Abstract
A pot experiment was carried out to evaluate the effect of Pseudomonas fluorescens and Trichoderma harzianum inoculation on the uptake of zinc (Zn) and cadmium (Cd) by Indian mustard (Brassica juncea) from the soil having three different concentrations of Zn (300, 600, 900 mg/kg) and Cd (5, 10, 15 mg/kg) separately. Microbial inoculation resulted in significantly better plant growth, available metal content and their uptake than control (without microbes). Available Zn was enhanced, ca.1.6- and 1.4-fold and Cd ca. 2.5- and 1.8-fold, by P. fluorescens and T. harzianum, respectively. P. fluorescens resulted in an increase in Zn uptake by 113.9, 51.9 and 58.4% and T. harzianum by 42.6, 32.1 and 33.9% over control from soils having 300, 600 and 900 mg Zn, respectively, while of the corresponding results for Cd were 110.2, 48.9 and 58.1% with P. fluorescens and 42.6, 30.9 and 33.4% with T. harzianum from soil having 5, 10 and 15 mg Cd, respectively, after 90 days of treatment. In general the rate of metal uptake was higher during the initial 30 days and declined later.
Introduction
Soil pollution by heavy metals, due to the application of metal-rich industrial wastes, fertilizers, pesticides, mining activities, etc. (Garbisu and Alkorta Citation2003; Halim et al. Citation2003), assumes great significance owing to the fact that metals do not degrade and are highly mobile through the soil-plant system, resulting in biomagnifications in the food chain (Ryan et al. Citation1982), and hence may affect the living organisms (Nogawa et al. Citation1987). The industrial and agricultural revolution in India has resulted in metal contamination of the soil (Chatterjee and Banerjee Citation1999; Banerjee Citation2003; Krishna and Govil Citation2004; Kumar et al. Citation2005), especially the soil of the western U.P. and Tarai-bhawar regions of Uttarakhand, which are regularly polluted by heavy loads of metals such as zinc (Zn), cadmium (Cd), nickel (Ni), lead (Pb), etc. (Jain et al. Citation2005; Saraswat et al. Citation2007), due to the installation of a variety of brass, electroplating and metallurgical industries, which have adversely impaired the soil fertility status of the region.
Phytoextraction, which makes use of the harvestable part of plants to sequester pollutants, represents a green and environment-friendly tool for cleaning metal-polluted soil and water at low cost (Cunningham and Berti Citation1993; Kumar et al. Citation1995; Raskin et al. Citation1997; Salt et al. Citation1998; Blaylock et al. Citation2000) as opposed to conventional chemical and physical remediation technologies that are generally too costly and often harmful to soil characteristics (i.e. texture and organic matter) (Baker et al. Citation1994; Luo et al. Citation2005). An ideal plant for metal clean-up is one with high biomass, deep root system and high tolerance for metal accumulation and the capacity to translocate in the above-ground portion. However, regardless of the plant used, an efficient metal phytoextraction is often limited by the availability of metals for root uptake, particularly in neutral and alkaline soils. Phytoavailability of metals is strongly influenced by soil characteristics such as pH, cation exchange capacity (CEC), or organic matter content, any of which may limit successful soil remediation (Kayser et al. Citation2000). One promising strategy to improve all these soil characteristics is microorganism-assisted phytoextraction, which rapidly ensures availability of metals by releasing compounds that can desorb metals from the soil matrix to form water-soluble metal complexes into the soil solution for plant uptake (Gadd Citation1993,Citation2004; Guo et al. Citation1996; Jing et al. Citation2007). Although recent studies have revealed that rhizospheric microorganisms are capable of enhancing metal phytoremediation (Carlot et al. Citation2002; Abou-Shanab et al. Citation2003a), as they are able to tolerate, survive and succeed when used in phytoremediation practices (Lucy and Glick Citation2004), the search for efficient plant-microbe interaction for this purpose is warranted. The present study was therefore undertaken to assess the role of microorganisms (Trichoderma harzianum and Pseudomonas fluorescens) assisting Zn and Cd extraction by Indian mustard (Brassica juncea).
Materials and methods
Plant
Seeds of Indian mustard (B. juncea Var. Kranti) were collected from the Seed Processing Centre of the G.B. Pant University of Agriculture and Technology, Pantnagar (29° 01′ 469′' N latitude, 79° 29′ 393′' E longitude and 243.8 masl), Uttarakhand, State of India, and stored before use.
Screening of isolates
Ten isolates of each of the two most common soil bacterium Pseudomonas sp. and fungus Trichoderma sp. were obtained from the Department of Microbiology and Department of Plant Pathology, G.B. Pant University of Agriculture and Technology, Pantnagar, respectively. Isolates of Pseudomonas sp. were maintained in nutrient agar (Hi-Media, India), while that of Trichoderma sp. in potato dextrose agar (PDA, Hi-Media, India) at 28±1°C. These isolates were screened for their optimum tolerance levels against different concentrations of Zn (200, 400, 600, 800, 1000, 1200 mgl-1) as zinc sulfate and Cd (0, 5, 10, 15, 20, 25 mgl-1) as cadmium carbonate. For this, the heavy metals (Zn and Cd) were separately added into the respective media and tolerance was determined by calculating the colony-forming units/ml and spore forming units/ml for Pseudomonas and fungus Trichoderma species, respectively. Based on screening, P. fluorescens was found to tolerate the highest concentration of Zn (1200 mgl-1) and Cd (25 mgl-1). Similarly among the Trichoderma isolates, T. harzianum tolerated 1000 mgl-1 Zn and 20 mgl-1 Cd. These isolates were taken for further study.
Preparation of inoculum
Inoculum of P. fluorescens was grown in 100 ml nutrient broth (Hi-Media) at 28°C and 120 rpm for 18 h until stationary phase was attained. T. harzianum was grown on PDA in roux bottles and incubated for 24 h, after which the greenish spores were extracted using sterilized glass-beads into 100 ml autoclaved distilled water. The respective cultures were then mixed thoroughly with 50 g of talc powder as carrier. Following this, the final population of P. fluorescens was calculated to be 6.2 × 107 cfu/ml and that of T. harzianum spores was 1.3×108 cfu/g of talc. The inoculum was sealed in sterilized plastic bags and stored at 4°C until use.
Pot experiment
The experiment was conducted in greenhouse condition (temperature 22–25°C, light intensity 250 µmol m-2 S-1; under a 14:10 h light/dark cycle). The seeds of B. juncea were surface disinfected for 30 min in 10% (v/v) hydrogen peroxide solution and then washed with deionized water. The seeds were pre-germinated in sterilized moist soil for three weeks, and one healthy plant was transplanted into each pot (15 cm inner diameter) containing 1 kg sterilized soil (pH, 6.5; organic matter, 4.9%; total N (%) 24; K (%) 31; Zn 25 mg/kg; Cd 0.8 mg/kg; Fe 33.7 mg/kg), and mixed thoroughly with prepared talc carrier inoculum. Each pot was fertilized with nitrogen (N), phosphorus (P) and potassium (K) using urea (120 mg N/kg), calcium phosphate (100 mg/kg), and potassium sulphate (50 mg/kg) as a basal fertilizing agent. The pots were irrigated daily with deionized water. The plants were allowed to acclimatize for four days from transplantation. The experiment was designed with 162 pots, 81 pots were treated with Cd (nine pots inoculated with T. harzianum, nine with P. fluorescens and nine as control for each of the three concentrations (5, 10, 15 mg/kg soil) of Cd as cadmium carbonate, and another 81 pots were prepared for three concentrations (300, 600, 900 mg/kg) of Zn as zinc sulfate. As such, each treatment was triplicated. The three concentrations of each metal were selected on the basis of their availability in Indian soil. Fifty four pots were harvested after 30, 60 and 90 days of metal treatment. On each harvest, pot soil and plant of a given treatment were collected for further analysis.
Chemical and biological analysis
The soil samples drawn from various treatments were air-dried and passed through a 2-mm sieve, and chemical properties were determined. Soil pH was measured with a pH meter (McNeal Citation1982). Total N was determined using the Kjeldahl procedure (Bremner and Mulvaney Citation1982) and K was measured using flame photometer (Page et al. Citation1982). Soil organic matter was determined by the Walkley-Black wet combustion method (Page et al. Citation1982). The total soil metal content was determined using an atomic absorption spectrophotometer after digesting 0.5 g of dried soil samples with 15 ml of HNO3, H2SO4 and HClO4 in 5:1:1 ratio at 80°C (Allen et al. Citation1986) and filtered through Whatman No. 42 filter paper followed by dilution up to 50 ml with triple distilled water. The total available metal, i.e. DTPA (diethylene triamine pentaacetic acid)-extractable metals (Cd and Zn) was determined by adding 0.005 M DTPA, 0.01M CaCl2 and 0.1 M triethanolamine (pH 7.3) to give a 1:2 (w/v) soil:solution ratio. After shaking for 60 min, tubes were centrifuged at 17,400 g for 10 min and the supernatants collected after filtering through a Whatman No. 41 filter paper (pore size 20–25 lm) (McGrath and Cunliffe Citation1985). Analysis of the filtrate for Cd and Zn was performed by AAS.
After 30, 60 and 90 days of treatment, plants were uprooted, cleaned with tap water, washed three times with deionized water and oven-dried to a constant weight at 75°C. Then 0.5 g of milled plant material was digested with a mixture of concentrated HCL/HNO3 (4:1 v/v) (American Public Health Association [APHA] Citation1995) and the solution was processed for analysis of total Zn and Cd using Atomic Absorption Spectrophotometer (AAS-SpectrA/A-20, Varian). The availability of heavy metals in soil solution was determined by adding distilled water in the ratio of 1:1 (w/v) (Novozamsky et al. Citation1993). The filterate was analyzed for Zn and Cd using AAS. The plant biomass was estimated on dry weight basis after drying the plants in an oven at 80°C for 48 h. Phytoextraction coefficients (i.e. the ratios between mg metal/g DM of plant and mg metal/g DW of soil) (Kumar et al. Citation1995) and Tolerance Index (TI) (i.e. the ratio between plant biomass measured in treated and the same measured in the control expressed as percentage) (Wilkins Citation1978) was calculated for both metals. Metal removal from the soil was calculated by dividing the total metal uptake per plant with total soil metal concentration (including metal originally present in it) and was expressed in terms of percentage.
Experimental data are presented as mean values±standard error using MS-Excel (2003). To verify the statistical significance of difference among various treatments, the data are analyzed using t-test and one-way analysis of variance as available in the SPSS statistical package (Statgraphics Plus v. 11) and significant level (p<0.05) has been given in appropriate places.
Results
Metal uptake by B. juncea
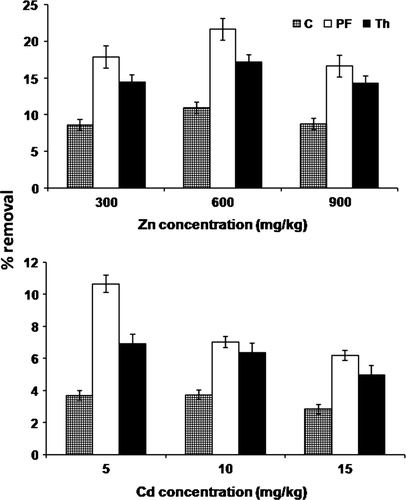
Zinc uptake by plants was at a maximum from the highest concentration, i.e. 900 mg for all the treatment followed by 600 mg and 300 mg (). A similar trend was recorded for Cd, although its accumulation was relatively low as compared to Zn (). A significant increase (p<0.05) in the availability of metals (Zn and Cd) in soil was found after application of microbes. Available Zn was enhanced ca.1.6- and 1.4-fold and Cd ca. 2.5- and 1.8-fold by P. fluorescens and T. harzianum, respectively ( and ). The metal uptake by B. juncea also increased with the inoculation of microbes. Moreover, the percentage increase in Zn uptake due to P. fluorescens inoculation was 113.9, 51.9 and 58.4%, and due to T. harzianum inoculation was 42.6, 32.1 and 33.9% from soils having 300, 600 and 900 mg Zn, respectively, after 90 days of treatment. Similarly for Cd, the percentage increase in uptake was 110.2, 48.9 and 58.1% with P. fluorescens, and 42.6, 30.9 and 33.4% with T. harzianum from soils having 5, 10 and 15 mg Cd, respectively, after 90 days of treatment (). In general, the uptake of both metals was at a maximum in the first 30 days, which declined at subsequent harvests. However, the cumulative uptake of both metals was duration-dependent.
Figure 1. Percent increase in (a) Zn and (b) Cd uptake by B. juncea inoculated with P. fluorescens (Pf) and T. harzianum (Th) as compared with control (without inoculation) at the 90th day of treatment.
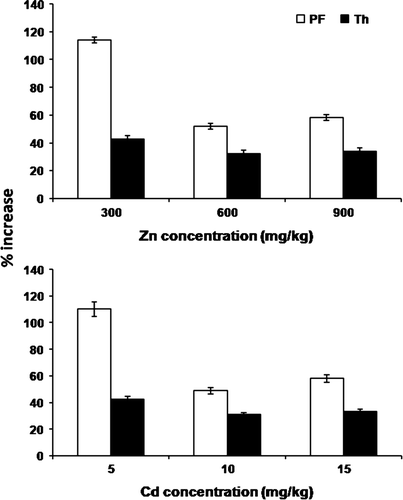
Table 1. Zinc uptake by B. juncea after 30, 60 and 90 days of metal treatment in response to Pf = P. fluorescens, Th = T. harzianum inoculation. Mean values (± SE) marked with different letters in a column differed significantly at p <0.05 (n=3).
Table 2. Cadmium uptake by B. juncea after 30, 60 and 90 days of treatment in response to Pf = P. fluorescens, Th = T. harzianum inoculation. Mean values (± SE) marked with different letters in a column differed significantly at p <0.05 (n=3).
Table 3. Available Zn in soil as influenced by Pf = P. fluorescens, Th = T. harzianum inoculation, during experimental period. Mean values (± SE) marked with different letters in a column differed significantly at p <0.05 (n=3).
Table 4. Available Cd in soil as influenced by Pf = P. fluorescens, Th = T. harzianum inoculation, during experimental period. Mean values (± SE) marked with different letters in a column differed significantly at p <0.05 (n=3).
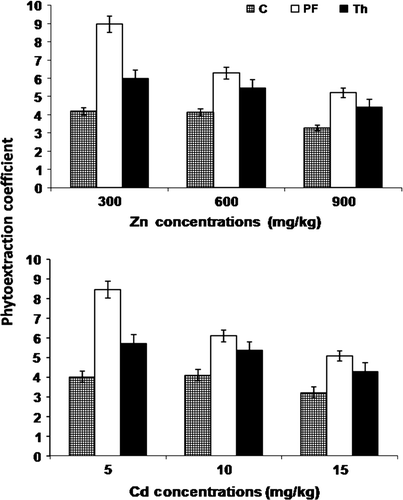
On per plant basis, the percentage removal of Zn was 12.75, 8.81, and 7.21% by P. fluorescens-treated and 7.92, 7.13 and 5.64% by T. harzianum-treated B. juncea from the pot soil with 300, 600 and 900 mg Zn treatments, respectively, after 90 days. Similarly for Cd, the percentage removal was 10.64, 7.01 and 6.19% with P. fluorescens and 6.90, 6.38 and 4.95% with T. harzianum for 5, 10 and 15 mg, respectively. These values were significantly (p<0.05) greater than that observed in control, i.e. without microbes (). As compared to control, the phytoextraction coefficient (PC) for Cd was at a maximum at the lowest concentration, i.e. 5 mg, while the corresponding maximum value for Zn was observed at 300 mg treatment (). Although the PC was enhanced by both bacterial and fungal inoculation, the significant difference (p<0.05) was observed only with P. fluorescens.
Effect of metals on B. juncea growth
The plant biomass exhibited a soil metal concentration-dependent decrease. However, Zn treatments had relatively less effect on biomass production than Cd treatments. Inoculation of microorganism significantly (p<0.05) increased the plant biomass as compared to control; the increase being more pronounced with P. fluorescens (). Furthermore, the plants inoculated with P. fluorescens also exhibited higher Tolerance Index (TI) than that inoculated with T. harzianum (). TI of plants against Zn stress (ca. 143%) was higher than that against Cd (ca.136%).
Figure 4. Tolerance index of B. juncea for (a) Zn and (b) Cd under different treatments at the 90th day of experiment. Pf = P. fluorescens, Th = T. harzianum.
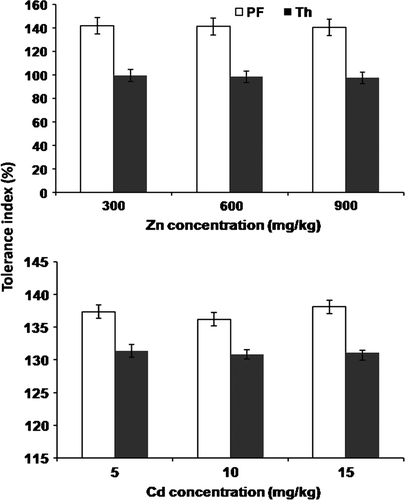
Table 5. Average biomass of B.juncea as influenced by varied soil metal regimes and inoculation by Pf = P. fluorescens and Th = T. harzianum. Mean values (± SE) marked with different letters in a column differed significantly at p <0.05 (n=3).
Discussion
For phytoextraction to occur, metals must be bioavailable, i.e. ready to be absorbed by plant roots. But only small fractions are in accessible form (in soil solution, as free metal ions and soluble metal complexes) and are readily available for plant uptake (Rao et al. Citation2008). The substantial increase in bioavailabilty of Zn and Cd in response to bacterial and fungal inoculation, as observed in the present study, underlines the role of rhizospheric microbes in the synthesis of siderophores and chelators to solubilize and sequester metals from soil (Gadd Citation2004; Braud et al. Citation2007). Microbial inoculation has also been observed to produce several organic acids and phytochelatins ensuring easy availability of metals to plants (Kamnev et al. Citation2005), which in turn brings down the pH of soil towards acidic nature and thereby enhances metal bioavailability (Kunito et al. Citation1998). Besides, the microbial inoculation is noticed to protect the plant against the toxic effects of heavy metals and other abiotic stresses (Belimov et al. Citation2005). In the present study as well, the inoculation with P. fluorescens and T. harzianum enhanced the tolerance index and metal uptake (measured in terms of phytoextraction coefficient) of B. juncea apart from producing significantly more biomass (Egamberdiyeva Citation2007). As such, the increased availability of metals in the presence of microbial inoculation coupled with better metal uptake, as observed in the present study, strengthen the hyperaccumulator behavior of B. juncea for Zn and Cd from metal-rich habitats, as argued by Ebbs et al. (Citation1997), Kumar et al. (1995) and Liphadzi and Kirkham (Citation2005).
Relatively higher metal uptake by B. juncea in response to P. fluorescens inoculation, recorded in the present study, showed conformity with the observation of Rajkumar et al. (Citation2005) and could be ascribed to its plant growth promoting properties such as synthesis of organic acids, chelators, phytochelatins, siderophores (Banchio et al. Citation2008; Dell'Amico et al. Citation2008). However, T. harzianum assisted moderate enhancement in metal uptake is perhaps due to increasing root absorption area and stimulating the acquisition of plant nutrients including metals ions (Khan et al. Citation2000). In general, maximum phytoextraction of both the metals, as observed during the first 30 days of the experiment, depicted the plant age specific metal uptake by B. juncea (Saraswat and Rai Citation2009), which is due to vigorous vegetative growth in the said period. However, the saturation of metal binding sites in response to slow vegetative growth in later phases may also be viewed as one of the factors reducing plant metal uptake during second and third harvests.
Increased soil metal concentration did not exhibit visible symptoms of toxicity except reduced plant growth and biomass production. Elevated levels of metals in the soil lead to excessive absorption by roots and translocation to shoots, and might have resulted in impaired metabolism and reduced plant growth (Foy et al. Citation1978; Bingham et al. Citation1986; McGrath et al. Citation1995). Apart from this, the high concentration of Zn and Cd in the soil might have competed out iron, leading to its deficiency and thus inhibiting both chloroplast development and chlorophyll biosynthesis in plant (Imsande Citation1998). A relatively moderate reduction in plant growth due to increased concentration of Zn in soil, as observed in the present investigation, highlighted it as a plant nutrient, unlike Cd. A substantial increase in plant biomass due to P. fluorescens or T. harzianum inoculation, depicted microbes mediated improvement in plant nutrition (Clark and Zeto Citation2000). Furthermore, P. fluorescens, a known plant growth-promoting bacterium, has also ensured bioavailability of phosphorus for plant uptake, fixing nitrogen for plant use, sequestering essential trace elements like iron by siderophores and producing plant hormones like auxins, cytokinins and gibberellins, beside cleaving the plant ethylene precursor ACC and thus lowering the ethylene level in growing and/or stressed plants due to presence of 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase (Glick et al. Citation1999). Naiman et al. (Citation2009) have also reported that inoculants of Azospirillum brasilense Az1 and Az2 and P. fluorescens Pf increased average aerial biomass by 12%, root biomass by 40% and grain yield of wheat by 16%. Further, the occurrence of induction in the plant's resistance against fungal, bacterial and viral diseases (Maurhofer et al. Citation1998) and enhancement of tolerance against biotic and abiotic stresses, including heavy metals (Whiting et al. Citation2001) in response to PGPR (P. fluorescens) cannot be ignored in the present study. This is also strengthened by the observed higher tolerance index of P. fluorescens treatments. However, such tendency shown by T. harzianum up to a limited extent showed conformity with the observations of Leyval et al. (Citation2002), Malcová et al. (Citation2003) and Zhender et al. (Citation1997) elsewhere.
In general, the percentage increase in Zn uptake by B. juncea was greater when compared to that of Cd, which may be due to the fact that, unlike Cd, Zn is an essential element required for catalyzing numerous enzymes or redox reactions in electron transfer, and has a structural function in nucleic acid metabolism and hence is taken up by specific uptake systems along with some non-specific transports especially at high concentration (Hall and Williams Citation2003). On the contrary, Cd being a non-essential and toxic element, might have imparted toxic effects in terms of low mitotic index, cell division and cell proliferation, chromosomal aberrations, alteration in the synthesis of RNA and slowing down of ribonuclease activity and thus have resulted in greater reduction in B. juncea biomass, as has been opined by Liu and Kottke (Citation2004) and Shah and Dubey (Citation1995) for other crops. The phytoextraction coefficient for Cd was higher at the lowest concentration, i.e. 5 mg, owing to its lowest toxicity at this concentration. On the other hand, Zn – a less toxic, essential nutrient – exhibited a higher phytoextraction coefficient at 300 mg concentration. However, such behavior of these metals observed in the present study, needs fine tuning for better understanding of insights of plant-microbes responses on metal-contaminated sites.
Conclusion
The study clearly demonstrated that a fast growing and high biomass accumulating B. juncea coupled with P. fluorescens or T. harzianum inoculation could be an excellent tool for efficient phytoextraction of heavy metals, especially Zn and Cd from the contaminated soil. However, a thorough understanding of the physical, chemical and biological aspects of rhizospheric process vis-à-vis hyperaccumulator plant species is needed to optimize the phytoremediation process at field level.
Acknowledgements
Research facilities by G.B. Pant University of Agriculture & Technology, Pantnagar, and financial assistance from the Indian Council of Agricultural Research, Govt. of India, in the form of research project are gratefully acknowledged.
References
- Abou-Shanab , RA , Angle , JS , Delorme , TA , Chaney , RL , Van Berkum , P , Moawad , H , Ghanem , K and Ghozal , HA . 2003a . Rhizobacterial effects on nickel extraction from soil and uptake by Alyssum murale . New Phytol. , 158 ( 1 ) : 219 – 224 .
- Allen , SE , Grimshaw , HM and Rowland , AP . 1986 . “ Methods in plant ecology ” . In Chemical analysis , Edited by: Moore , PD and Chapman , SB . 285 – 344 . Oxford : Blackwell Scientific Publications .
- American Public Health Association (APHA) . 1995 . Standard methods for examination of water and wastewater , 17th ed Washington, DC : APHA .
- Baker , AJM , McGrath , SP , Sidoli , CMD and Reeves , RD . 1994 . The possibility of in situ heavy metal decontamination of polluted soils using crops of metal-accumulating plants . Resour Conserv Recy. , 11 ( 1 ) : 41 – 49 .
- Banchio , E , Bogino , PC , Zygadlo , J and Giordano , W . 2008 . Plant growth promoting rhizobacteria improve growth and essential oil yield in Origanum majorana L . Biochem Systemat Ecol. , 36 ( 10 ) : 766 – 771 .
- Banerjee , ADK . 2003 . Heavy metal levels and solid phase speciation in street dusts of Delhi, India . Environ Pollut. , 123 ( 1 ) : 95 – 105 .
- Belimov , AA , Hontzeas , N , Safronova , VI , Demchinskaya , SV , Piluzza , G , Bullitta , S and Glick , BR . 2005 . Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.) . Soil Biol Biochem. , 37 ( 2 ) : 241 – 250 .
- Bingham , FT , Pereyea , FJ and Jarell , WM . 1986 . Metal toxicity to agricultural crops . Metal ions in biological systems , 20 ( 1 ) : 119 – 156 .
- Blaylock MJ , Huang JW , Raskin I . 2000 . Phytoremediation of toxic metals: Using plants to clean up the environment . 53 . New York : John Wiley and Sons Inc .
- Braud , A , Jezequel , K and Lebeau , T . 2007 . Impact of substrates and cell immobilization on siderophore activity by Pseudomonads in a Fe and/or Cr, Hg, Pb containing medium . J Hazard Mater. , 144 ( 1–2 ) : 229 – 239 .
- Bremner , JM and Mulvaney , CS . 1982 . “ Nitrogen-total ” . In Methods of soil analysis , Edited by: Page , AL . Madison, WI : American Society of Agronomy .
- Carlot , M , Giacomini , A and Casella , S . 2002 . Aspects of plant-microbe interactions in heavy metal polluted soil . Acta Biotechnol. , 22 ( 1–2 ) : 1 – 13 .
- Chatterjee , A and Banerjee , RN . 1999 . Determination of lead and other metals in a residential area of greater Calcutta . Sci Total Environ. , 277 ( 2–3 ) : 175 – 185 .
- Clark , RB and Zeto , SK . 2000 . Mineral acquisition by arbuscular mycorrhizal plants . J Plant Nutr. , 23 ( 7 ) : 867 – 902 .
- Cunningham , SD and Berti , WR . 1993 . Remediation of contaminated soils with green plants: An overview . In Vitro Cell Dev Biol. , 29 ( 4 ) : 207 – 212 .
- Dell'Amico , E , Cavalca , L and Andreoni , V . 2008 . Improvement of Brassica napus growth under cadmium stress by cadmium-resistant rhizobacteria . Soil Biol Biochem. , 40 ( 1 ) : 74 – 84 .
- Ebbs , SD , Lasat , M , Brady , D , Cornish , J , Gordon , R and Kochian , LV . 1997 . Phytoextraction of cadmium and zinc from a contaminated soil . J Environ Qual. , 26 : 1424 – 1430 .
- Egamberdiyeva , D . 2007 . The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils . Appl Soil Eco. , 36 ( 2–3 ) : 184 – 189 .
- Foy , CD , Chaney , RL and White , MC . 1978 . The physiology of metal toxicity in plants . Annu Rev Plant Physiol. , 29 ( 1 ) : 511 – 566 .
- Gadd , GM . 1993 . Interactions of fungi with toxic metals . New Phytol. , 124 ( 1 ) : 25 – 60 .
- Gadd , GM . 2004 . Microbial influence on metal mobility and application for bioremediation . Geoderma. , 122 ( 2–4 ) : 109 – 119 .
- Garbisu , C and Alkorta , I . 2003 . Basic concepts on heavy metal soil bioremediation . Eur J Min Proc Environ Protect. , 3 ( 1 ) : 58 – 66 .
- Glick , BR , Pattern , CL , Holguin , G and Penrose , DM . 1999 . Biochemical and genetic mechanism used by plant growth promoting bacteria , London : Imperial College Press .
- Guo , Y , George , E and Marschner , H . 1996 . Contribution of an arbuscular mycorrhizal fungus to the uptake of cadmium and nickel in bean and maize plants . Plant Soil. , 184 ( 2 ) : 195 – 205 .
- Halim , M , Conte , P and Piccolo , A . 2003 . Potential availability of heavy metals to phytoextraction from contaminated soils induced by exogenous humic substances . Chemosphere. , 52 ( 1 ) : 265 – 275 .
- Hall , JL and Williams , LE . 2003 . Transition metal transporters in plants . J Exp Bot. , 54 ( 393 ) : 2601 – 2613 .
- Imsande , J . 1998 . Iron, sulfur and chlorophyll II deficiencies: A need for an integrative approach in plant physiology . Physiologia Plantarum. , 103 ( 1 ) : 139 – 144 .
- Jain , CK , Singhal , DC and Sharma , MK . 2005 . Metal pollution assessment of sediment and water in the river Hindon, India . Environ Monitor Assess. , 105 ( 1–3 ) : 193 – 207 .
- Jing , YD , He , ZL and Yang , XE . 2007 . Role of soil rhizobacteria in phytoremediation of heavy metal contaminated soils . J Zhejiang Univ Sci B. , 8 ( 3 ) : 192 – 207 .
- Kamnev , AA , Tugarova , AV , Antonyuk , LP , Tarantilis , PA , Polissiou , MG and Gardiner , PH . 2005 . Effects of heavy metals on plant-associated rhizobacteria: Comparison of endophytic and non-endophytic strains of Azospirillum brasilense . J Trace Elem Med Biol , 19 ( 1 ) : 91 – 95 .
- Kayser , A , Wenger , K , Keller , A. , Attinger , W. , Felix , H , Gupta , SK and Schulin , R . 2000 . Enhancement of phytoextraction of Zn, Cd, and Cu from calcareous soil: The use of NTA and sulfur amendments . Environ Sci Technol. , 34 ( 1 ) : 1778 – 1783 .
- Khan , AG , Kuek , C , Chaudhry , TM , Khoo , CS and Hayes , WJ . 2000 . Plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation . Chemosphere. , 41 ( 1–4 ) : 197 – 207 .
- Krishna , A and Govil , PK . 2004 . Heavy metal contamination of soil around Pali K Industrial Area, Rajasthan, India . Environ Geol. , 54 ( 1 ) : 1465 – 1472 .
- Kumar , PBAN , Dushenkov , V , Motto , H and Raskin , I . 1995 . Phytoextraction: The use of plants to remove heavy metals . Environ Sci Technol. , 29 ( 5 ) : 1232 – 1238 .
- Kumar MP , Reddy TM , Nithila P , Reddy SJ. 2005 . Distribution of toxic trace metals Zn, Cd, Pb, and Cu in Tirupati soils, India . Soil Sediment Contam . 14 6 : 471 478 .
- Kunito , T , Oyaizu , H and Matsumoto , S . 1998 . Ecology of soil heavy metal-resistant bacteria and perspective of bioremediation of heavy metal-contaminated soils . Recent Res Devel Agric Biol Chem. , 2 : 185 – 206 .
- Leyval , C , Jones , EJ , Val , CD and Haselwandter , K . 2002 . “ Potential of arbuscular mycorrhizal fungi for bioremediation ” . In Mycorrhizal technology in agriculture , Edited by: Gianinazzi , S , Schuepp , H , Barea , JM and Haselwandter , K . 175 – 186 . Boston, Berlin : Birkauser Verlag, Basel .
- Liphadzi , MS and Kirkham , MB . 2005 . Phytoremediation of soil contaminated with heavy metals: A technology for rehabilitation of the environment . S Afr J Bot. , 71 ( 1 ) : 24 – 37 .
- Liu , D and Kottke , I . 2004 . Subcellular localization of cadmium in the root cells of Allium cepa by electron energy loss spectroscopy and cytochemistry . J Biosci. , 29 ( 3 ) : 329 – 335 .
- Lucy , MR and Glick , BR . 2004 . Application of free-living plant growth promoting rhizobacteria . Antonie von Leeuwenhoek. , 86 ( 1 ) : 1 – 25 .
- Luo , C , Shen , Z and Li , X . 2005 . Enhanced phytoextraction of Cu, Pb, Zn and Cd with EDTA and EDDS . Chemosphere. , 59 ( 1 ) : 1 – 11 .
- Malcová , R , Vosátka , M and Gryndler , M . 2003 . Effects of inoculation with Glomus intraradices on lead uptake by Zea mays L. and Agrostis capillaris L . Appl Soil Eco. , 23 ( 1 ) : 55 – 67 .
- Maurhofer , M , Reimmann , C , Sacherer , SP , Heeb , S , Haas , D and Defago , G . 1998 . Salicylic acid biosynthesis genes expressed in Pseudomonas fluorescens strain P3 improve the induction of systemic resistance in tobacco against tobacco necrosis virus . Phytopathology. , 88 ( 7 ) : 678 – 684 .
- McGrath , SP , Chaudri , AM and Giller , KE . 1995 . Long term effects of metals in sewage sludge on soils, microorganism and plants . J Ind Microbiol. , 14 ( 2 ) : 94 – 104 .
- McGrath , SP and Cunliffe , CH . 1985 . A simplified method for the extraction of the metals Fe, Zn, Cu, Ni, Cd, Pb, Ni, Cr, Co, and Mn from soils and sewage sludge . J Sci Food Agric. , 36 ( 1 ) : 794 – 798 .
- McNeal , EO . 1982 . “ Soil pH and lime requirement ” . In Methods of soil analysis. Part 2. Chemical and microbiological properties , Edited by: Page , AL , Miller , RH and Keeney , DR . 199 – 224 . Madison, WI : ASA and SSSA .
- Naiman , A , Latronico , A , Tovagliari , IE and de Salamone , G . 2009 . Inoculation of wheat with Azospirillum brasilense and Pseudomonas fluorescens: Impact on the production and culturable rhizosphere microflora . Eur J Soil Biol. , 45 ( 1 ) : 44 – 51 .
- Nogawa , K , Honda , R , Kido , T , Tsuritani , I and Yamada , Y . 1987 . Limits to protect people eating cadmium in rice, based on epidemiological studies . Trace Subst Environ Health. , 21 : 431 – 439 .
- Novozamsky , J , Lexmond , TM and Houba , VJG . 1993 . A single extraction procedure of soil for evaluation of uptake of some heavy metals in plant . Int J Environ. Anal. Chem. , 51 ( 1–4 ) : 47 – 58 .
- Page AL , Miller RH , Keeney DR. 1982 . Chemical and microbiological properties . Methods of Soil Analysis. , 2nd ed . Madison, WI : American Society of Agronomy .
- Rajkumar M , Nagendran R , Kui Jae Lee , Wang Hyu L , Sung Zoo K. 2005 . Influence of plant growth promoting bacteria and Cr6 + on the growth of Indian mustard . Chemosphere . 62 5 : 741 748 .
- Rao , CRM , Sahuquillo , A and Lopez Sanchez , JF . 2008 . A review of the different methods applied in environmental geochemistry for single and sequential extraction of trace elements in soils and related materials . Water Air Soil Pollut. , 189 : 291 – 333 .
- Raskin , I , Smith , RD and Salt , DE . 1997 . Phytoremediation of metals: Using plants to remove pollutants from the environment . Curr Opin Biotechnol. , 8 ( 2 ) : 221 – 226 .
- Ryan , J , Pahren , H and Lucas , J . 1982 . Controlling cadmium in the human food chain. A review and rationale based on health effects . Environ Res. , 27 : 251 – 302 .
- Salt , DE , Smith , R and Raskin , I . 1998 . Phytoremediation . Annu Rev Plant Physiol. , 49 : 643 – 668 .
- Saraswat , S and Rai , JPN . 2009 . Phytoextraction potential of six plant species grown in multimetal contaminated soil . Chemistry and Ecology. , 25 ( 1 ) : 1 – 11 .
- Saraswat , S , Tewari , S and Rai , JPN . 2007 . Impact of brass and electroplating industry effluent on some physico-chemical and biological properties of soil . J Sci Ind Res. , 66 ( 11 ) : 957 – 962 .
- Shah , K and Dubey , RS . 1995 . Effect of cadmium on RNA level as well as activity and molecular forms of ribonuclease in growing rice seedlings . Plant Physiol Biochem. , 33 ( 5 ) : 577 – 584 .
- Whiting , SN , Souza , MP and Terry , N . 2001 . Rhizosphere bacteria mobilize Zn for hyperaccumulation by Thlaspi caerulescens . Environ Sci Technol. , 35 ( 15 ) : 3144 – 3150 .
- Wilkins , DA . 1978 . The measurement of tolerance to edaphic factors by means of root growth . New Phytologist. , 80 : 623 – 633 .
- Zhender , G , Kloepper , J , Yao , C and Wei , G . 1997 . Induction of systemic resistance in cucumber against beetles by plant growth promoting rhizobacteria . J Eco Entomol. , 90 ( 2 ) : 391 – 396 .