Abstract
Plants of chickpea were exposed to varied levels of cobalt (Co) and sampled at the 60-day stage. Cobalt at concentration <100 µM significantly increased the number of nodules, their dry mass, leghemoglobin concentration and the activity of nitrogenase. Similarly, the activities of glutamate dehydrogenase, glutamine synthetase and glutamate synthase also exhibited an increase in the presence of Co <100 µM, in nodules and leaves, respectively. The various photosynthetic attributes in leaves and the activity of antioxidative enzymes both in nodules and leaves were inhibited by Co in a concentration-dependent manner. However, the lipid peroxidation and the content of proline exhibited a significant increase in response to Co and were at a maximum in the plants exposed to 250 µM concentration of cobalt. Since most of the parameters showed a significant increase in response to 50 µM cobalt, this concentration may be regarded as a threshold concentration.
Introduction
Nitrogen is one of the most abundant mineral elements, which is the component of all the enzymes, nucleic acids and a number of other metabolites. It is obtained by the plants from soil in the form of nitrate, ammonia or by fixation of the atmospheric nitrogen (N2) by leguminous plants. In either of these cases, the fixed nitrogen or the assimilated NO3 is converted to ammonia, which at a physiological pH changes to ammonium ion (). The presence of NH3 or
ions is quite toxic to the plants, which inhibits the activity of nitrogenase (Marschner Citation2003) and degrades the ATP molecule (Hopkins Citation1995). Therefore, it is rapidly exported from bacteroid into the surrounding nodule cells and assimilated into organic compounds, which are ultimately translocated to other plant parts (Udvardi and Day Citation1997) through xylem stream. This is achieved by the concerted action of two pathways (Marschner Citation2003): (i) glutamate dehydrogenase pathway, which synthesizes glutamate from 2-oxoglutarate, and (ii) glutamine synthetase (GS)-glutamate synthase (GOGAT) cycle. GS catalyzes the ATP-dependent amination of glutamate producing glutamine, while GOGAT catalyzes the reductive transfer of the amino group of glutamine to the α-keto position of 2-oxoglutarate yielding two molecules of glutamate (Lancien et al. Citation2000). GOGAT together with GS maintains the flow of
into glutamine and glutamate, which are then used in several other aminotransferase reactions for the synthesis of amino acids.
Cobalt has been categorized as a beneficial element for plants (Marschner Citation2003; Pilon-Smits et al. Citation2009). It is an integral component of the coenzyme cobalamine (vitamin B12 and its derivatives). Its deficiency has been found to affect nodule development and function at different levels (Dilworth et al. Citation1979; Dilworth and Bisseling Citation1984). Supplying the plants of Vicia faba with cobalt was found to increase their growth, nodulation and yield characteristics (Hala and Kandil Citation2007). Similar beneficial effects of lower concentrations of cobalt were reported by Jayakumar et al. (Citation2009) in soybean. In Rhizobium and Bradyrhizobium species, three enzymes – methionine synthase, ribonucleotide reductase and methyl malonyl-coA mutase – are known to be cobalamine-dependent, and cobalt-induced changes in their activities are primarily responsible for nodulation and N2 fixation in legumes (Dilworth and Bisseling Citation1984). Out of these enzymes, methylmalonyl coA mutase is involved in the synthesis of leghemoglobin. Therefore, under deficiency of cobalt, the synthesis of leghemaglobin is directly impaired, which ultimately affects the nitrogen-fixing capacity of plants, because leghemoglobin protects nitrogenase from oxygen by keeping O2 supply low (Hopkins Citation1995). However, the elevated concentration has been found to cause toxicity in plants. It causes a marked inhibition of growth together with chlorosis and necrosis (Vanselow Citation1966), declines the Hill activity, catalase activity and deteriorates the quality of produce (Chatterjee et al. Citation2006). Excess cobalt also causes enzyme inhibition (Shalygo et al. Citation1999). The heavy metals, including cobalt, also alter the photosynthetic activity of the plants at multiple levels such as pigments, stomatal functioning, electron transport chain, enzymes, and thylakoid membrane (Mysliva-Kurdziel et al. Citation2004). Lower concentrations of cobalt enhanced the antioxidative enzyme activities whereas higher concentrations generated negative effects in Arachis hypogea (Jaleel et al. Citation2008) and in Vigna radiata plants (Jaleel et al. Citation2009).
The present investigation was carried out with the objective to study the effects of Co stress on morphological parameters, nitrogen fixation and assimilation and photosynthetic attributes with specific emphasis on antioxidative enzyme activities in chickpea (Cicer arietinum L.) plants, which are the defense mechanism to any type of abiotic stress.
Materials and methods
Plant material and growth conditions
Seeds of chickpea (Cicer arietinum L.) cv. Navoday were surface sterilized with 5% (v/v) sodium hypochlorite for 10 min followed by repeated washings with distilled water. These seeds were inoculated with 108 cells ml−1 Rhizobium and were sown in plastic pots consisting of sand moistened with distilled water. The resulting seedlings were allowed to grow in a net house at an average temperature, humidity and day/night photoperiod of 25±2°C, 60±5% and 12/12 h, respectively. The pots were supplemented with 0 (control), 50, 100, 150, 200 or 250 µM of cobalt through nutrient solution (Hewitt Citation1966) at day 7, after sowing. The plants were supplemented with nutrient solution only on alternate days. Ten pots were maintained per treatment, in a completely randomized block design and each pot possessed two plants. The plants were sampled at 60 DAS to assess various parameters.
Leghemoglobin content
The leghemoglobin content, in fresh nodules, was estimated following the method described by Thimmaiah (Citation1999). The leghemoglobin was extracted with 0.1M phosphate buffer, followed by filtration through two layers of cheese cloth. The turbid reddish brown filtrate was centrifuged at 10,000 g, and 3 ml of pyridine reagent was added to 3 ml of extract. The greenish yellow hemochrome formed was divided equally into two test tubes. To these test tubes, 50 mg of potassium hexacyanoferrate and sodium dithionate were added and the absorbance was read on a spectrophotometer (Spectronic 20D, Milton Roy, USA).
Nodule nitrogen content
The nodule nitrogen content was estimated by employing the method of Lindner (Citation1944). The plant material was digested with concentrated H2SO4, followed by neutralizing it with NaOH and sodium silicate solutions. Nesslers reagent was added to this solution and samples were read on a spectrophotometer (Spectronic 20D, Milton Roy, USA).
Assay of the enzymes of nitrogen metabolism
The nitrogenase activity was assayed by acetylene reduction method (Hardy et al. Citation1968). Gas chromatograph (Nucon Series 5250, New Delhi), equipped with a flame ionization detector to quantify the ethylene produced was used for the purpose. The result was expressed in terms of nano moles of ethylene formed/g (nodule fresh mass)/hour.
Extracts for determination of GDH and GOGAT activities were prepared from nodules and leaves homogenized in 3 ml of extraction buffer containing 0.05M Tris HCl (pH 7.5), 0.4M sucrose and 0.01M β-mercaptethanol. Homogenates were centrifuged at 10,000 g for 20 min and the supernatant was used for assays, according to the method described by Thimmaiah (Citation1999). Then, 1 ml of the extract was added to the reaction mixtures consisting of 0.1 M Tris-HCl buffer (pH 7.5), 0.33M 2-oxoglutarate (pH 6.0), 1 mM NADH and 3 M NH4Cl or 0.3 M L-glutamine for GDH and GOGAT, respectively. The decrease in absorbance due to oxidation of NADH in the reaction medium was read at 340 nm on a UV spectrophotometer (Elico, Hyderabad, India). One unit of GDH or GOGAT oxidized 1 µM of NADH per min under assay conditions. GS from the nodules and leaves were extracted by grinding them in an extraction medium containing 0.1 M potassium phosphate buffer (pH 7.8), 0.4 M sucrose, 10 mM dithiothreitol, 10 mM KCl, 1 mM MgCl2 and 10 mM EDTA. The homogenate was centrifuged at 10,000 g for 20 min and the supernatant was used for the enzyme assay, according to the method of Thimmaiah (Citation1999). Enzyme extract (50 ml) was added to a reaction mixture containing 50 mM Tris-maleate buffer (pH 7.5), 67 mM hydroxylamine, 80 mM L-glutamine, 8 mM ATP and 4 mM EDTA. The absorbance was read at 540 nm on a spectrophotometer and was compared with a calibration curve plotted by using pure γ-glutamyl hydroxamate.
The activity of NR was measured following the method laid down by Jaworski (Citation1971). A mixture of fresh leaf samples, phosphate buffer (pH 7.5), KNO3 and isopropanol was incubated at 30°C for 2 h. Sulphanilamide and N-1-napthylethylenediamine hydrochloride solutions were added to the incubated mixture. The absorbance was read at 540 nm on a spectrophotometer.
The in vivo nitrite reductase was assayed according to the method of Srivastava et al. (Citation1979). The 2 ml reaction mixture consisted of 0.05 M Tris-HCL buffer (pH 7.8), sodium dithionite (200 mM), glutamic acid (25 mM) and NaNO2 (60 mM). The NH3 released was reacted with Nesselers reagent and the absorbance was recorded spectrophotometrically.
Carbonic anhydrase (CA) activity
The CA activity in the leaves was measured by the method described by Dwivedi and Randhawa (Citation1974). The leaf samples were cut into small pieces in cysteine dihydrochloride solution. These leaf samples were blotted and poured into a test tube, followed by the addition of phosphate buffer (pH 6.8), 0.2 M NaHCO3, bromothymol blue and the methyl red indicator. This reaction mixture was titrated against HCl and the activity of the enzyme was expressed on a fresh mass basis.
Photosynthetic pigments and photosynthesis measurements
The chlorophyll from fresh leaf samples was extracted with 80% acetone. The absorbance of the extract was measured at 663 nm and 645 nm on a spectrophotometer (Spectronic 20D, Milton Roy, USA). The quantity of pigment in the samples was calculated by using the formula proposed by Lichtenthaler (Citation1987). The stomatal conductance (gs), intercellular CO2 concentration (Ci) and net photosynthetic rate (PN) in intact leaves were measured by the LI-6400 portable photosynthesis system (LI-COR Lincoln, NE, USA), between 11:00 and 12:00 h.
Proline content
The proline content in fresh leaves was estimated following the procedure used by Bates et al. (Citation1973).
Lipid peroxidation
Lipid peroxidation was estimated by measuring the malondialdehyde formed according to Hodges et al. (Citation1999). The leaf sample (0.5 g) was homogenized in a mortar with 80% ethanol which was centrifuged at 3000 g for 10 min at 4°C. The pellet was extracted twice with the same solvent. The supernatants were pooled and 1 ml of this sample was added to a test tube with an equal volume of the solution comprised of 20% trichloroacetic acid, 0.01% butylated hydroxy toluene and 0.65% thiobarbituric acid. Samples were heated at 95°C for 25 min and cooled to room temperature. Absorbance of the samples was recorded at 440, 532 and 600 nm. Lipid peroxidation (n mol malondialdehyde ml−1) was calculated by using the formula given by Hodges et al. (Citation1999).
Assay of antioxidative enzymes
The leaf tissue was homogenized in 50 mM phosphate buffer (pH 7.0) containing 1% soluble polyvinylpyrolidine. The homogenate was centrifuged at 15,000 rpm for 10 min at 5°C and the supernatant obtained was used as an extract for peroxidase (POX), catalase (CAT), superoxide dismutase (SOD) and glutathione reductase.
The reaction mixture for CAT consisted of phosphate buffer (pH 6.8), 0.1 M H2O2 and enzyme extract (1.0 ml). H2SO4 was added to the reaction mixture, after incubating it for 1 min at 25°C, and was titrated against potassium permanganate solution (Chance and Maehly Citation1956).
For the estimation of POX activity (Chance and Maehly 1956), the enzyme extract (0.1 ml) was added to the reaction mixture consisting of pyrogallol phosphate buffer (pH 6.8) and 1% H2O2. The change in the absorbance was read for 2 min at the interval of 20 sec, at 420 nm on a spectrophotometer. A control set was prepared by adding double distilled water instead of enzyme extract.
The activity of SOD was measured by the method of Beauchamp and Fridovich (Citation1971). A 3 ml of reaction mixture containing 50 mM phosphate buffer (pH 7.8), 13 mM methionine, 75 mM nitroblue tetrazolium, 2 mM riboflavin, 0.1 mM EDTA and 0–50 µM of enzyme extract was prepared. Riboflavin was added last. This reaction mixture was exposed to low fluorescent light and the decrease in absorbance of the reaction mixture was read at 560 nm on a spectrophotometer. 50% inhibition was considered as one enzyme unit.
Glutathione reductase was assayed as per the method of Smith et al. (Citation1988). The reaction mixture contained, 66.67 mM potassium phosphate buffer (pH 7.5), 0.33 mM EDTA, 0.5 mM 5,5-dithiobis-2-nitrobenzoic acid in 0.01 M potassium phosphate buffer (pH 7.5), 66.67 mM NADPH, 66.67 mM oxidized glutathione and 0.1 ml enzyme extract. The reaction was started by adding oxidized glutathione and the increase in absorbance at 412 nm was recorded spectrophotometrically.
Statistical analysis
Each observation was replicated five times. The values for various parameters of the plants were subjected to statistical analysis following the standard procedure described by Gomez and Gomez (Citation1984). The means were compared by LSD test to study the significance at 5% level of probability.
Results
Plant dry mass and nodulation
The plants treated with different levels of cobalt exhibited a response dependent on its concentration (). The plant dry mass was significantly increased by 50 µM of Co, which declined as the concentration increased further in the nutrient medium. However, nodule number and their dry mass exhibited a concomitant increase up to 100 µM and declined significantly when the concentration of Co in the nutrient medium was elevated up to 250 µM. At this concentration, Co decreased plant dry weight by 55%, nodule number by 50% and nodule dry weight by 60%, below that of the control.
Table 1. Effect of cobalt (0, 50, 100, 150, 200 and 250 µM) on dry mass (g) plant−1, nodule number, nodule dry mass (mg) plant−1, and leghemoglobin content [mg g−1 (F.M.)] in Cicer arietinum L. cv. Navoday, at 60 days after sowing.
Leghemoglobin content and nitrogenase activity
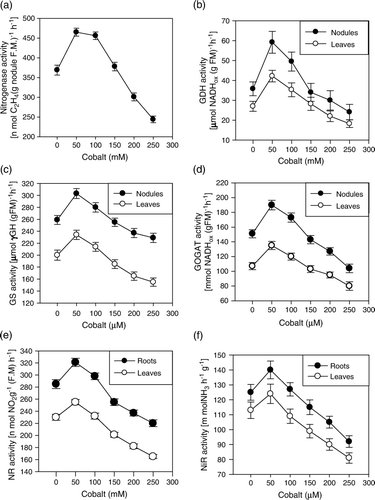
Both these parameters exhibited similar trends at all the concentrations of Co (; a). The lowest concentration (50 µM) of Co resulted in a maximum elevation of the parameters, which was 37% (leghemoglobin) and 60% (nitrogenase) higher than that of the control. This response was closely followed by 100 µM of Co, although the values were slightly lower than those of 50 µM concentration. The concentration>150 µM proved inhibitory and the extent of inhibition was proportionate to the concentration of the metal. The maximum concentration (250 µM) was the most severe that caused 47% and 58% decrease in leghemoglobin content and nitrogenase activity, respectively, as compared to the control.
Figure 1. Effect of cobalt (0, 50, 100, 150, 200 and 250 µM) on nitrogenase activity (a), glutamate dehydrognase (GDH) activity (b), glutamine synthetase (GS) activity (c), glutamate synthase (GOGAT) activity (d), nitrate reductase (NR) activity (e), and nitrite reductase (NiR) activity (f) in Cicer arietinum L. cv. Navoday, at 60 days after sowing.
Enzymes of nitrogen assimilation
The activities of different enzymes involved in nitrogen assimilation were higher in nodules than that in leaves (b–d). The plants treated with 50 or 100 µM of Co significantly enhanced the activities of GDH, GS and GOGAT, both in nodules and leaves, compared to those of the control. However, no significant change was observed at 150 µM level of Co. The other two concentrations (200 and 250 µM) caused a concomitant reduction in the activities of these enzymes, where the values were least in the plants grown in the presence of 250 µM of Co.
Nitrate reductase and nitrite reductase
The activity of NR and NiR was significantly increased by 50 µM of Co (e–f). The presence of 100 µM concentration had no significant impact on the activities of these enzymes. However, the concentration>100 µM concomitantly reduced their activities in roots as well as in leaves. The inhibition was more conspicuous in roots than in leaves, which was 56% and 41% (NR) and 39% and 30% (NiR) lower than the control, in roots and leaves, respectively, in the presence of 250 µM of cobalt.
Carbonic anhydrase (CA) activity and chlorophyll content
The exposure of the plants to lowest concentration (50 µM) of Co did not have a significant effect on these parameters (). However, the concentrations >50 µM significantly decreased the CA activity and chlorophyll concentration, which was proportionate to the concentration of the metal. 100 µM and 150 µM did not differ significantly from each other in their effects. However, 250 µM concentration was the most toxic that decreased the values by 51% (CA) and 60% (chlorophyll), compared to the plants grown without Co in the nutrient medium.
Table 2. Effect of cobalt (0, 50, 100, 150, 200 and 250 µM) on total chlorophyll content [mg g−1 (F.M.)], stomatal conductance (gs), intercellular CO2 concentration (Ci) (ppm), carbonic anhydrase (CA) activity [mol CO2 kg−1 (F.M.) s−1] and net photosynthetic rate (PN) [mM CO2 m−2s−1] in Cicer arietinum L. cv. Navoday, at 60 days after sowing.
Photosynthetic characteristics
Stomatal conductance (gs), intercellular CO2 concentration (Ci) and net photosynthetic rate (PN) exhibited a significant decline in response to the presence of Co. The decrease was proportionate to the concentration of cobalt. Moreover, the maximum concentration (250 µM) was the most severe that decreased the above parameters by 62%, 52% and 34%, respectively, below the control ().
Lipid peroxidation and proline content
In comparison to other parameters, lipid peroxidation and proline content followed an increasing trend in response to the presence of Co in the nutrient medium (a, and b). Cobalt, at a concentration of 50 µM, was not too effective. The response generated by this concentration was close to the control. However, as the concentration of Co was increased, these parameters also increased concomitantly. Compared with the control, the values were 48% and 32% (lipid peroxidation) and 122% and 90% (proline) higher, in nodules and leaves, respectively, at 250 µM concentration of cobalt.
Antioxidative enzymes
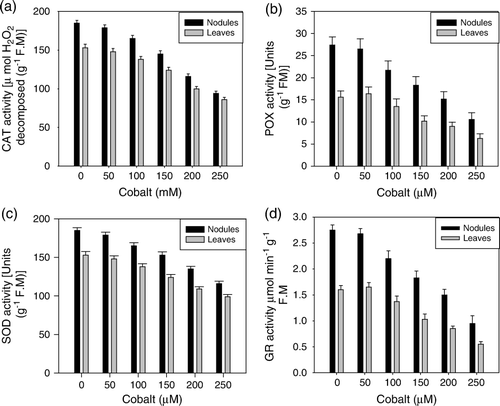
The activity of the antioxidative enzymes (CAT, POX, SOD and GR) was significantly higher in nodules than leaves, irrespective of the concentration of Co in the nutrient medium (a–d). The response generated by 50 µM of Co was close to that of control. However, when the plants were exposed to Co>50 µM, the values exhibited a sharp decrease that was proportionate to the concentration of the metal. 250 µM concentration of Co caused the maximum inhibition, which was 50% (CAT), 57% (POX), 37% (SOD) and 61% (GR) in nodules and 44% (CAT), 52% (POX), 46% (SOD) and 47% (GR) in leaves, as compared to the control.
Discussion
Cobalt, at the lowest level (50 µM), significantly enhanced the number of nodules and their dry mass, leghemoglobin concentration () and nitrogenase activity (). Co is an integral component of coenzyme, cobalamine (vitamin B12), which regulates at least three important enzymes associated with nodular activity (Dilworth and Bisseling Citation1984). These enzymes are (a) Methionine synthase, which contributes to the size of bacteroid and participates in protein synthesis, (b) ribonucleotide reductase, which determines DNA synthesis and rhizobial cell division (Chatel et al. Citation1978), and (c) methylmalonyl-coA mutase, which is involved in the leghemoglobin synthesis. The activities of the former two enzymes in response to 50 µM of cobalt resulted in an increase in the nodulation and the dry mass of nodules, whereas the latter one enhanced the level of leghemoglobin which is a hemochrome protein pigment that surrounds the oxygen labile nitrogenase enzyme and regulates the optimum supply of O2 that keeps the enzyme active in the nitrogen fixing nodule. Therefore, the activity of nitrogenase was elevated with an increased level of leghemoglobin, in the presence of 50 µM of Co (), which is also confirmed by positive correlation between nitrogenase and leghemoglobin (). Many reports have confirmed a positive correlation between leghemoglobin and nitrogenase activity (Comba et al. Citation1998). However, when the metal was supplemented in higher concentration (>100 µM), it declined the nodulation as well as the level of leghemoglobin and nitrogenase (; ) activity. At excessive concentrations, heavy metals cause oxidative stress that induces the degradation of biologically important molecules such as lipids, amino acids, proteins (enzymes) and carbohydrates, resulting in the release of malondialdehyde (TBARS formation) (Alaiz et al. Citation1999). Therefore, TBARS formation is a precise indicator of oxidative stress. In the present research, Co stress also generated an oxidative stress which is evident from increased lipid peroxidation (TBARS formation) (a). It is also noteworthy that the lipid peroxidation was higher in the nodules than in the leaves, which could be due to direct exposure of nodules to Co. Oxidative stress generated by Co depleted the level of leghemoglobin that resulted in an exposure of nitrogenase to excess O2 and hence declined its activity. Another possible explanation in this connection is that oxidative stress also causes damage to the plasma membrane. The damaged plasma membrane prevents the export of fixed nitrogen to the adjoining tissues, which could be in the form of ammonia or glutamine. Accumulation of both these metabolites slows down the activity of nitrogenase (Shanmugam et al. Citation1978). Therefore, the activity of nitrogenase was lower in the nodules of the plants exposed to excess (>150 µM) cobalt, compared to control (a).
Figure 3. Effect of cobalt (0, 50, 100, 150, 200 and 250 µM) on lipid peroxidation (a) and proline content (b) in Cicer arietinum L. cv. Navoday, at 60 days after sowing.
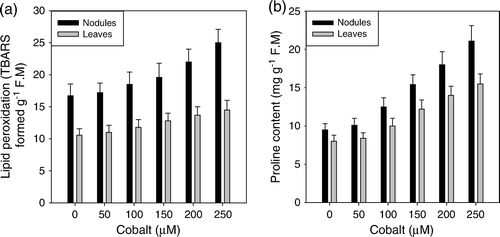
Table 3. Correlation coefficient between different parameters.
The nitrogen fixed in the bacteroids of the host nodules is released as NH3 to the host cytosol by simple diffusion across the peribacteroid membranes (Udvardi and Day Citation1997). In the host cytosol, NH3 is assimilated via two pathways (Marschner Citation2003): (i) glutamate dehydrogenase (GDH)-NADH pathway, which synthesizes glutamate from 2-oxoglutarate and , and (ii) the glutamine synthetase (GS)-glutamate synthase (GOGAT) cycle. GS catalyzes the ATP-dependent amination of glutamate producing glutamine while GOGAT catalyzes the reductive transfer of the amide group of glutamine to 2-oxoglutarate, yielding two molecules of glutamate (Lancien et al. Citation2000). The decline in the activities of enzymes of these two nitrogen assimilation systems in response to toxic concentration of Co might be mediated through the depletion in leghemoglobin () and consequently the excess O2 supply. The high O2 consumption in nodules has a great potential for production of toxic oxygen species. This is true in particular for leghemoglobin which is also subjected to autoxidation in which O2
·−and H2O2 are released (Marschner Citation2003) generating oxidative stress. Since oxidative stress has an adverse impact on enzymes (protein), therefore, the activities of these enzymes declined in the presence of excess Co. Photosynthates availability is another important factor that determines the efficiency of nitrogen fixation and assimilation (Pate and Herridge Citation1978). The declined photosynthesis () can also be regarded as a reason for decreased activity of the enzymes of nitrogen assimilation. In addition to this, the metal induced oxidative stress could be another cause of the enzyme inhibition including those of nitrate reductase and nitrite reductase (e–f). Ortega et al. (Citation1999) have demonstrated oxidative inactivation of GS in soybean roots and oxidized GS has been shown to be inactive and more susceptible to degradation than the non-oxidized form. Similarly, the nodular activity and nitrogen assimilation was affected by other bivalent ions like Cd in rice (Chien et al. Citation2004) and Al in soybean (Balestrasse et al. Citation2006).
Photosynthesis is one of the important physiological processes most susceptible to heavy metal toxicity. They (heavy metals) affect it at multiple levels such as pigment biosynthesis/degradation, stomatal functioning, enzyme inhibition, alteration in chloroplast membranes structure/function and photosystems (Mysliva-Kurdziel et al. Citation2004). Heavy metal stress causes the activation of chlorophyll degrading enzyme, chlorophyllase (Abdul-Basset et al. Citation1995) and in particular Co inhibits those involved in chlorophyll biosynthesis, such as 5-aminolevulenic acid (ALA) synthase, ALA dehydratase, prophobilinogenase and unporphyrinogen III decarboxylase (Shalygo et al. Citation1999). Therefore, the concentration of chlorophyll exhibited a decrease in response to Co stress (). The stress generated by Co also caused the decrease in stomatal conductance and consequently the intercellular CO2 concentration and carbonic anhydrase (CA) activity (). The CA catalyzes the interconversion of CO2 and HCO3 −, and its activity is to a large extent regulated by CO2, besides zinc and light (Tiwari et al. Citation2005). Thus, the decreased CO2 concentration was accompanied with a concomitant decline in CA activity. The decreased chlorophyll concentration, stomatal conductance, intercellular CO2 concentration and CA activity () together with decreased Hill reaction activity (Chatterjee et al. Citation2006) ultimately hampered the net photosynthetic rate () which is also confirmed by positive correlation between CA activity and net photosynthetic rate as well as between chlorophyll and net photosynthetic rate ().
The antioxidative enzymes, CAT, POX, SOD and GR were concomitantly declined by the Co stress, both in nodules and leaves (a–d). A similar decrease in the activity of various antioxidative enzymes in response to excess cobalt was observed in Vigna radiata (Jaleel et al. Citation2009). The observed decrease is also symptomatic of oxidative stress as stated earlier (increased TBARS formation). The oxidative stress induced decline in the activities of antioxidative enzymes has also been reported in rice seedlings (Boo and Jung Citation1999). Similarly, Balestrasse et al. (Citation2006) also suggested that decrease in the antioxidant enzymes in Al-stressed soybean plants was mediated through the generation of oxidative stress. However, contradictory to the response of enzymatic antioxidants to Co, the level of proline exhibited a concomitant increase as the metal concentration was increased in the nutrient medium (b). The increase in the proline pool is a general response of plants to various abiotic stresses, including that of heavy metals (Schat et al. Citation1997). The mechanism and functional significance of proline accumulation in plants under heavy metal stress has been controversial. Most of the studies have demonstrated the increased level of proline as a biochemical adaptation of the plants to maintain water balance and scavenge ROS generated through water stress caused by heavy metals (Schat et al. Citation1997). However, Kastori et al. (Citation1992) observed proline accumulation in metal exposed leaf discs and argued that this was due to metal uptake per se, rather than to water deficit stress. In the present research, the increased level of proline could be a biochemical adaptation to scavenge the active oxygen species, which remained there in the cells due to inhibition of the antioxidative enzymes (a–d).
In conclusion, the results presented here suggest that Co at lower concentration (>50 µM) favors the nodulation, nitrogen fixation and assimilation. The higher concentration (>100 µM) generated oxidative stress, which was noticed on the basis of increased lipid peroxidation. The induced oxidative stress impaired the nitrogen metabolism, photosynthesis and the antioxidant system which resulted in a decrease in dry matter accumulation in chickpea (Cicer arietinum L.). However, the increased level of proline was a biochemical adaptation to scavenge the active oxygen species, which remained there in the cells due to inhibition of the antioxidative enzymes.
References
- Abdul-Basset , R , Iss , AA and Adam , MS . 1995 . Chlorophyllase activity: Effects of heavy metals and calcium . Photosynthetica. , 31 : 421 – 425 .
- Alaiz , M , Hidalgo , FJ and Zamera , R . 1999 . Effect of pH and temperature on comparative antioxidant activity of nonenzymatically browned proteins produced by reaction with oxidized lipids and carbohydrates . J Agr Food Chem. , 47 : 478 – 752 .
- Balestrasse , KB , Gallego , SM and Tomaro , ML . 2006 . Aluminium stress affects nitrogen fixation and assimilation in soybean (Glycine max L.) . Plant Growth Regul. , 48 : 271 – 281 .
- Bates , LS , Waldren , RP and Teare , ID . 1973 . Rapid determination of free proline for water stress studies . Plant Soil. , 39 : 205 – 207 .
- Beauchamp , CO and Fridovich , I . 1971 . Superoxide dismutase: Improved assays and assay applicable to acrylanide gels . Ann Biochem. , 44 : 216 – 287 .
- Boo , YC and Jung , J . 1999 . Water deficit-induced oxidative stress and oxidative defenses in rice plants . J Plant Nutr. , 29 : 127 – 136 .
- Chance , B and Maehly , AC . 1956 . Assay of catalase and peroxidase . Methods Enzymol. , 2 : 764 – 775 .
- Chatel , DL , Robson , AD , Gartrell , JW and Dilworth , MJ . 1978 . The effect of inoculation and cobalt application on the growth and nitrogen fixation by sweet lupinus . Aust J Agric Res. , 29 : 1191 – 1202 .
- Chatterjee , C , Gopal , R and Dube , BK . 2006 . Physiological and biochemical responses of Frenchbean to excess cobalt . J Plant Nutr. , 29 : 127 – 136 .
- Chien , HF , Lin , CC , Wang , JW , Chen , CT and Kao , CH . 2004 . Changes in ammonium ion content and glutamine synthetase activity in rice leaves caused by excess cadmium are a consequence of oxidative damage . Plant Growth Regul. , 36 : 41 – 47 .
- Comba , ME , Benavides , MP and Tomaro , ML . 1998 . Effect of salt stress on antioxidant defense system in soybean root nodules . Aust J Plant Physiol. , 25 : 665 – 671 .
- Dilworth , MJ and Bisseling , T . 1984 . Cobalt and nitrogen fixation in Lupinus angustifolius L. III DNA and methionine in bacteroids . New Phytol. , 98 : 311 – 316 .
- Dilworth , MJ , Robson , AD and Chatel , DL . 1979 . Cobalt and nitrogen fixation in Lupinus angustifolius L. II. Nodule formation and functions . New Phytol. , 83 : 63 – 73 .
- Dwivedi , RS and Randhawa , NS . 1974 . Evolution of a rapid test for hidden hunger of zinc in plants . Plant Soil. , 40 : 445 – 451 .
- Gomez , KA and Gomez , AA . 1984 . Statistical procedures for agricultural research , New York : John Wiley & Sons .
- Hala , Kandil . 2007 . Effect of cobalt fertilizer on growth, yield and nutrients status of Faba bean (Vicia faba L.) plants . J App Sci Res. , 3 ( 9 ) : 867 – 872 .
- Hardy , RWE , Holstein , EK , Jakson , EK and Burns , RS . 1968 . The acetylene, ethylene assay for nitrogen fixation. Laboratory and field evaluation . Plant Physiol. , 43 : 1185 – 1207 .
- Hewitt EJ. 1966 . Sand and water culture methods used in the study of plant nutrition , 2nd ed. . Wallingford, , UK : Commonwealth Agricultural Bureau International .
- Hodges , MD , DeLong , JM , Forney , CF and Prange , RK . 1999 . Improving the thiobarbituric acid-reactive-substances assay for lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds . Planta. , 207 : 604 – 611 .
- Hopkins , WJ . 1995 . Introduction to plant physiology , 3rd ed , New York : John Wiley and Sons .
- Jaleel , CA , Jayakumar , K , Chang-xing , Z and Azooz , MM. 2008 . Effect of soil applied cobalt on activities of antioxidant enzymes in Arachis hypogaea . Global J Mol Sci , 3 ( 2 ) : 42 – 45 .
- Jaleel , CA , Jayakumar , K , Chang-xing , Z and Azooz , MM . 2009 . Antioxidant potentials protect Vigna radiata (L.) Wilczek plants from soil cobalt stress and improve growth and pigment composition . Plant Omics J. , 2 ( 3 ) : 120 – 126 .
- Jayakumar , K , Jaleel , CA , Azooz , MM , Vijayarengan , P and Gomathinayagam , Panneerselvam . 2009 . Effect of different concentrations of cobalt on morphological parameters and yield components of soybean . Global J Mol Sci. , 4 ( 1 ) : 10 – 14 .
- Jaworski , EG . 1971 . Nitrate reductase assay in intact plant tissues . Biochem Biophys Res Commun. , 43 : 1274 – 1279 .
- Kastori , R , Petrovic , M and Petrovic , N . 1992 . Effect of excess lead, cadmium, copper and zinc on water relations in sunflower . J Plant Nutr. , 15 : 2427 – 2439 .
- Lancien , M , Gadal , P and Hodges , M . 2000 . Enzyme redundancy and the importance of 2-oxoglutarate in higher plant ammonium assimilation . Plant Physiol. , 123 : 817 – 824 .
- Lichtenthaler , HK . 1987 . Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes . Methods Enzymol. , 148 : 350 – 382 .
- Lindner , RC . 1944 . Rapid analytical method for some of the more common inorganic constituents of plant tissues . Plant Physiol. , 19 : 76 – 89 .
- Marschner H. 2003 . Mineral nutrition of higher plant , 2nd ed. . Amsterdam : Academic Press . 201 228 , 405–435.
- Mysliva-Kurdziel B , Prasad MNV , Strzalka K. 2004 . Photosynthesis in heavy metal stressed plants . In: Prasad MNV Heavy metal stress in plants: From biomolecules to ecosystems . Berlin : Springer-Verlag . 146 181 .
- Ortega , JL , Roche , D and Sengupta-Gopalan , A . 1999 . Oxidative turnover of soybean root glutamine synthetase. In vitro and in vitro studies . Plant Physiol. , 119 : 1483 – 1495 .
- Pate , JS and Herridge , DF . 1978 . Partitioning and utilization of net photosynthate in nodulated annual legumes . J Exp Bot. , 29 : 401 – 412 .
- Pilon-Smits , EAH , Quinn , CF , Tapken , W , Malagoli , W and Schiavon , M . 2009 . Physiological functions of beneficial elements . Curr Opin Plant Biol. , 12 : 267 – 274 .
- Schat , H , Sharma , SS and Vooijs , R . 1997 . Heavy metal-induced accumulation of free proline in a metal-tolerant and non-tolerant ecotype of Silene vulgaris . Physiol Plant. , 101 : 477 – 482 .
- Shalygo , NV , Kolesnikova , NV , Voronetskaya , VV and Averina , NG . 1999 . Effect of Mn2+, Fe2+, Co2+ and Ni2+ on chlorophyll accumulation and early stages of chlorophyll formation in greening barley seedlings . Russ J Plant Physiol. , 46 : 496 – 501 .
- Shanmugam , KT , O'Gara , F , Andersen , K and Valentine , RC . 1978 . Biological nitrogen fixation . Annu Rev Plant Physiol. , 29 : 263 – 276 .
- Smith , IK , Vierheller , TL and Thorne , CA . 1988 . Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis (2-nitrobenzoic acid) . Ann Biochem. , 175 : 408 – 413 .
- Srivastava , RC , Mukherji , D , Mathur , SN and Srivastava , HS . 1979 . Measurement of nitrate reductase in leaf tissue of Vigna mungo . Planta. , 147 : 196 – 198 .
- Thimmaiah , SR . 1999 . Standard methods of biochemical analysis , New Delhi : Kalyani Publishers .
- Tiwari , A , Kumar , P , Singh , S and Ansari , SA . 2005 . Carbonic anhydrase in relation to higher plants . Photosynthetica. , 43 : 1 – 9 .
- Udvardi , MK and Day , DA . 1997 . Metabolite transport across symbiotic membranes of legume modules . Ann Rev Plant Physiol Mol Biol. , 48 : 493 – 523 .
- Vanselow AP. 1966 . Cobalt . In: Chapman HD Diagnostic criteria of plants and soils . Riverside, CA : University of California, Division of Agricultural Science . 142 156 .