treated plants followed by NT1-treated plants (), whereas minimum enhancement was reported in T5-treated plants, which was less than even untreated tomato plants. Maximum shoot length was observed in C4, which was about 25% higher than untreated plants. The lowest shoot length was reported in C5-treated plants, which was about 20% less than that of untreated plants. All plants showed a longer root length as compared to shoot length, except the plants grown in the presence of T15 (). Similar results were also stated by Munns (Citation2002) mentioning suppression of plant growth under saline stress, which may be due to the decreasing availability of water or toxicity of high salt concentration. A significant decrease in root length and shoot length was also reported by Kashyap and Sharma (Citation2006) in the presence of high NaCl concentration.
Reduction in primary root length and lateral root count may cause a reduction in nutrient uptake by plants. This may eventually result in reduced plant growth in the form of reduced shoot length as well as the number of leaves. The root elongation might be attributed to the decreased ethylene levels due to the presence of the selected PGPR containing ACC deaminase producing efficiency even in the presence of 6% NaCl. It may be postulated that inoculation with these rhizobacterial strains might have decreased endogenous ethylene levels because of ACC deaminase activity, thereby resulting in the formation of longer roots. This also subsequently results in promoting shoot length. Similar elongation in root and shoot length of maize under 6 dsms−1 NaCl stress in the presence of PGPR was observed by Nadeem et al. (Citation2006). Contrarily, Contesto et al. (Citation2008) and Desbrosses et al. (Citation2009) have reported results that showed that ACC deaminase does have a negative effect on root hair elongation as expected from a decreased ethylene production, but that PGPR triggered-root hair elongation also involves an ethylene-independent pathway, and that this latter effect is stronger than the ethylene-dependent, ACC deaminase-susceptible, pathway. In addition, they show that while it affects root hair growth, PGPR ACC deaminase activity does not affect lateral root growth. An increase (45%) in root length along with shoot length, fresh weight and the number of leaves per plant of pea was also reported by Zahir et al. (Citation2008) when inoculated with ACC deaminase containing P. fluorescens. This result is in accordance with our results where an increase in root length, shoot length, the number of leaves and lateral root count of tomato when inoculated with C4 and T15 under salt stress was observed. Vivas et al. (Citation2003) also reported enhanced root and shoot growth of lettuce when inoculated with Bacillus spp. under dry salt stress conditions.
Maximum increase in fresh weight was reported in plants treated with C4 () and T15, which was equal to that of uninoculated NaCl-added plants. Maximum dry weight was reported in C5-treated plants. Chlorophyll a content was at a maximum in C4- and C5-treated plants. Chlorophyll b content was at a maximum in C4- and T15-treated plants, which was less than NaCl-treated uninoculated plants but higher than untreated uninoculated plants of tomato. On the other hand, NT1-treated plants showed the lowest chlorophyll a and b content, which was almost equal to that of uninoculated untreated plants (). A decrease in overall root and shoot weight has also been reported by Soussi et al. (Citation1998) in chickpea. This reduction in weight of plants under salt stress may be due to the inhibition or hydrolysis of reserved synthesizing food and its translocation to growing shoot parts (Singh et al. Citation2008). Results show an increase in fresh weight and dry weight of plants grown in the presence of NaCl. This must be due to the high uptake of NaCl by plants from the rhizosphere soil, which contained externally added 2% NaCl.
An increase in chlorophyll and carotenoid content in all plants treated with PGPR under saline stress has also been reported by Nadeem et al. (Citation2006), whereas Han and Lee (Citation2005) reported increased chlorophyll a and b in lettuce plants grown in the presence of NaCl stress. Increased chlorophyll content may be the result of increased photosynthetic area of plant leaves at high salt stress by PGPR inoculation compared to control untreated plants where leaf area was reduced due to stress. According to Cheeseman (Citation1988) salinity stress diverts metabolic carbon to storage pools and, as a result, less carbon is available for growth leading to reduced growth of plants.
Analysis of Na estimation in plants and residual Na+ in soil shows that the lowest transfer rate was in plants treated with T15 and C4. This can be considered as one of the factors due to which plants treated with these two isolates were better grown as compared to untreated plants grown in the presence of NaCl. Transfer factors of NT1, T5 and C5 were almost the same but lower than that of untreated plants grown in the presence of NaCl. The tolerance index of C4 was also highest among all treatments making it the best treatment of growth promotion of tomato plants under salinity stress (). Salt stress increases the concentrations of Na+ in the plant tissues. The higher the Na uptake and accumulation, the higher the amount reported as toxic. Similar reports were also observed by Sheldon et al. (Citation2004) where growth of wheat and chickpea under salt stress resulted in Na accumulation up to 1 mg gm−1 plant and 15 mg gm−1 plant at 0.3% NaCl concentrations in soil. He further concluded that accumulation of salt may have also served to decrease the osmotic potential of the plant tissues and facilitates uptake. This high accumulation of Na+ in chickpea appeared to have resulted in reduced growth of plants.
Treatment with NT1 shows lower sodium uptake by plants, whereas high sodium retention in soil reveals that, although plants took up sodium along with other nutrients, PGPR retained most of the sodium in soil thereby lowering the deleterious effects of Na+ accumulation in plants. Similar reports were also quoted by Han and Lee (Citation2005) as well as Vivas et al. (Citation2003) where they mentioned that the presence of PGPR strains could improve production of plant growth regulators and thus enhance plant growth by reducing toxic ion uptake and the formation of stress-specific proteins in plants.
Many studies have shown that high concentrations of sodium and chloride in soil may depress nutrient ion activities and produce extreme ratios of Na/Ca+2 and Na+/K+ in the plants causing the plants to be susceptible to osmotic and specific ion injury as well as to nutritional disorders resulting in reduced yield and quality (Grattan and Grieve Citation1999). The results obtained show that salinity caused an increase in Na+ concentration, but the amount accumulated in the plant differed depending on the treatment.
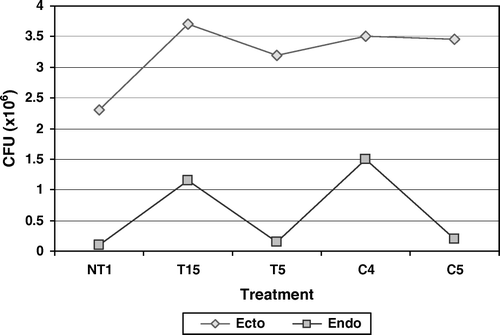
Maximum colonization in ectorhizosphere was reported by T15, which also showed second best colonization at endorhizosphere level. C4 was found to be the second best colonizer of tomato ectorhizosphere, whereas it showed maximum colonization at endorhizosphere level (). Enhanced colonization potential, at endorhizosphere as well as ectorhizosphere level, helps the PGPR to become a dominating population in the root rhizosphere. A PGPR can express its plant growth, promoting potential only if it is able to inhabit the roots of plants. All the plant nutrients released by a PGPR are released in its nearby outer surroundings. If it is not able to colonize the roots, plant roots are not able to absorb these nutrients and nutrients are lost in the surrounding area and no growth promotion effect could be exhibited by the PGPR. Therefore, a colonization pattern may be responsible for enhanced plant growth by a PGPR.
Figure 8. Colonization of PGPR in tomato rhizosphere under 2% NaCl stress. Results showed significant difference at 1% level.
Studies of various vegetative parameters, i.e. leaf size, number of leaves, number of branches and number of lateral roots, show that C4-treated plants best resisted the toxicity of salinity. The plants showed an almost equal number of leaves, branches and lateral roots when treated with C4 as compared to plants grown in the absence of NaCl. The rest of all isolates also showed enhanced number of leaves, branches and lateral roots as compared to NaCl-untreated plants (). It is well documented that the PGPR have a strong effect on lateral root formation as compared to primary root.
Conclusion
Salinity is one of the most critical constraints which hampers agricultural productions in many areas worldwide. However, utilization of salt-affected soils for agriculture is also indispensible to feed the burgeoning population of the world. Although many new physical and chemical methods are being used along with traditional methods to bring salt-affected landmass under irrigation, not much success is viewed anywhere. However, the use of soil rhizosphere bacteria possessing the traits of plant growth promotion under saline stress is becoming prevalent worldwide to achieve sustainable agriculture along with soil reclamation through phytoremediation as well as bioremediation. The selected PGPR in this study adapted to 6% NaCl stress, were able to enhance plant growth efficiently in the presence of 2% NaCl. Fewer reports are available regarding the siderophore and ACC deaminase enzyme production potential of PGPR under saline stress. This work reports that the isolates selected were able to produce siderophores as well as ACC deaminase enzyme, which helped the plants to resist salinity stress. T15 and C4 showed leading results in protecting tomato plants against salinity stress. T15 showed the lowest NaCl uptake rate as a major mechanism of plant growth promotion. C4 also showed the highest tolerance ratio as a mechanism of plant growth promotion. C5 helped in enhancing the biomass of both the plants, thereby minimizing the toxicity of the stress conditions on plant growth as well as reclamation of salt-affected soil. The data represented here is quite encouraging to use these PGPR for enhancing plant growth under saline stress conditions. Results suggest that selected PGPR isolated from the study would be able to regenerate the saline lands and maximize agricultural benefits.
Acknowledgements
Our sincere thanks go to UGC for providing us with the financial help for conducting this study. We also wish to thank the Head of the Department of Microbiology for allowing us to carry out this work at the department of Microbiology.
References
- Arnow , LE. 1937 . Colorimetric determination of the components of 3,4-hydroxyphenylalanine-tyrosine mixtures . Annu Rev Biochem. , 118 : 531 – 537 .
- Cheeseman , JM. 1988 . Mechanisms of salinity tolerance in plants . J Plant Physiol. , 87 : 547 – 550 .
- Cicek , N and Cakirlar , H. 2002 . The effect of salinity on some physiological parameters in two maize cultivars . Bulg J Plant Physiol. , 28 ( 1–2 ) : 66 – 74 .
- Contesto , C , Desbrosses , G , Lefoulon , C , Béna , G , Borel , F , Galland , M , Gamet , L , Varoquaux , F and Touraine , B. 2008 . Effects of rhizobacterial ACC deaminase activity on Arabidopsis indicate that ethylene mediates local root responses to plant growth-promoting Rhizobacteria . Plant Sci. , 175 : 178 – 189 .
- Csaky , TZ. 1948 . On estimation of bound hydroxylamine in biological materials . Acta Chem Scand. , 2 : 450 – 454 .
- Desbrosses , G , Contesto , C , Varoquaux , F , Galland , M and Touraine , B. 2009 . PGPR-Arabidopsis interaction is a useful system to study signaling pathways involved in plant developmental control . Plant Signaling Behav. , 4 : 319 – 321 .
- Egamberdiyeva , D and Hoflich , G. 2003 . Influence of growth promoting bacteria on the growth of wheat in different soils and temperatures . Soil Biol Biochem. , 35 : 973 – 978 .
- Glick , BR , Liu , C , Ghosh , S and Dumbrof , EB. 1997 . Early development of canola seedlings in the presence of the plant growth-promoting rhizobacteria Pseudomonas putida GR12-2 . Soil Biol Biochem. , 29 ( 8 ) : 1233 – 1239 .
- Grattan , SR and Grieve , CM. 1999 . Salinity-mineral nutrient relations in horticultural crops . Sci Hortic. , 78 : 127 – 157 .
- Grichko , VP and Glick , BR. 2001 . Amelioration of flooding stress by ACC deaminase-containing plant growth promoting bacteria . Plant Physiol Biochem. , 39 : 11 – 17 .
- Han , HS and Lee , KD. 2005 . Plant growth promoting Rhizobacteria effect on antioxidant status, photosynthesis, mineral uptake and growth of lettuce under soil stress . Res J Agri Biol Sci. , 1 ( 3 ) : 210 – 215 .
- Hontzeas , N , Saleh , SS and Glick , BR. 2004 . Changes in gene expression of canola roots induced by ACC-deaminase containing plant growth promoting bacteria . Mol Plant Microbe Interact. , 17 ( 8 ) : 865 – 871 .
- Kashyap , S and Sharma , S. 2006 . In vitro selection of salt tolerant Morus alba and its field performance with bioinoculants . Hort Sci (Prague) , 33 ( 2 ) : 77 – 86 .
- Kausar , R and Shahzad , SM. 2006 . Effect of ACC-deaminase containing rhizobacteria on growth promotion of maize under salinity stress . J Agric Soc Sci. , 2 ( 4 ) : 216 – 218 .
- Marschner , H. 1995 . Mineral nutrition of higher plants , 889 London : Academic Press .
- Mayak , S , Tirosh , T and Glick , BR. 2004 . Plant growth-promoting bacteria that confer resistance in tomato plant to salt stress . Plant Physiol Biochem. , 142 : 565 – 572 .
- Munns , R. 2002 . Comparative physiology of salt and water stress . Plant Cell Environ. , 25 : 239 – 250 .
- Nadeem , SM , Hussain , I , Naveed , M , Asghar , HN , Zahir , ZA and Arshad , M. 2006 . Performance of plant growth promoting rhizobacteria containing ACC-deaminase activity for improving growth of maize under salt stressed conditions . Pak J Agric Sci. , 43 ( 3–4 ) : 114 – 121 .
- Nautiyal , CS. 1997 . A method for selection and characterization of rhizosphere-competent bacteria of chickpea . Curr Microbiol. , 34 : 12 – 17 .
- Penrose , DM and Glick , BR. 2003 . Methods of isolating and characterizing ACC deaminase-containing plant growth promoting rhizobacteria . Physiol Planta. , 118 : 10 – 15 .
- Pesqueira , J , García , MD , Staltari , S and Molina , MC. 2006 . NaCl effects in Zea mays L.×Tripsacum dactyloides (L.) L. hybrid calli and plants . Elect J Biotechnol. , 9 ( 6 ) : 286 – 290 .
- Pikovskyaya , RI. 1948 . Mobilization of phosphorus in soil in connection with vital capacity of source microbial species . Microbiologia , 17 : 326 – 370 .
- Sarwer , M and Kremer , RJ. 1995 . Enhanced suppression of plant growth through production of L-tryptophan derived compounds by deleterious rhizobacteria . Plant Soil. , 172 : 261 – 269 .
- Satti , SME and Lopez , M. 1994 . Effect of increasing potassium levels for alleviating sodium chloride stress on the growth and yield of tomato . Commun Soil Sci Plant Anal. , 25 : 2807 – 2823 .
- Sheldon A Menzies NW , Bing SH , Dalal RC. 2004 . The effect of salinity on plant available water . Supersoil 2004: 3rd Australian New Zealand soil conference .
- Singh , N , Pandey , P , Dubey , RC and Maheshwari , DK. 2008 . Biological control of root rot fungus Macrophomina phaseolina and growth enhancement of Pinus roxburghii (sarg.) by rhizosphere competent Bacillus subtilis BN1 . World J Microbiol Biotechnol. , 24 : 1669 – 1679 .
- Soussi , M , Ocana , A and Lluch , C. 1998 . Effect of salt stress on growth, photosynthesis and nitrogen fixation in chickpea (Cicer arietinum L.) . J Expt Bot. , 49 : 1329 – 1337 .
- Suarez , N and Medina , E. 2005 . Salinity effect on plant growth and leaf demography of the mangrove, Avicennia germinans L . Trees , 19 : 721 – 727 .
- Vivas A , Marulanda A , Ruiz-Lozano JM , Barea JM , Azcón R. 2003 . Influence of Bacillus spp on physiological activities of two arbuscular mycorrhizal fungi and plant responses to PEG-induced drought stress . Mycorrhiza 13 : 249 256 .
- Xu , G , Magen , H , Tarchirky , J and Kafkafy , U. 2000 . Advances in chloride nutrition in plants . Adv Agron. , 68 : 97 – 150 .
- Yildirim , E , Taylor , AG and Spittler , TD. 2006 . Ameliorative effects of biological treatments on growth of squash plants under salt stress . Scientia Hort. , 111 : 1 – 6 .
- Zahir , ZA , Munir , A , Asghar , HN , Shaharoona , B and Arshad , M. 2008 . Effectiveness of Rhizobacteria containing ACC deaminase for growth promotion of peas (Pisum sativum) under drought conditions . J Microbiol Biotechnol. , 18 ( 5 ) : 958 – 963 .