Abstract
A comparative account of the polymorphic expression of two antioxidative enzymes (Peroxidase and Superoxide dismutase), two hydrolyzing enzymes (Esterase and Acid phosphatase) and total proteins was estimated both qualitatively and quantitatively from the leaves of five mangroves (Bruguiera gymnorrhiza, Excoecaria agallocha, Heritiera fomes, Phoenix paludosa and Xylocarpus granatum) from two different habitats (in situ habitat of Sundarbans and their replicas from ex situ habitat in fresh water condition) and discussed. The higher amount of total protein occurred in fresh water plants compared to their Sundarbans counterparts. The increment of total protein ranged among 156% to 5.7%. Gel electrophoresis experiments revealed that in most of the cases there were extra numbers of protein bands expressed with relatively low molecular weight in saline habitat. In all salinity imposed plants, there were sharp increases in band intensity and the number of isoforms of investigated enzyme. Peroxidase increment in saline plants ranged from 257–139%. Similarly, SOD was estimated at 247–147% in saline habitats. Increments of Esterase and Acid phosphatase varied from 287–154% and 293–139%, respectively.
Introduction
The environment and ecosystem of the tropical and subtropical coastal zone is marked with unique geophysical characters like sea surges with tidal waves, upland discharges, rapid sedimentation, substrate erosion and episodic cyclones. The main feature of mangroves is in their ability for successful colonization under constant physiological stress (Chaudhury Citation1996). These plants grow by developing some morphological, reproductive and physiological adaptation (Zimmermann Citation1983; Das Citation1999). This formation has a multifunctional beneficial impact on the coastal ecosystem in the form of production and protection. This vast greenery nurses several estuarine habitats and mitigates the violence of cyclonic effect (Hogarth Citation1999). Recently, this economic and ecologically utility plant community has come under severe threat worldwide. Conservation of biodiversity of such an ecosystem is a prime issue in the scientific world. It is well established that the biodiversity of mangrove vegetation is being degraded to a large extent all over the world due to human interference and tectonic activities. Spalding et al. (Citation1997) reported that 181,399 km2 of mangrove vegetation was only about 50% of the past vegetation coverage. Changed ecology and over-exploitation caused the depletion of mangrove formation, which is estimated to be more than 1% per annum (Hatcher et al. Citation1989).
In the Indian subcontinent (which extends between 21°31′, 22°30′ N and 88°10′, 89°51′ E), two important river systems – the Ganga and Brahmaputra – constitute the largest delta formation where the vast mangrove vegetation thrives with maximum species diversity. In the Sundarbans delta, there has been a very slow tilting of the coast due to tectonic uplift in the northwestern part (India) and subsidence in the east (Bangladesh). It has a major impact on mangrove species distribution as increased salinity prevails in the western part (India). This forest area (Indian territory) covers approximately 2195 km2 (Sanyal Citation1996) excluding the anastomosing network of creeks and backwaters. The soil salinity ranges between 15 and 27 PPT and maximum available irradiance is approximately 2000 µmol m−2s−1 (Nandy (Datta) et al. Citation2007). The flora comprises 36 true mangroves, 28 associates and seven obligatory mangrove species representing 29 families and 49 genera (Naskar and Guha Bakshi Citation1983). Unfortunately, excessive demographic pressure, over-harvesting for timber and fuel-wood, poaching, reclamation for aquaculture and industrial pollution have become detrimental to these coastal resources. Since the 17th century, damming on the lower Ganges cut off an ample fresh water influx through Hooghly and its tributaries to the islands of Sundarbans. Moreover, silting in the riverbed due to sewage and industrial pollution elevates the salinity level of water. These manual and environmental adversities have proved disastrous for some important plant species including Heritiera fomes, Nypa fruticans and Xylocarpus mekongensis (Banerjee Citation1999). These species predominate in between the Raimangal and Matla rivers, where fresh water influx from the Ichamati river towards Raimangal is much better (in Bangladesh part). Especially H. fomes prefers a slightly and/or moderately saline zone and the ridges of higher elevation that are inundated only during spring tide (Alim Citation1979). Previously, in West Bengal, these trees used to be 2 m in girth, but over 1 m girth is no longer common and top dying of H. fomes is very frequent in the Sundarbans forest (Curtis Citation1993).
Unlike morphological markers, molecular markers are not prone to environmental influences and provide some vital information towards the priority areas for conservation strategies. Therefore, the use of molecular markers (enzymes, DNA) might enhance the understanding of such situations. Enzyme analysis is an added tool for detecting this diversity (Zeidler Citation2000). The International Union for Protection of New Varieties of Plants (UPOV) have harmonized and adopted test guidelines and procedures for the use of isozyme electrophoresis as a characteristic for establishing the uniqueness of the plants (UPOV Citation1997).
Mangroves have to cope with considerably high soil salinity and consequently, a physiologically dry substrate. As such they are confronted with the problem of maintaining adequate turgor pressure within the cell sap because of high salt concentrations in the growth medium and thus protecting their metabolic activity (Flowers et al. Citation1977). This leads to accumulation and/or synthesis of organic substances in the form of compatible solutes within the vacuole (Hasegawa et al. Citation2000). Cheeseman et al. (Citation1997) experimentally showed that ascorbate peroxidase and SOD synthesis are much higher in field grown mangroves. Superoxide dismutase (SOD) and several antioxidant enzymes are potentially involved in H2O2 metabolism leading to photoprotection. Parida et al. (Citation2002) reported that sugar, prolein and some polyphenolic compounds accumulate in the cell sap of Bruguiera parviflora to restore the water potential to more negative. Experimental works reported that in mangroves, the synthesis of these osmolytes, specific proteins and translatable mRNA induced and increased by salt stress (Hurkman et al. Citation1989; Bray Citation1993; Xu et al. Citation1996; Swire-Clark and Marcotte Citation1999; Xu et al. Citation2001). A positive linear relationship between peroxidase activity and leaf tissue metal concentrations were reported in Avicennia marina (Macfarlane and Burchett Citation2001). An in vitro experiment on B. parviflora resulted in differential changes in the levels of the isoforms of antioxidative enzymes due to NaCl treatment which may be useful as markers for recognizing salt tolerance in mangroves and suggested that the elevated levels of the antioxidant enzymes protect the plants against the reactive oxygen species (ROS) thus avoiding lipid peroxidation during salt stress (Parida et al. Citation2004a, Citation2004b). An increased level of peroxidase and SOD accumulation was reported in water logging stress in Kandelia candel and Bruguiera gymnorrhiza (Ye et al. Citation2003).
In obligate halophytes, reverse adaptation often provokes significant metabolic shifts that can be partially characterized by isozyme study. Peroxidase (in different isoforms) is widely distributed throughout the growing phase and has great biological importance. In plants, peroxidase is either bound to cell wall or located in the protoplast (Mader Citation1976). Cell wall bound peroxidases are probably involved in lignification while other isoenzymes have the regulatory role in plant senescence or in the destruction of auxins (Frenkel Citation1972; Stonier and Yang Citation1973). Due to changed ecology, isoforms of these stress-related enzymes were differentially expressed. There are hardly any reports dealing with a comparative account of quantitative and qualitative analysis of antioxidant and hydrolyzing enzymes in Indian context. In view of the above, this work aims to understand the extent of changes of isoforms of two antioxidant enzymes (peroxidase and superoxide dismutase) and two important hydrolyzing enzymes (esterase and acid phosphates) in five true mangrove species grown in the natural field condition (in Sundarbans) and their counterparts grown in the fresh water condition in the garden of ISI Kolkata. The comparative assessment, both gel electrophoretic study and quantitative estimation of total leaf protein and enzyme, would provide some important clues towards their reverse adaptability to mesophytic condition for postulating proper conservation techniques in ex situ condition.
Materials and methods
Five species of true mangroves (Bruguiera gymnorrhiza, Excoecaria agallocha, Heritiera fomes, Phoenix paludosa and Xylocarpus granatum) were selected for this experiment; Heritiera and Xylocarpus are very much stressed and the remaining three are profusely grown in western Sundarbans. The youngest leaf buds were collected in ice from properly identified and well matured in situ (from Sundarbans forest, where salinity ranges from 15–27 PPT) plants (about 10–12 years old) and their counterparts from ex situ (grown in fresh water condition in the premises of the Indian Statistical Institute, of all most same age, salinity ranging from 2–2.5PPT) conditions.
Protein estimation and SDS-PAGE analysis
Total protein estimation was carried out for five mangrove taxa from both habitats following the methods of Lowry (Citation1951). Extraction of proteins for gel electrophoresis was done from 2 g of fresh leaf. Leaf samples were macerated in a mortar-pestle, and to this was added 5 ml of extraction buffer (containing 10% (w/v) SDS, 10 mM β- Marcaptoethanol, 20% (v/v) glycerol, 0.2 M Tris/HCl (pH 6.8) and 0.05% Bromophenol blue). The mixture was centrifuged at 10000 rpm for 20 min. Supernatants were used as samples. Protein samples were resolved in 12.5% SDS-PAGE gels following the procedure of Laemmli (Citation1970) and stained with Coomassie Brilliant Blue R-250 (Sigma). Molecular weights of the different protein bands were determined in respect to standard protein markers (Bioline Hyper Page prestained protein marker, 10–200 kDa) with the Kodak MI software after documentation of the gel slab with Gel-Doc system (Biostep GmbH, Germany).
Extraction of enzymes for native gel electrophoresis and PAGE analysis
Two grams of young leaf buds were macerated to powder with liquid nitrogen with a mortar-pestle; then 0.1 g PVP and 5 ml of extraction buffer (consisting of 1 M Sucrose, 0.2 M Tris-HCL and 0.056 M β-Marcaptoethanol; pH adjusted at 8.5) was added and homogenized. The extractants were centrifuged at 10,000 rpm for 20 min at 4°C; supernatants were used as samples for gel electrophoresis. Isozyme analysis of four enzymes – Peroxidase, Superoxide dismutase, Esterase and Acid phosphatase – was performed for the investigated five taxa. Equimolar amounts of enzymes were loaded in each well. Samples from the saline and non-saline environment were loaded side by side for precision of polymorphic band expression. Slab gels were stained for definite enzymes following the procedure by Das and Mukherjee (Citation1997). Gels were documented with a Gel-Doc System (Biostep GmbH, Germany) and analysis for band intensity and Relative Mobility Factor (Rmf) were estimated with Kodak-MI software.
Enzyme assay
Peroxidase (PRX, E.C.1.11.1.7)
A total of 200 mg fresh leaf sample was extracted in 1–1.5 ml 0.9% KCl and centrifuged at 12,000 rpm for 15 min at 4°C; the supernatant was used as enzyme sample. Absorbances were taken by Helios γ spectrophotometer (Thermo electron Corporation, USA) at 460 nm in respect to the standard curve prepared following the methods of Shannon et al. (Citation1966) with minute modification.
Superoxide dismutase (SOD, E.C.1.15.1.1)
Cell sap was extracted from 200 mg of leaf and 1–1.5 ml 50 mM Phosphate buffer, ph adjusted to 7.0; centrifuged at 12,000 rpm for 15 min at 4°C. Supernatants were used for enzyme samples. Different aliquots (50, 100, 150, 200, 250 µg/ml) of the standard enzyme samples were also used for preparing the standard curve, and absorbance were measured at 550 nm following the protocol described by Keith et al. (Citation1983) with minute modification.
Esterase (EST, E.C.3.1.1.1)
Enzyme sample was prepared from 200 mg fresh leaf sample extracted with 1–1.5 ml ice cold 0.1 M Tris/HCl buffer adjusted pH 8.0. Extractants were centrifuged at 12,000 rpm for 15 min at 4°C. The supernatant was used as sample. Absorbances were noted at 322 nm in respect to the prepared standard curve following the procedure described by Balen et al. (Citation2004).
Acid Phosphatse (ACP, E.C.3.1.3.2)
200 mg fresh leaf sample was extracted in 1–1.5 ml 40 mM succinic acid /NaOH buffer, pH adjusted to 4.0; centrifuged at 12,000 rpm for 15 min at 4°C. Supernatant was taken for enzyme assay. A standard curve was prepared with the known enzyme samples and absorbances were taken at 322 nm following Huttová et al. (Citation2002).
The data presented was the average of 20 readings for each plant, and standard errors were also depicted in the figures. SPSS 12.0 version was used for statistical analysis towards estimating the correlation value, if any, between the total protein amount and quantitatively assayed enzymes. For each enzyme, the pure samples (Sigma chemicals) were used for preparing the standard curves.
Results
Total protein
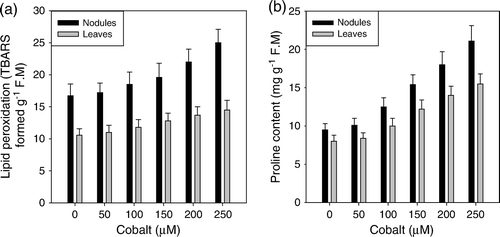
Total leaf protein was estimated from the five mature enough taxa, grown in both saline and fresh water environments. In all five species, the total protein content showed higher amounts in fresh water grown plants compared to their Sundarban counterpart (salt stress environment). The highest amount was estimated in B. gymnorrhiza (125.82 mg/g fr. wt) and E. agallocha (123.2 mg/g fr. wt) and the minimum was in X. granatum (73.96 mg/g fr. wt) grown in ex situ conditions. The increment of total protein was estimated highest in P. paludosa (156%) and lowest in X. granatum (5.7%). In H. fomes, fresh water habitat showed 57% more protein content than that of the in situ habitat (A).
Figure 1. (A) Bar diagram of total leaf proteins with standard errors from the plants grown in saline and non-saline habitat. (B) SDS-PAGE documentation of five taxa grown in saline and non-saline habitat; in the pairs of lanes, the left lane represents saline and the right one forms non-saline habitat plants. M – Protein marker, 1 – B. gymnorrhiza, 2 – E. agallocha, 3 – H. fomes, 4 – P. paludosa and 5 – X. granatum.
SDS-PAGE analysis
This analysis revealed that the numbers of protein bands were expressed differentially in the same species from two different habitats. The molecular weights of these bands were calculated in respect to standard marker, run in the same gel. The result revealed that in Bruguiera, the saline habitat individual showed one extra band than its non-saline replica, and molecular weight ranged from 169.1–66.67 kDa (non-saline) and 210.7–66.11 kDa (saline). Excoecaria showed the same number of bands in both habitats having molecular weights ranging from 205.8–65.55 kDa (non-saline) and 213.2–77.72 kDa (saline). The highest number of protein bands appeared in Heritiera from both the environments, nine bands in each having molecular weights of 211.2–26.71 kDa in non-saline and 212.2–37.0 kDa in saline taxa. One extra band appeared in non-saline Phoenix than its saline pair and the molecular weight ranged from 201.3–46.43 kDa and 213.2–46.0 kDa, respectively. In it, one more band was expressed in saline plant, having 202.8–50.57 kDa (non-saline) and 197.3–58.27 kDa (saline) (B).
Native gel electrophoresis: Peroxidase (PRX)
Band expression obtained from gel electrophoresis revealed that H. fomes and X. granatum showed the same number of isoforms in two different habitats, whereas in B. gymnorrhiza, P. paludosa and E. agallocha, the numbers of isoforms were higher in Sundarbans species than that of their replicas from fresh water conditions (). But the Rmf and band intensity were different to a large extent in all the five species. In Bruguiera, the saline plant showed eight isoforms with the highest OD 163.5 (0.07 Rmf), whereas the fresh water individual showed five isoforms where the highest OD was 51.37 (0.68 Rmf). In Heritiera and Xylocarpus, the numbers of isoforms were the same but the highest OD obtained 206.0 (0.18 Rmf) and 180.0 (0.68 Rmf) from the saline individual and from fresh water habitats, the highest OD values were 166.0 (0.07 Rmf) and 89.9 (0.07 Rmf), respectively. Non-saline Phoenix and Excoecaria showed three and two isoforms of PRX and saline partners expressed four and three isoforms, respectively ( and A).
Figure 2. (A–D) Documented native gel electrophoresis. (A) Peroxidase; (B) Superoxide dismutase; (C) Esterase; (D) Acid phosphatase. In all cases, left lane from non-saline and right one from saline plants. 1 – B. gymnorrhiza, 2 – E. agallocha, 3 – H. fomes, 4 – P. paludosa and 5 – X. granatum. (E–H) Bar diagrams of quantitative estimation of enzymes with standard errors bars.
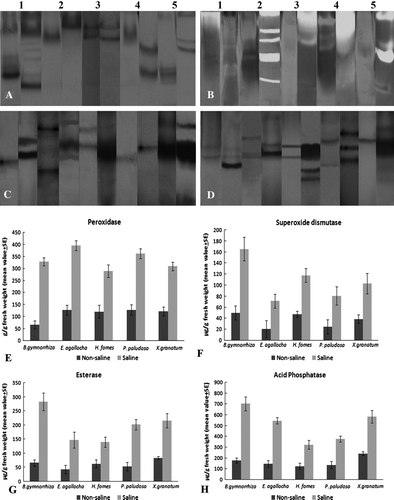
Table 1. Band intensity (OD) and Relative Mobility Fraction (RMF) of isozymes from mangroves in two environments.
Superoxide dismutase (SOD)
The experimental data showed that in all five species, isoforms of SOD were expressed in less numbers from the fresh water grown individuals than that of their saline replica. All four species expressed three isoforms in the non-saline environment, except Phoenix, where it was two. The plants from the saline habitat, Heritiera, Phoenix and Xylocarpus, showed five isoforms, and Bruguiera and Excoecaria had two. The densitometric scanning showed that the band intensity of each isoform was much higher in saline habitats. In Heritiera, highest intensity (138.7 OD) occurred with Rmf value 0.78 in saline individuals, where in the reverse habitat, it was much less (6.48 OD and 0.63 Rmf). Similarly, in Bruguiera, it was 142.0 OD with 0.73 Rmf in saline and 41.53 OD at 0.18 Rmf in non-saline habitat. In Xylocarpus and Phoenix, saline and fresh water condition showed the highest peak as 147.0 OD (0.87 Rmf) and 12.0 OD (0.49 Rmf) and 184.0 OD (0.78 Rmf) and 42.0 OD (0.26 Rmf), respectively. In Excoecaria, the highest peak of intensity were observed in saline and non-saline habitats as 170.07 OD (0.31 Rmf) and 163.0 OD (0.33 Rmf) (; B).
Esterase (EST)
From the gel staining it revealed that EST expression in all species from fresh water habitats were two isoforms, except Xylocarpus (single band) and Excoecaria (three bands). The comparative band intensity was also remarkably high from all saline habitat taxa except in Phoenix, where it was slightly higher (222.0 OD at 0.48 Rmf in saline plants and 177.0 OD at 0.3 Rmf in non-saline habitat). In Heritiera, among the four expressed bands in the saline habitat, the highest band intensity occurred at 226.0 OD (0.48 Rmf) and it was 53.6 OD (0.36 Rmf) in the reverse habitat. Bruguiera showed as high as 221.0 OD (0.53 Rmf) in saline (expressed number of isoforms was three) and 20.0 OD (0.48 Rmf). In Xylocarpus, out of five isoforms in saline condition, the highest OD was 223.0 (0.37 Rmf) in saline and the other side it was 59.5 OD (0.3 Rmf). Out of three isoforms, in saline species of Excoecaria, highest OD obtained 214.0 (0.17 Rmf) and in non-saline it was 102.27 OD (0.24 Rmf) (; C).
Acid phosphatase (ACP)
Among the five investigated taxa, all four species showed excess number isoforms of ACP in saline individuals except in Excoecaria, where it was single band in both the environments, although the band intensity was higher in saline plants (196.0 OD, 0.4 Rmf) than their non-saline partners (26.15 OD, 0.53 Rmf). In Bruguiera, the saline habitat expressed two isoforms of ACP with higher intensity of 157.0 (0.49 Rmf) and 148.6 OD (0.32 Rmf) but the freshwater plant had only one band with 124.0 OD (0.53 Rmf). In both Xylocarpus and Phoenix, saline environment expressed one more isoform than that of their reverse habitat (three isoforms were expressed in fresh water habitat in each). The highest band intensity in in situ Xylocarpus occurred with 247.0 OD (0.49 Rmf), and in the reverse condition the highest band intensity and Rmf value was almost the same (248.0 OD and 0.46). In the ex situ plant of Phoenix, the highest intensity was observed at 96.03 OD (0.17 Rmf) and in its counterpart, it was 227.0 at 0.12 Rmf. Among the three expressed bands, the highest OD value occurred as 168.4 (0.49 Rmf) in Heritiera (saline) and 145.0 OD (0.37 Rmf) in the non-saline plant (; D).
Quantitative assay of enzymes
The plant species from the saline environment showed the all four (PRX, SOD, EST and ACP) investigated enzymes were in higher quantities than that of their fresh water grown individuals. Increase in PRX quantity (µg/g) was highest in Bruguiera (257%), then Xylocarpus (209%), Phoenix (181%) and Heritiera (176%) while the increment was 139% in Excoecaria (E). In the case of SOD, the highest increment occurred in Heritiera (241%), then Bruguiera and Phoenix (229 and 224%, respectively) and lowest in Excoecaria (147%) (F). Similarly, EST was its highest increase in Phoenix (287%), Bruguiera (257%) and Heritiera (241%), and lowest in Excoecaria (154%) (G). ACP reached its maximum increment in Bruguiera (293%) and Xylocarpus (267%) and lower in Excoecaria (139%) (H).
Statistical analysis
Estimated total protein and four enzymes from two habitats were taken into account. A two-tailed bivariate correlation coefficient (Pearson coefficient) was calculated among the each parameter (). The analysis showed that in the case of the relationship between Protein and SOD, all species in the saline environment had an inverse relationship (at 0.01% level) except Bruguiera, wherein it was significant at 0.05% level. In PRO vs. PRX, a significant inverse relationship was observed only in Bruguiera (0.05%) and Phoenix (0.01%) whereas the other three plants (Excoecaria, Heritiera and Xylocarpus) showed no statistically significant relationship. Correlation between PRO and EST obtained a significant positive relationship at 0.01% level only in Bruguiera and Excoecaria in saline inhabitants and others did not show any relationship. The only inversely correlation was obtained in Excoecaria (saline plant) at 0.01% level, whereas in the case of other plants it showed no relationship.
Table 2. Correlations among the different enzymes and total proteins in the plants of two habitats.
Discussion
All the five investigated mangrove taxa from fresh water habitat showed an increased amount of total leaf protein than that of their saline replica. It was noted that the percentage of increment varied in a wide range from 5–36%, in which the highest increment occurred in Excoecaria and Phoenix while the lowest was seen in Heritiera and Xylocarpus (6.05 and 5.7%, respectively). This occurred probably as salinity-imposed plants are adversely affected in their growth and metabolism due to the osmotic effect of salt, nutritional imbalance, accumulation of incompatible toxic ions. The decreased protein content in saline environment might be due to the enhanced activity of protease (Parida et al. Citation2002). The present result was in accordance with Rajesh et al. (Citation1999), who experimentally reported that in Ceriops, the total leaf protein decreased under higher concentrations of saline treatment. Raymond et al. (Citation1994) recorded that stress-induced protein degradation may be essential, which provided amino acids for synthesis of new proteins suited for growth or survival under the modified condition. Mansour (Citation2000) reported that protein biosynthesis declined under salt-stress condition, while cells preferentially synthesize some specific stress proteins. Stress-induced proteins accumulated in the cell which might be synthesized de novo in response to salt or might be present constitutively at low level (Pareek et al. Citation1997). In the present investigation the degradation of proteins in salt habitat, Heritiera and Xylocarpus showed a lesser amount than the other three taxa investigated, probably leading to synthesis of a lesser amount of compatible amino acids in salt habitat. Parida et al. (Citation2002) reported that the total soluble leaf proteins decreased in Bruguiera parviflora under NaCl treatment. This decrease might have the outcome of adverse effect of NaCl treatment resulting in a synthesis of certain low molecular weight proteins which are yet to be elucidated.
Among the various antioxidant enzymes, in this paper we estimated two: Peroxidase (PRX) and Superoxide dismutase (SOD). A qualitative and quantitative study of two antioxidant enzymes (PRX and SOD) and two other important (hydrolyzing) enzymes (EST and ACP) from saline and fresh water grown plants revealed that in most of the cases, the numbers of isoforms, band intensity and enzyme expression were higher in salt-stressed plant. It has been proved that during electron transport in the mitochondria and chloroplasts, some leakage of electrons occurs and these leaked electrons react with O− during aerobic metabolism to produce Reactive Oxygen Species (ROS) such as superoxide (O2 −), hydrogen peroxide (H2O2) and the hydroxyl radical (−OH) (Halliwell and Gutteridge Citation1985). These cytotoxic ROS may seriously affect the normal metabolism through oxidative damage of lipids, proteins and nuclic acids (Fridovich Citation1986). During photosynthesis, the internal O2 level becomes high and chloroplasts are prone to generate ROS at that time (Foyer and Mullineaux Citation1994). Plants synthesize a number of antioxidative enzymes to counteract these ROS, especially SOD converts O2 − into H2O2 and PRX catalyze H2O2 (Asada Citation1994). In salinity-imposed plants, the balance between the production of ROS and the scavenging activity of the antioxidants becomes disrupted, which ultimately results in oxidative damage. Plants with high levels of antioxidants, either constitutive or induced, have been reported to provide sufficient resistance against oxidative damage (Parida et al. 2004a). The present work showed that both PRX and SOD expressions were high in saline plants and the increment ranged from 139–257% in the case of PRX and 147–241 in SOD. The present result was in accordance with earlier works (Cheeseman et al. Citation1997; Takemura et al. Citation2000). In both cases, the increments were lower in Heritiera (139% in PRX, 147% in SOD) and Xylocarpus (142% in PRX, 166% in SOD) than that of the other three species of saline habitat. Parida et al. (2004b) concluded that high salt concentration enhanced the accumulation of free amino acids and polyphenols. Thus, NaCl stress not only imposes alterations in antioxidative metabolism, but also accumulation of osmolytes as adaptive measures. The numbers of isoforms were also increased in the case of PRX and SOD in saline habitat plants. In Bruguiera (saline), the highest numbers of isoforms were expressed in the case of PRX, but were unchanged in the case of Heritiera and Xylocarous (three isoforms in each habitat). This might be due to the relatively less suitability of those plants in the saline environment. SOD showed the excess isoforms in all saline plants compared to their fresh water counterparts. Therefore, it is evident that the salt-imposed production of toxic ROS is mostly regulated by upregulation of antioxidative enzymes like PRX and SOD. Sahu and Mishra (Citation1987) reported changes in enzymatic activity of peroxidase during senescence of rice leaves when submitted to salt stress. They observed that NaCl increased peroxidase activity which could be related to regulation of membrane permeability, cell wall formation and oxidation of accumulated substances due to salt stress. It was also proved that peroxidases are enzymes related to polymer synthesis in cell wall (Bowles Citation1990), as well as with prevention of oxidation of membrane lipids (Kalir et al. Citation1984).
Biosynthesis of esterase (EST) revealed that, in all five species, it is in the higher amount in the in situ taxa investigated. The fresh water grown plants synthesized esterase enzyme with less numbers of isoforms except Excoecaria, where the numbers of isoforms were the same (3), but band intensity was more in saline plants. The highest number of isoforms occurred in Heritiera (saline – 4; in non-saline – 2) and Xylocarpus (saline – 5; in non-saline – 1). Still, the percentage of increment was lower in the above two taxa than the other three from saline habitat (123 and 156%, respectively), the other three species ranged from 241–287% of esterase increment. This result supplemented by Hassanein (Citation1999), where he experimentally proved that nine different esterase isoenzymes were detected in embryos of seeds germinated in 105 mM NaCl, whereas only five of them were detected in the embryos of untreated seeds. Pectins are major components of the primary plant cell wall. They can be both methylesterified and acetylesterified, and de-esterification occurs by specific esterases (Cécile et al. Citation2006). Al-Hakimi and Hamada (Citation2001) reported that the contents of cellulose, lignin of either shoots or roots, pectin of root and soluble sugars of shoots were lowered with the rise of NaCl concentration. Hence, esterases play a major role in counteracting the salt-induced imbalance in cell wall formation.
Acid Phosphatases (ACP) are a group of enzymes that catalyze the hydrolysis of a variety of phosphate esters. These enzymes are widely distributed in plants and are related to phosphate supply and metabolism from a vast array of phosphate esters which are essential for normal growth and development of plant organs (Olczak et al. Citation2000). The present work revealed that the amount of increment in saline grown plants occurred ranging from 139–293%. It may be due to the fact that under conditions of stress, growth is restricted and delivery of phosphate is impaired, thus resulting in the activation of the cellular phosphatases that release soluble phosphate from its insoluble compounds inside or outside of the cells thereby modulating osmotic adjustment by free phosphate uptake mechanism (Fincher Citation1989). Jain et al. (Citation2004) also demonstrated that the endosperm acid and alkaline phosphatase activities were significantly higher after salt treatment, than that of the control in pearl millet. Olmos and Hellin (Citation1997) observed that acid phosphatases are known to act under salt and water stress by maintaining a certain level of inorganic phosphate which can be co-transported with H+ along a gradient of proton motive force. Hence, the plants in which the ACP increments were observed to be lower might be less suited in higher salt environments.
The present investigation revealed that a significant inverse correlation occurred between the concentration of the antioxidative enzymes, peroxidase and SOD with total protein in the case of B. gymnorrhiza, E. agallocha and P. paludosa in saline habitat. This elevation in the antioxidant enzyme concentration level may have taken place to scavenge more numbers of free radicals that are produced during stress (Davies Citation2000) and the decrease in protein concentration might be the result of formation of more compatible osmolytes to restore more negative water potential in cell sap. Both these phenomenon might provide some combat force to the plants against salinity stress. On the other hand, no such statistical significant relationship between antioxidant enzymes and total protein concentration was found in the case of H. fomes and X. granatum. This relationship, as discussed above, may provide some important clues towards the proper salt management mechanism for sustainable existence in the hostile environment. Therefore, the absence of it might be one of the reasons towards less adaptibility for the plants in the present situation. Although there are scopes yet to elucidate in detail regarding the significance of increment of these enzymes in salt-imposed plants, the present work might provide baseline information and a system necessary to conduct future research in relation to the genetic basis of salt tolerance.
References
- Al-Hakimi , AMA and Hamada , AM . 2001 . Counteraction of salinity stress on wheat plants by grain soaking in ascorbic acid, thiamin or sodium salicylate . Biolog Plantarum. , 44 ( 2 ) : 253 – 261 .
- Alim A. 1979 . Instruction manual for plantations in coastal areas . In: White KL Research considerations in coastal afforestation . Food and Agricultural Organization, UNDP/FAO Project BDG/72/005 . Chittgong, , Bangladesh : Forest Research Institute . 65 75 .
- Asada , K . 1994 . “ Production and action of active oxygen species in photosynthetic tissues ” . In Causes of photooxidative stress and amelioration of defense systems in plants , Edited by: Foyer , CH and Mullineaux , PM . 77 – 104 . Boca Raton : CRC Press .
- Balen , B , Krsnik-Rasol , M and Zadro , I . 2004 . Eserase activity and isozymes in relation to morphogenesis in Mammillaria gracillis Pfeiff. tissue culture . Acta Bot Croat. , 63 ( 2 ) : 83 – 91 .
- Banerjee LK. 1999 . Mangroves of Orissa coast and their ecology . Bishen Singh Mohendra Pal singh , Dehra Dun , 41 .
- Bowles , DJ . 1990 . Defense-related proteins in higher plants . Annu Re Biochem. , 59 : 873 – 907 .
- Bray , EA . 1993 . Molecular responses to water deficit . Plant Physiol. , 103 : 1035 – 1040 .
- Cécile , T , Francoise , L and Pierre , VC . 2006 . Polymorphism and modulation of cell wall esterase enzyme activities in the chicory root during the growing season . J Exp Bot. , 57 ( 1 ) : 81 – 89 .
- Chaudhury JK. 1996 . Mangrove forest management. Mangrove rehabilitation and management project in Sulawesi . 297 .
- Cheeseman , JM , Herendeen , LB , Cheeseman , AT and Clough , BF . 1997 . Photosynthesis and photoprotection in mangroves under field conditions . Plant Cell Environ. , 20 : 579 – 588 .
- Curtis SJ. 1993 . Working plan for the forests of the Sundarbans Division for the period from 1st April 1931 to 31st March 1961, Calcutta, India . Bengal Government Press . 175 .
- Das , S . 1999 . An adaptive feature of some mangroves of Sundarbans, West Bengal . J Plant Biol. , 42 ( 2 ) : 109 – 116 .
- Das , S and Mukherjee , KK . 1997 . Morphological and biochemical investigations on Ipomea seedlings and their species interrelationships . Annu Bot. , 79 : 565 – 571 .
- Davies , KJA . 2000 . Oxidative stress, antioxidant defenses and damage removal, repair and replacement systems . IUBMB Life , 50 : 279 – 289 .
- Fincher , GB . 1989 . Molecular and cellular biology association with endosperm mobilization in germinating cereal grains . Annu Rev Plant Physiol Plant Mol Biol. , 40 : 305 – 346 .
- Flowers , TJ , Troke , PF and Yeo , AR . 1977 . The mechanism of salt tolerance in halophytes . Annu Rev Plant Physiol. , 28 : 89 – 121 .
- Foyer , CH and Mullineaux , PM . 1994 . Causes of photooxidative stress and amelioration of defense systems in plants , 343 – 364 . Boca Raton : CRC Press .
- Frenkel , C . 1972 . Involvement of peroxidase and Indole-3-acetic acid oxidase isozymes from pear, tomato, and blueberry fruit in ripening . Plant Physiol. , 49 : 757 – 763 .
- Fridovich , I . 1986 . Biological effects of the superoxide radical . Arch Biochem Biophys. , 247 : 1 – 11 .
- Halliwell , B and Gutteridge , JMC . 1985 . Free radicals in biology and medicine , Oxford, , UK : Clarendon Press .
- Hasegawa , PM , Bressan , RA , Zhu , JK and Bohnert , HJ . 2000 . Plant cellular and molecular responses to high salinity . Annu Rev Plant Physiol Plant Mol Biol. , 51 : 463 – 499 .
- Hassanein , AM . 1999 . Alterations in protein and esterase patterns of peanut in response to salinity stress . Biolog Plantarum. , 42 ( 2 ) : 241 – 248 .
- Hatcher , BG , Johannes , RE and Robertson , AI . 1989 . Review of research relevant to the conservation of shallow tropical marine ecosystems . Oceanogr Mar Biol Rev , 27 : 337 – 414 .
- Hogarth , PJ . 1999 . The biology of mangroves , London : Academic Press .
- Hurkman , WJ , Fornia , CS and Tanaka , CK . 1989 . A comparison of the effects of salt on polypeptides and translatable mRNAs in roots of a salt-tolerant and salt-sensitive cultivar of barley . Plant Physiol. , 90 : 1444 – 1456 .
- Huttová , J , Tamás , L and Mistrik , I . 2002 . Aluminium induced acid phosphatase activity in roots of Al-sensitive and Al-tolerant barley varieties . Roslinná Výroba , 48 ( 12 ) : 556 – 559 .
- Jain , A , Sharmai , AD and Singh , K . 2004 . Plant growth hormones and salt stress-mediated changes in acid and alkaline phosphatase activities in the pearl millet seeds . Int J Agric Biol. , 6 ( 6 ) : 960 – 963 .
- Kalir , A , Omri , G and Poljakoff-Matber , A . 1984 . Peroxidase and catalase activity in leaves of Halimione portulacoides exposed to salinity . Physiolog Plantarum. , 62 : 238 – 244 .
- Keith , H , Emmanuelle , V , Hélène , B and Charistian , A . 1983 . Superoxide dismutase assay using alkaline dimethylsulfoxide as superoxide anion-generating system . Analyt Biochem. , 135 : 280 – 287 .
- Laemmli , UK . 1970 . Cleavage of structural proteins during the assembly of the head of bacteriophage T4 . Nature , 227 : 680 – 685 .
- Lowry , OH , Rosebrough , N , Farr , AL and Randall , RJ . 1951 . Protein measurements with folin phenol reagent . J Biol Chem. , 193 : 265 – 275 .
- Macfarlane , GR and Burchett , MD . 2001 . Photosynthetic pigments and peroxidase activity as indicators of heavy metal stress in the grey mangrove, Avicennia marina (Forsk.) Vierh . Marine Pollut Bull. , 42 ( 3 ) : 233 – 240 .
- Mader , M . 1976 . Die Localization der Peroxidase Iso-. enzymgruppe G, in der Zellwand von Tabak-Geweben . Planta. , 131 : 11 – 15 .
- Mansour , MMF . 2000 . Nitrogen containing compound and adaptation of plants to salinity stress . Biol Plantarum , 43 ( 3 ) : 491 – 500 .
- Nandy (Datta) , P , Das , S , Ghose , M and Spooner-Hart , R . 2007 . Effects of salinity on photosynthesis, leaf anatomy, ion accumulation and photosynthetic nitrogen use efficiency in five Indian mangroves . Wetl Ecol Manage. , 15 : 347 – 357 .
- Naskar , KR and Guha Bakshi , DN . 1983 . A brief review on some less familiar plants of the Sundarbans India . J Econ Taxonomic Bot. , 4 ( 3 ) : 699 – 712 .
- Olczak , M , Kobialka , M and Watorek , WX . 2000 . Characterization of diphosphonucleotide phosphatase/phosphodiesterase from yellow lupin (Lupinus luteus) seeds . Biochimica Biophysica Acta. , 1478 ( 2 ) : 239 – 247 .
- Olmos , E and Hellin , E . 1997 . Cytochemical localization of ATPase plasma membrane and acid phosphatase by cerium based in a salt-adapted cell line of Pisum sativum . J Exp Bot. , 48 : 1529 – 1535 .
- Pareek , A , Singla , SL and Grover , A . 1997 . “ Salt responsive proteins/genes in crop plants ” . In Strategies for improving salt tolerance in higher plants , Edited by: Jaiwal , PK , Singh , RP and Gulati , A . 365 – 391 . New Delhi, , India : Oxford and IBH Publishing Co .
- Parida , A , Das , AB and Das , P . 2002 . NaCl stress causes changes in photosynthetic pigments, proteins and other metabolic components in the leaves of a true mangrove, Bruguiera parviflora, in hydroponic cultures . J Plant Biol. , 45 : 28 – 36 .
- Parida , AK , Das , AB and Mohanty , P . 2004a . Defense potentials to NaCl in a mangrove, Bruguiera parviflora: Differential changes of isoforms of some antioxidative enzymes . J Plant Physiol. , 161 ( 5 ) : 531 – 542 .
- Parida , AK , Das , AB and Mohanty , P . 2004b . Investigations on the antioxidative defence responses to NaCl stress in a mangrove, Bruguiera parviflora: Differential regulations of isoforms of some antioxidative enzymes . Plant Growth Regulat. , 42 ( 3 ) : 213 – 226 .
- Rajesh , A , Arumugam , R and Venkatesalu , V . 1999 . Response to ceriops roxburghiana to NaCl stress . Biol Pantarum. , 42 ( 1 ) : 143 – 148 .
- Raymond , P , Broquisse , R , Chevalior , C , Couee , I , Dieuaide , M , James , F , Just , D and Pradet , A . 1994 . “ Proteolysis and proteolytic activities in the acclimation to stress: The case of sugar starvation in maize root tips ” . In Biochemical and cellular mechanisms of stress tolerance in plants , Edited by: Cherry , JH . 325 – 334 . Berlin : Springer-Verlag .
- Sahu , AC and Mishra , D . 1987 . Changes in some enzyme activities during excised rice leaf senescence under NaCl-stress . Biochem Physiolog Pflanzen. , 182 : 501 – 505 .
- Sanyal P. 1996 . Sundarbans: The largest mangrove diversity on the globe . In: Willium Roxburgh Memorial Seminar on Sundarban Mangals , Calcutta 11 26 .
- Shannon , LM , Key , E and Lew , JY . 1966 . Peroxidase isozyme from Horseraddish root. I. Isolation and physical properties . J Biol Chem. , 249 ( 9 ) : 2166 – 2172 .
- Spalding , M , Blasco , F and Field , C . 1997 . World Mangrove Atlas. The International Society for Mangrove Ecosystems , 178 Japan : Okinawa .
- Stonier T , Yang Hsin-Mei 1973 . Studies on Auxin protectors: XI. Inhibition of peroxidase-catalyzed oxidation of glutathione by auxin protectors and o dihydroxyphenols . Plant Physiol. 51 : 391 395 .
- Swire-Clark , GA and Marcotte , WR Jr . 1999 . The wheat LEA protein Em functions as an osmoprotective molecule in Saccharomyces cerevisiae . Plant Mol Biol. , 39 : 117 – 128 .
- Takemura , T , Hanagata , N , Sugihara , K , Baba , S , Karube , I and Dubinsky , Z . 2000 . Physiological and biochemical responses to salt stress in the mangrove, Bruguiera gymnorrhiza . Aquat Bot. , 68 : 15 – 28 .
- Union for Protection of New Varieties of Plants (UPOV) 1997 . Adopted report of the Technical committee . 33rd session, Geneva; October 16–18, 1996. UPOV TC/33/11 .
- Xu , D , Duan , X , Wang , B , Hong , B , Ho , TDH and Wu , R . 1996 . Expression of a late embryogenesis abundant protein gene, HVA1, from barley confers tolerance to water deficit and salt stress in transgenic rice . Plant Physiol. , 110 : 249 – 257 .
- Xu , XY , Abo , M , Okubo , A and Yamazaki , S . 2001 . Salt-stress responsive membrane proteins in Rhodobacter sphaeroides f. sp denitrificans IL106 . J Biosc Bioeng. , 91 : 228 – 230 .
- Ye , Y , Tam Nora , FY , Wong , YS and Lu , CY . 2003 . Growth and physiological responses of two mangrove species (Bruguiera gymnorrhiza and Kandelia candel) to water logging . Envrion Exp Bot. , 49 ( 3 ) : 209 – 221 .
- Zeidler , M . 2000 . Electrophoretic analysis of plant . Acta Univ Palacki Olomuc Fac Rerum Nat Biol. , 38 : 7 – 16 .
- Zimmermann , MH . 1983 . Xylem structure and the ascent of sap , Berlin : Springer-Verlag .