Abstract
A high frequency and rapid regeneration protocol was developed from leaf and nodal explants of Enicostemma littorale Blume on MS medium supplemented with 6-benzyl amino purine (BAP; 3 mg/l) and naphthalene acetic acid (NAA; 1 mg/l). Maximum number of shoots was obtained on medium containing BAP. The regenerated shoots were further elongated on the medium having GA3. In vitro shoots were then excised and transferred to rooting medium containing IAA or IBA (0.5–1.0 mg/l). The rooted plantlets were hardened in polycups containing sterile soil and vermiculite (1:1). Plantlets, thus obtained were finally transferred to a greenhouse. The plantlets showed high survival rate (80%) in the soil.
Introduction
Enicostemma littorale Blume (White Head) is a perennial glabrous medicinal herb (Gentianaceae). It is found distributed throughout the greater part of India and common in coastal areas. The plant is pungent and very bitter, antihelmintic, cures fever and vata diseases. It is also used as stomachic, laxative, antidiabetic, and crushed plant material is applied to snake-bites (Kirtikar and Basu Citation1935).
Seed setting of the plant is very high, yet germination frequency is too poor under natural conditions. Although it is found abundantly in dry lands, the drug content of the plant is very low (about 0.3%). Therefore, a large amount of plant material is required for drug extraction. Commercial exploitation and elimination of natural habitats due to urbanization has led to the gradual extinction of several medicinal plants. This medicinal herb being so important needs conservation as well as propagation both in vivo and in vitro. Micropropagation is one of the effective approaches to conserving such germplasms. Furthermore, genetic improvement is another approach to augmenting drug yielding capacity of the plant. Since there is no report on in vitro propagation of this plant through callogenesis, an attempt of callus culture as the first step was undertaken. The present communication reports the standardization of explant for callusing and regenerate plantlets of E. littorale from nodal and leaf explants.
Materials and method
Plant material
Leaves and stems of Enicostemma littorale (a three-month-old mature plant) were excised from their natural habitat in the Mysore district, Karnataka. They were first washed thoroughly in running tap water for 30 min and then treated with a detergent teepol 5% (v/v) for 3 min. Materials were surface sterilized with 0.1% (m/v) HgCl2 for 4 min and then washed at least three times with sterile distilled water. Leaf and stem explants were cut into small pieces and cultured on solid Murashige and Skoog's (Citation1962) basal medium supplemented with 3% (w/v) sucrose and different concentrations of 6-benzyl amino-purine (BAP) 0.5–3.0 mgl−1, naphthalene acetic acid (NAA) 0.5–1.0 mgl−1, kinetin (Kn) 2.0–3.0 mgl−1 and 6-γγ-dimethyl amino purine (2-iP) 2.0–3.0 mgl−1. The cultures were observed constantly for any response.
Callogenesis and shoot induction
Induction of callus was achieved from leaf and stem explants of Enicostemma littorale on MS medium supplemented with BAP (0.5–3.0 mgl−1) and NAA (0.5–1.0 mgl−1), 2,4-D (0.5–4.0 mgl−1) and BAP (0.5–3.0 mgl−1) and Kn (0.5–3.0 mgl−1) and IAA (0.5–1.0 mgl−1) either alone or in combination. Direct multiple shoots were obtained from leaf and nodal explants on MS medium supplemented with BAP, Kn and 2-ip after 10–15 days of incubation ().
Table 1. Effect of cytokinins and naphthalene acetic acid (NAA) on multiple shoot induction and regeneration of plantlets from leaf and nodal explants of E. littorale.
Shoot elongation and rooting of shoots
The multiple shoots produced on the leaf and stem callus were excised from the parent culture and transferred to MS basal medium containing GA3 (0.5–1.0 mgl−1) for shoot elongation. After three weeks of culture, the elongated shoots (2–3 cm) were excised and transferred for rooting in half strength MS basal medium supplemented with IBA (0.5–1.5 mgl−1) and IAA (0.5–1.5 mgl−1).
Acclimatization
Rooted shoots were carefully taken out of the medium and washed thoroughly in running tap water to remove the traces of medium attached to the roots without damaging. The plantlets were planted in 5 cm poly cups containing sterile soil and vermiculite in the ratio of 1:1. The cups were covered with polythene bags to maintain humidity and kept in a shade house where they were observed for further growth and establishment. After 20–25 days the established plants were transplanted to pots containing garden soil.
Culture conditions and data analysis
Culture conditions were applied in all the experiments uniformly. The pH of the medium was adjusted to 5.8 using 0.1 N NaOH, prior to autoclaving at 121°C for 20 min. The cultures were maintained at 25±2°C under a 12-h photoperiod with a light intensity of 35–40 m-2 S-1 (Philips India Mumbai, India).
All the experiments were repeated at least three times with more than 12 replicates. All the data were subjected to statistical analysis of mean and standard deviation (SD).
Results and discussion
Direct multiple shoot buds were emerged from leaf and nodal explants on MS medium supplemented with BAP, Kn and 2-ip (). Shoot buds were emerged from leaf (A) and nodal (B) explants, after 10–15 days of culturing. A leaf segment of 0.5 cm2 elicited more numbers of multiple shoots when compared to nodal explants in medium containing BAP, Kn and 2-ip. This is quite contrary to the work of Seetharam et al. (Citation2002) in Enicostemma hyssopifolium where they reported that the nodal explants were far better than leaf in inducing multiple shoots. In the present investigation, the regeneration of shoot buds all over the surface of the leaf explants is similar to the work of Venkatachalan et al. (Citation1999) wherein the leaflets of Arachis hypogaea produced shoot buds all over the surface with highest frequency.
Figure 1. In vitro plant regeneration of Enicostema littorale Blume. (A) and (B) Direct multiple shoots from leaf and stem segments on MS medium + 3.0 mgl−1+1.0 mgl−1 NAA, respectively. (C) Initiation of multiple shoots from leaf callus on MS medium + 3.0 mgl−1+1.0 mgl−1 NAA, respectively. (D) Multiple shoot induction from stem callus on MS medium + 3.0 mgl−1+0.5 mgl−1 NAA. (E) Direct root initiation from regenerated shoot on half strength MS medium containing 1.0 mgl−1 IAA after 10 days of culture. (F) Plantlet potted in vermiculate medium in moist chamber.
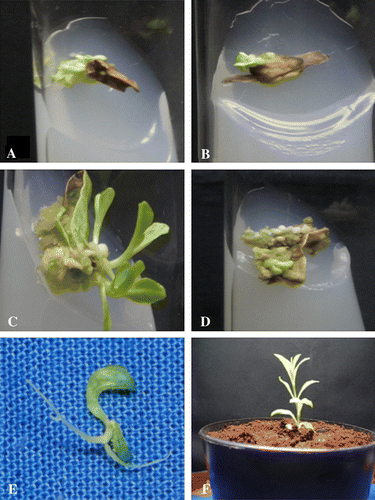
Cytokinins such as BAP, Kn and 2-ip were reported to overcome apical dominance and to promote shoot formation. The highest number of shoots per explant was formed on medium containing 3.0 mg/l BAP and 1.0 mg/l NAA. The average number of shoots per explant was 52±2.83 ().
Medium with 3.0 mg/l Kn and 0.5 mg/l NAA induced only a mean of 19±1.49 shoots per explant. The shoots induced on 2-ip fortified medium yielded a very low percentage of plants. The shoots induced on BAP enriched medium were healthier and thicker than the shoots recovered either on Kn or 2-ip containing medium which showed thin shoots with higher shoot length. Multiple shoot formation was also reported in other medicinal plants, like Phyllanthus caroliniensis (Catapan et al. Citation2000), Bacopa monnieri (Tejavathi and Shailaja Citation1999), Mentha piperata, Curculigo orchioides (Bhavisha and Jasrai Citation2003), Desmodium gangeticum (Asmita and Thirunavoukkarasu Citation2006) and Anthocephalus cadamba (Prem Apurva and Thakur Citation2009).
Callogenesis and shoot induction
Leaf and stem explants cultured on MS medium supplemented with various concentrations of auxin (2,4-D, NAA and IAA) alone or in combination with cytokinin (BAP and Kn) started swelling after five days of culturing. Friable callus was obtained within a week on MS medium supplemented with 2,4-D (1.0 mgl−1) and BAP (0.5 mgl−1). After three to four weeks of culturing, compact green callus, interspersed with dense and glossy texture, was observed.
The callus subcultured on MS medium containing various concentrations of BAP or Kn (0.5–5.0 mg/l) alone or in combination with an auxin NAA showed varied response with respect to the number of shoot buds obtained ().
Table 2 Effect of growth regulators on indirect organogenesis from leaf and stem explants.
However, the best results were obtained with BAP and NAA in combination at various concentrations. The combination of BAP (3.0 mg/l) and NAA (1.0 mg/l) proved optimal for maximum shoot differentiation. This is in agreement with the reports in Jatropha curcas (Rajore and Batra Citation2007). The presence of BAP is obligatory for the induction and proliferation of shoot buds. Sinha et al. (Citation2000) reported the necessity of BAP for shoot induction of Albizia chinesis. The differential effect of various concentrations of BAP on the stimulation of shoot bud formation from various medicinal plant species has been reported before (Ravishankar and Venkataraman Citation1988; Rani and Grover Citation1999). BAP was reported to be the most effective growth regulator for shoot differentiation in other species. The survival rate obtained in the present study was 80% from the leaflet explants.
The three-week-old callus was subcultured onto the regeneration medium fortified with a range of 2 and 3 mgl−l BAP and 0.5 and 1.0 mgl−l NAA. Within 15 days of incubation, fleshy primary callus became hard and nodular and showed the organization of photosynthetic nodules, from which shoot buds were differentiated. The interaction of higher levels of BAP or Kn with lower levels of NAA induces the caulogenic potency of the callus and the same has been reported in some of the medicinal species (Yusuf et al. Citation2001; Nagaraja et al. 2003). In E. littorale leaf callus culture a high frequency of shoot bud differentiation was noticed at the concentration of 3.0 mg/l BAP and 1.0 mg/l NAA. From each segmented leaf callus, 86.8±3.9 shoots were observed (C). At 2.0 mg/l BAP and 0.5 mg/l NAA, the number of shoots per callus was reduced to 52.4±2.9. Likewise high frequency shoot bud differentiation from stem callus was observed at 3.0 mg/l Kn and 0.5 mg/l NAA and it was 51.4±3.2 shoots from each stem callus (D). The number of shoots per explant was reduced to 16.8±1.8 shoots at 2.0 mg/l Kn and 0.5 mg/l NAA.
Shoot elongation and Rhizogenesis
The differentiated shoots either directly or indirectly were transferred to MS medium augmented with different concentrations of GA3 (0.0–3.0 mgl−l) for shoot elongation. At 1.0 and 1.5 mg/l GA3, elongation of shoots was observed up to 8–10 cm, similar to the reports by other workers (Nirmal et al. Citation2000; Xie and Hong Citation2001). At other concentrations of GA3, there is a considerable decrease in the frequency of shoot length.
Individual shoots were excised and implanted in half strength MS basal liquid medium as well as with auxins such as IAA and IBA. MS medium containing either IAA or IBA enhanced the rate of frequency of root induction. At 1.0 mg/l of IAA, root initiation was observed after 10 days of inoculation (E).
Acclimatization
Plantlets measuring 10–12 cm were subsequently transferred to polycups (F) containing sterile soil and vermiculite (1:1). These plantlets after acclimatization were transferred to a greenhouse with 80% survivability. In conclusion, the above prime protocol describes large scale production of plantlets through leaf and stem explants, which can ensure a stable supply of this medicinally important plant irrespective of any seasonal variation and may serve as a source for biologically active compounds. The observations in E. littorale clearly indicate the high regenerative potential in culture, which can be exploited to obtain somaclones within a short span of time.
References
- Asmita , B and Thirunavoukkarasu . 2006 . In vitro micropropagation of Desmodium gangeticum (L.) DC through nodal explants . Ind J Plant Physiol. , 11 ( 1 ) : 83 – 88 .
- Bhavisha , BW and Jasrai , YT. 2003 . Micropropagation of an endangered medicinal plant Curculigo orchioides Gaertn . Plant Tiss Cult. , 13 ( 1 ) : 13 – 19 .
- Catapan , E , Otuki , MF and Viana , AM . 2000 . In vitro culture of Phyllanthus caroliniensis . Plant Cell Tiss Organ Cult. , 62 : 195 – 202 .
- Kirtikar KR , Basu BD. 1935 . Indian medicinal plants III . Saujanya Books , Dehradun, , India .
- Murashige , T and Skoog , F . 1962 . A revised medium for rapid growth and bioassays with tobacco tissue culture . Physiologia Plant. , 15 : 473 – 497 .
- Nagaraja , YP , Krishna , V and Maruthi , KR. 2000 . Rapid micropropagation of Andrographis alata through leaf culture . Plant Cell Biotech Mol Biol. , 4 : 3 – 4 .
- Nirmal , BK , Anu , A , Ramashree , AB and Praveen , K . 2000 . Micropropagation of curry leaf tree . Plant Cell Tiss Organ Cult. , 61 : 199 – 203 .
- Prem , Apurva and Thakur , PC . 2009 . Somatic embryogenesis and root proliferation from internode of Anthocephalus cadamba . In vitro Asian J Exp Sci. , 23 ( 1 ) : 99 – 122 .
- Rani , G and Grover , IS . 1999 . In vitro callus induction and regeneration studies in Withania somifera . Plant Cell Tiss Organic Cult. , 1 : 23 – 27 .
- Rajore , S and Batra , A . 2007 . An alternative source for regenerable organogenic callus induction in Jatropha curcas L . Ind J Biotech. , 6 : 545 – 548 .
- Ravishankar , GA and Venkataraman , L . 1988 . Rapid multiplication of plants from cultures of auxillary bud of Mentha piperata . Philipp J Sci. , 117 : 121 – 130 .
- Seetharam , YN , Barad , A , Gururaj , C and Jyothishawaran , G . 2002 . In vitro shoot regeneration from leaf and nodal explants of Enicostemma hyssopifolium (Wild) Verd. A vulnerable medicinal plant . Indian J Biotechnol. , 1 : 401 – 404 .
- Sinha , RK , Majumdar , K and Sinha , S . 2000 . In vitro differentiation and plant regeneration of Albizia chirensis (OSB) Merr . In vitro Cell Div Biol Plant. , 36 : 370 – 373 .
- Tejavathi , DH and Shailaja , KS. 1999 . Regeneration of plants from the cultures of Bacopa monnieri (L) Pennell . Phytomorphology. , 49 ( 4 ) : 447 – 452 .
- Venkatachalan , P , Geetha , N , Sankara Rao , K , Jayabalan , N and Saravanababu , S . 1999 . BAP-regulated direct shoot organogenesis from cultured seedling explants of groundnut (Arachis hypogaea L) . Indian J Experim Biol. , 37 : 807 – 812 .
- Xie , D and Hong , Y . 2001 . In vitro regeneration of Acacia mangium through Organogenesis . Plant Cell Tiss Organ Cult. , 66 : 167 – 173 .
- Yusuf , A , Tyagi , RK and Mallik , SK . 2001 . Somatic embryogenesis and plantlet regeneration from leaf segments of Piper colubrinum . Plant Cell Tiss Organ Cult. , 65 : 255 – 258 .