Abstract
Caffeic acid (CA) sometimes behaves as a potent phytotoxin affecting plant and fungi growth and physiology. The aim of the present study was to investigate whether CA at a concentration range similar to that found in sugarcane leaves, had any effect against different phases of Sporisorium scitamineum growth cycle. Leaf CA concentration from two different sugarcane cultivars, Mayari (My) 55-14, resistant, and Barbados (B) 42231, susceptible to smut, was chromatographically quantified by HPLC. A smut elicitor promoted an increase of CA concentration in the resistant cv. while no effect was produced in the susceptible one. The effect of CA upon S. scitamineum growth cycle showed to be dependent of both concentration and time. At 5.0 µg ml−1, CA produced and inhibition of teliospore germination, haploid sporidia production and dikaryotic mycelium appearance. At 30 µg ml−1, CA produced similar effects to these just described. Inhibition was more evident after 24 h or 28 h incubation of teliospores in CA solution than after 48 h. CA at 20 µg ml−1 reduced both germination of teliospores and production of haploid sporidia but it had no significant effect on dikaryotic mycelium appearance after 24-h incubation.
Introduction
Sporisorium scitamineum (=Ustilago scitaminea) was reported to be the causal agent of sugarcane smut and it belongs to class Basidiomycetes, subclass Ustilaginomycetidae, order Ustilaginales, family Ustilaginaceae, genus Ustilago which includes a group of phytopathogenic fungi (Vanky Citation1999; Thaung Citation2005). Sugarcane smut was first reported from Natal in South Africa (McMartin Citation1945). The disease has been associated with specific problems in sugarcane-producing areas of the world (Singh et al. Citation2004), affecting both plant growth and juice quality (Martínez et al. Citation2000). The fungus is spread from plant to plant by airborne teliospores.
The sequence of nuclear events that characterize the life cycles of the various Ustilago species is likewise similar, and recent molecular genetic research has shown that homologous genes govern their sexuality and pathogenicity. At the teliospore germination time of S. scitamineum, spores extend in size, split its exosporidium (external wall of teliospores) and produce a probasidium from which, following meiosis, four sporidia emerge. Haploid sporidia represent the second cell type (Singh et al. Citation2004). Germination of sporidia leads to the production of a germ tube or same sporidial cells (Chona Citation1943). Heterothallism in S. scitamineum is bipolar. Sporidia belongs to two mating types; plus (+) and minus (−). Single haploid (n) sporidia continue to multiply by budding in a yeast-like manner. Mycelial dikaryotic (n + n) phase, third cell type appears following three consecutive ways: (a) Fusion of compatible sporidia; (b) promycelial anastomosis: The growth of two promycelium together and the lysis of their cell wall in crossed site; finally the migration of the nucleus to fused cell; and (c) hyphal anastomosis of two mycelia of different mating types arising from sporidial germination (Agnitori Citation1990; Moosawi-Jorf et al. Citation2006).
Teliospore germination, sporidial fusion and dikaryon formation normally take place on the epidermal surface of the plant, but only the dikaryon is capable to parasite the host. The main ways of entry into the plant are leaf stomata and epidermis (Santiago et al. Citation2008b) and buds (Alexander and Ramakrishnan Citation1980). From these points, the pathogen develops systemically throughout the stalk, but teliospores are formed only in peripheral tissues of the long whip-like, sorus-bearing structure (the ‘whip’, which is the primary symptom). When teliospores are deposited on a wet surface of the plant, germination is followed by the formation of appresoria, mainly on the inner scale of young buds and on the bases of emerging leaves (Waller Citation1970). Dikaryotic mycelia are capable to penetrate through buds at each sugarcane node and shortly reach apical meristem systemically. Entry into the meristem in the bud occurs between 6 and 36 h after the teliospores are deposited on the surface (Alexander and Ramakrishnan Citation1980).
Sugarcane plants develop different defence mechanisms to combat the invasion of pathogenic organisms. De Armas et al. (Citation2007) correlated the susceptibility or resistance to smut with changes in the level of free phenolic compounds and phenyalanine ammonia-lyase (PAL), and peroxidase activities in discs of sugarcane leaves as a response to elicitors extracted from the smut mycelium. According to the biosynthetic routes of the different phenolic acids, caffeic acid is derived from p-coumaric acid which is synthesized with PAL participation. Caffeic acid, C9H8O4, hereafter CA, a type of carboxylic acid is one of the most common cinnamic acids isolated from a variety of crops, weed residues, as well as other plants (Rice Citation1995). As an early intermediate of phenyl propanoid metabolism, it is a precursor for structural polyphenols and many biologically active secondary compounds that are important in the defence chemistry of plants. Many biological activities have been reported for free CA. In bioassay experiments, it inhibits the growth of plants (Singh et al. Citation1989), fungi (Bostock et al. Citation1999) and bacteria (Ravn et al. Citation1989). CA is one of many phenolics considered to be an important part of the general defence mechanism of plants against infection and predation (Faulds and Williamson Citation1999). CA was detected in soluble as well as in insoluble cell wall-bound fractions from sugarcane stems of young plants with values for smut inoculated plants higher than those found for uninoculated plants (Santiago et al. Citation2009).
The objectives of this study were to determine the CA content of sugarcane leaves from two different cultivars, one resistant, Mayarí 55-14 (My 55-14) and the other one susceptible, Barbados 42231 (B 42231), to smut after treatment with an elicitor suspension isolated from S. scitamineum mycelium. The action of CA against different phases of S. scitamineum growth cycle was assessed in order to determine if the level of CA in leaves contribute to modulate, in part, the defence response of resistant sugarcane cultivars.
Materials and methods
Isolation and culture of teliospores
Whips of smutted plants of sugarcane from the susceptible cv. B 42231 were collected from fields of the National Institute for Sugarcane Research (INICA) in Matanzas, Cuba. Parts belonging 15 cm from tip and below of whips were cut and removed and then the middle part of whips were introduced in non-vacuum desiccator for 48 h at room temperature. Whips containing teliospores were shaken and waste materials were removed. Teliospores were surface disinfected three times in sterile distilled water containing 50 µM streptomycin sulphate (Sigma Chem. Co.), transferred into sterile vials and maintained in sterile desiccators containing calcium chloride to reduce the humidity of teliospore mass (Izadi and Moosawi-Jorf Citation2007). Teliospores were kept in air-tight containers in the dark at room temperature for subsequent investigation.
One mg of teliospores were germinated on 0.5 ml of three different media, sterile distilled water, potato dextrose broth (PDB), or Lilly-Barnett (LB) containing 50 µM streptomycin sulphate, in the dark from 0–72 h at 25°C. The sequence of nuclear events that characterize the smut life cycle was examined microscopically at time intervals of about two hours. Aliquots (10 µl) of teliospore suspension were transferred by micropipette sterile tips on slides and stained with 0.1% cotton blue in lactophenol (Lloyd and Naidoo Citation1981) in a single step heating for 1 min. The fungi are stained dark blue and stand out well against the light blue background. Teliospore germination to form a promycelium, haploid sporidia and dicaryotic mycelium were observed microscopically in an Olympus DP50 microscope adapted with a CCD camera capturing images by using a Viewfinger Lite program.
Incubation of sugarcane leaves on smut elicitor
Field-grown 12 month-old Saccharum officinarum, My 55-14 cv. and B 42231 cv., were used throughout this work. Twenty discs of 1.0 cm of diameter were obtained from central part of first completely developed young leaves of different stalks and floated on 10 mM phosphate buffer containing 4% isopropylic acid for 2 h at 37°C in the dark to permeabilize leaf cells. Cells were permeabilized, as a general mechanism, to favour the entry of the elicitor molecules. Then, 0.5 ml of a smut elicitor solution was added to the discs and incubated in the dark from 0–72 h as previously described (de Armas et al. 2007). Three biological replications were made by using leaf samples from different stalks. Controls were performed in absence of smut elicitor.
Smut elicitor was prepared from teliospores of S. scitamineum isolated from diseased B 42231 plants. Teliospores were incubated in PDB medium at 38°C for five days. The mycelium formed was harvested and, after washing and drying, ground in liquid nitrogen. The powder was extracted with 10 mM Tris-HCl buffer and 80% (v/v) methanol according to de Armas et al. (2007). After different washes and extractions, a clear supernatant was used to elicit the response from sugarcane leaves.
Quantification of CA
After incubation of leaf discs with or without smut elicitor, samples were ground in a mortar with liquid nitrogen, solubilized with phosphate buffer and the suspension centrifuged at 10,000 g for 15 min at 2°C. The pellet was extracted with 5 ml 80% (v/v) methanol at 70°C for 1 h with continuously shaking in the dark and centrifuged (15,000 g for 15 min at 2°C). The supernatant was dried in a speed vac and dry precipitate was redissolved in 0.2 ml acetonitrile (ACN) and used for HPLC analysis. HPLC separation was carried out using a Spectra Physics 8810 liquid chromatograph equipped with a UV-Vis SP8490 detector. Analytical conditions were those described by de Armas et al. (2007). Quantitative estimation of methanol-soluble CA was carried out by using the slope of the straight line obtained by linear regression from different injected mass of standard CA and its corresponding area counts.
Experimental media and fungal growth measurements
Commercial CA was aseptically dissolved into sterile distilled water at specific concentrations to determine their effects upon growth of S. scitamieum. Smut teliospores (1.0 mg) were incubated either on 0.5 ml sterile distilled water (control) or on three different CA concentrations (5, 20 and 30 µgml−1) containing 50 µM streptomycin sulphate, in the dark at 25°C. CA and streptomycin sulphate were purchased from Sigma Chemical Co. (St Louis, MO, USA). The smut life cycle monitoring, it is, germinated teliospore (GT), haploid sporidia (HS) and dicaryotic mycelium (DM), was carried out at 24, 28 and 48 h incubation under light microscopy as described in the last paragraph of ‘Isolation and culture of teliospores’ in Materials and methods. For quantitative determination of the cell number, Neubauer chamber was used. Aliquots of suspension were put into the counting chamber by sterile tips micropipette. Number of TG, HS and DM were counted and the total number was determined mathematically ignoring those lying on the top and right margin.
Three biological replicates of each treatment were prepared and each Neubauer count was run 10 times.
Statistical analyses
For determination of CA concentration in leaves statistical analyses of the differences between the mean values measured from control and treated plants were performed using the Student's t-test. To analyze the effect of CA upon smut life cycle statistical analysis was performed using multipleway Analysis of Variance (ANOVA), followed by post-hoc analysis with Tukey-HSD (Honest Significant Differences) test. Differences were considered to be significant at p < 0.05.
Results
Sporisorium scitamieum life cycle
Pre-immersion of teliospores on streptomycin sulphate was done to avoid bacterial contaminations and the three media were amended with streptomycin sulphate to prevent bacterial growth.
Teliospores began to germinate after 4 h of culturing on PDB medium, 8 h on LB medium and 18 h on sterile distilled water at 25°C (). Haploid sporidia were produced after 10 h on PDB medium. However, sporidia formation occurred 20 h after spreading teliospores, on LB medium and sterile distilled water. DM formation occurred in parallel on the three culture media 24 h after spreading teliopores (), although the amount of specimens was different.
Table 1. Time-course of smut life cycle cultured on sterile distilled water, potato dextrose broth (PDB) and Lilly Barnett medium (LB) at 25°C. Germinated teliospores (GT), haploid sporidia (HS) and dikaryiotic mycelium (DM) were analyzed. -, absence of specimens; +, to 100,000 specimens; ++, from 100,000 to 300,000 specimens; +++, more than 300,000 specimens.
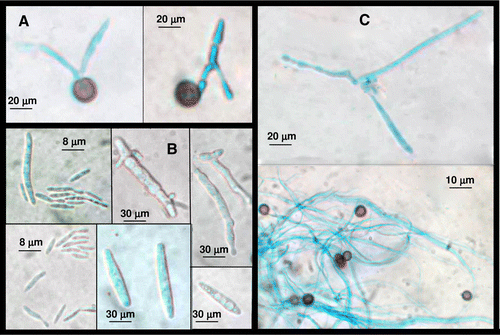
It is evident from that germination of teliospores and sporidia formation occurred earlier on PDB medium than on LB medium or distilled water, also the amount of specimens was higher than on LB medium. However, although the life cycle of S. scitamineum was delayed on sterile distilled water, a considerable amount of GT and HS occurred at 24 h. The amount of HS reached the maximum after 24 h incubation either on distilled water or on PDB medium and its presence was higher than that of other nuclear events. shows light micrographs of these nuclear events of smut life cycle grown on sterile distilled water for 48 h at 25°C. TG (A) and produced sporidia (B). Single celled sporidia measured between 6 and 15 µm and were elliptical in shape. Sporidial colony growth was yeast-like but following mating, mycelial hyphae developed (C).
The effect of smut elicitor on CA accumulation in sugarcane leaves.
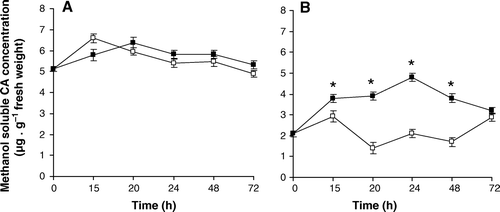
HPLC analysis of CA was performed in both control and inoculated with smut elicitor leaves of both resistant and susceptible cultivars. The time-course of CA accumulation in leaf was studied in methanolic extracts which yield soluble CA. Smut elicitor produced different accumulation patterns of CA in both cultivars. Similar concentration of CA was observed in the presence and in the absence of elicitor in leaves of smut-susceptible B 42231 cv. (A). The smut resistant cv., My 55-14 displayed significantly higher concentrations of CA when incubations were performed in the presence of the elicitor (B). However, concentration range of CA in this resistant cv. was lower than that found in the susceptible cv. Maximum CA level was detected after 24 h incubation and corresponds to 5 µgml−1 in My 55-14 cv (B).
Figure 2. Time-course of the accumulation of total, methanol-soluble CA in leaf discs from B 42231 cv. (susceptible) (A) and My 55-14 cv. (resistant) (B). Unfilled symbols represent the controls and filled symbols represent samples treated with smut elicitor. All values are the mean of two replicates from three biological samples. Vertical bars represent the standar error of the means. * indicates values significantly different from the control at p < 0.05.
In vitro effects of CA on fungal growth
According to the range of CA observed in control and inoculated sugarcane leaves of both resistant and susceptible cultivars (), three CA concentrations were chosen to study in vitro its effect on the smut life cycle. The culture medium used was sterile distilled water amended with 5, 20 and 30 µgml−1 CA, which dissolves in water better than in PDB or LB medium. In this study, we demonstrate that fungal growth occurs, even if it is delayed on time on sterile distilled water (). So the quantification of their possible biological actions was made at 24, 28 and 48 h.
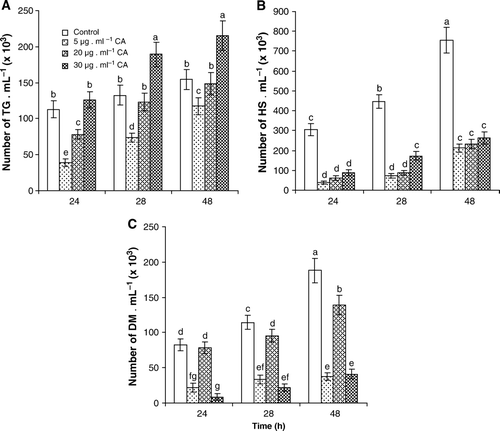
shows the effect of CA on GT (A), HS (B) and DM (C). GT did not significantly increased with time of incubation although CA at the lowest assayed concentration (5 µg·ml−1) significantly inhibited GT at any time. Increasing CA concentration to 20 µg·ml−1, it was produced inhibition of germination as compared with control, only after 24-h incubation. This concentration does not produce any significant reduction in the amount of GT at 28 or 48 h. In response to 30 µg·ml−1 CA, there was, however a sharp increase in the average number of GT, not significant at 24 h but significant at 28 and 48 h (A). The amount of HS (four from each GT) increased with the time of incubation in absence of CA. The number of HS significantly decreased in media containing CA at all the assayed concentrations (B). DM from HS was produced in control media and significantly increased with time. In response to 5 µg·ml−1 CA or 30 µg·ml−1 a significant reduction in the production of DM at 24, 28 and 48 h was found. However, the concentration of 20 µg·ml−1 produced no effect on DM appearance as compared with control plates at 24 h. After 28 and 48 h incubation, significant inhibition in the production of DM was observed. It is important to notice that 20 µg·ml−1 CA produced reduction of DM amount lower than that achieved at 5 or 30 µg·ml−1 (C).
Figure 3. The effect of different CA concentration (5, 20 and 30 µgml−1) upon S. scitamineum life cycle after 24, 28 and 48 h incubation. Amount of: GT (A), HS (B) and DM (C) production, in relation to control (sterile distilled water). The quantitative determination was carried out by a direct microscopic counting chamber Neubauer. All values are the mean of 10 counts done in three biological replicates Vertical bars represent the standard error of the means. Different letters indicate significant differences (p < 0.05).
Micrographs of quantitative determination using a Neubauer chamber show the CA effect on sporidia formation at 24-h incubation (). An inhibition was observed when phenolic was used in the bioassay. The effect was appreciable at all the concentrations of CA used, 5 µgml−1 (B), 20 µgml−1 (C) and 30 µgml−1 (D) in respect to the control treatment (A). We have chosen the best fields for micrographs with independence of the quantification process.
Discussion
The disinfection treatment for culturing teliospores (Izadi and Moosawi−Jorf 2007) leads to the full prohibition of bacterial contamination in the culture media (). Cultured of smut was successfully by the use of PDB, LB and sterile distilled water containing streptomycin sulphate. Although it has been observed that faster growth of smut was obtained on PDB medium, also on sterile distilled water the sequence of nuclear events in a high percentage occurred at 24 h (). Teliospore germination, continued budding of sporidia and dicaryon formation may all be carried out in vitro (), but the remainder of the fungal life cycle, nuclear fusion and teliospore formation, has never been observed to take place outside of the sugarcane plant (Schenck Citation1999). Results of cutting inoculations and detection of fungus in plantlets using PCR reaction indicated that, regardless of existence of the smut fungus in plants, infection was only produced by dicaryotic forms (Moosawi-Jorf and Izadi Citation2007). Therefore, any inhibition in the life cycle sequence of nuclear events leads to a decrease of pathogenicity.
Soluble elicitors produced by smut caused differential changes in the titre of CA when they interacted with sugarcane leaf discs. The principal change was achieved in My 55-14 cv. by the presence of the elicitor (B). There were no significant differences with the time of incubation either in the control or in inoculated plants from cv. Barbados 42231 (A). In previous studies we demonstrated that elicitor molecules derived from fungi (proteins, peptides, and glycopeptides of medium molecular mass) are able to evoke biochemical changes related to phenylpropanoid metabolism (de Armas et al. 2007; Santiago et al. Citation2008a).
It is well known that phenolic acids are precursors of a variety of antimicrobial compounds that play an important role in plant defence responses. The synthesis of these compounds has been associated to the resistance of plants to fungi and viruses (Barber et al. Citation2000; Ralph et al. Citation2006). The possibility that these compounds may protect plants against disease has intrigued biologists since the early part of the previous century (Link et al. Citation1929; Müller and Börger Citation1940). Any disruption of the organization and function of the internal cellular space results in the breakdown of normal metabolic processes.
Concentration of CA detected in the resistant cultivar was lower than that found in the susceptible cultivar (). Accumulation of CA produced a feedback inhibition of PAL activity in sugarcane (Santiago et al. Citation2008a). According to de Armas et al. (2007), PAL activity is fundamental to maintain or increase the synthesis of phenolics. In a previous study, working with sugarcane stems inoculated with smut sporidia, our group demonstrated that CA concentration detected in the free soluble fraction showed a significant decrease with time after treatment with smut elicitor in the resistant cultivar (Santiago et al. Citation2009). In the present study, we detected concentration of CA ranged 6 µg·CA·g−1 fresh weight in leaves of B 42231 with independence of the inoculation process. However, concentration of CA detected in My 55-14 leaves was around 2 µgml−1, three times lower than that detected in B 42231 leaves. Inoculation with smut elicitor produced a significant increase of this CA concentration to reach values about 5 µgml−1. Low molecular weight phenolic precursors and free radicals produced during polymerization may be directly toxic to the pathogen. Many phenolic compounds have been reported to have antimicrobial activity. This was demonstrated by Keen and Littlefield (Citation1979) who identified two antimicrobial lignin precursors, coniferyl aldehyde and coniferyl alcohols as phytoalexins in the flax-Melampsora lini interactions. They demonstrated that the degree of resistance was directly correlated with the amount of coniferyl aldehyde produced.
Here, we have examined in vitro effects of commercial CA on smut life cycle reproducing the amount of this phenolic detected on sugarcane leaf. Phenolic has inhibitory effects upon production of HS (B and 4) and DM formation (C). A significant inhibition was obtained with 5 µgml−1 CA upon the three cell types of smut cycle – TG, HS and DM formation – at 24, 28 and 48 h (). This was consistent with the observation that an Arbuscular Mycorrhizal (AM) fungus colonized asparagus grown in sand treated with CA less than it did controls (Pederson et al. Citation1991). Douds et al. (Citation1996) showed that CA, found in Daucus carota cytoplasm, also depressed the growth of Gigaspora gigantea and G. margarita. CA inhibited the growth of four sweet potato pathogenic fungi and the germination of proso millet seeds in bioassays (Harrison et al. Citation2003). Although CA produced inhibition of all the cell types, it seems that these cells were able to use CA as a carbon source since higher times of incubation produced less inhibition ().
With the extension of hyphal growth around the vascular tissues, there is the possibility that S. scitaminea spread quickly and systemically into the plant through the vascular system (Singh et al. Citation2004). However, if sugarcane is able to inhibit dycaryotic mycelium formation, infectivity would be reduced. Our current research confirms that levels of CA (around 5µg·ml−1), which are those detected after inoculation with smut elicitor in a resistant cv., produce inhibitory effect on the formation of HS and DM ( and ). Then, a CA concentration found in the resistant cv. lower than that found in the susceptible cv. could confer to the first cv., a better capacity for decreasing pathogenicity. Rather amazing was the effect of CA on DM production. At 20 µg·ml−1 CA inhibition of DM formation was lower than that achieved by 5µg·ml−1 CA. This fact could be explained on the basis of the existence of a receptor for CA with, at least, two classes of binding sites for the ligand. To our knowledge, this receptor has not been characterized in fungi at the moment. However, CA interacts with the multisubunit protein helianthinin from sunflower seeds (Suryaprakash et al. Citation2000). The authors demonstrated that helianthinin has two classes of binding sites for CA and that the phenylpropanoid induces destabilization of the subunit–subunit interaction in the protein. Also methaemoglobin has two binding sites for CA (Suryaprakash et al. Citation2000). If a specific protein receptor for CA exits in DM of S. scitamineum which would able to interact with CA is not known. But a receptor could explain that 5 µg·ml−1 CA saturate all the binding sites for the ligand, producing then a high inhibition whereas 20 µg·ml−1 oversaturate receptor molecules, producing bad unions that nullify inhibition. A concentration of CA higher than 20 µg·ml−1 would develop a catabolic pathway, as suggests, probably through fatty acid β-oxidation of the propanoid chain (Kawazu et al. Citation1996). This is, the excess of CA when added to a concentration of 30 µg·ml−1 is so high that, after the binding to the hypothetical receptor, is able to induce or activate degradative fungal enzymes.
It would be possible to hypothesize that in the present work, the low rate of dicaryon formation is produced because budding is inhibited, and cells start to make narrow filaments instead of which that are referred as mating hyphae or conjugates tubes. Different aspects of the cell cycle, including sporidial mating, are under the complex control of two loci, a and b. In Ustilago maydis b alleles encode polypeptides that, in combination, activate a series of genes governing pathogenicity (Froeliger and Leong Citation1991; Schenck Citation1999).
Acknowledgments
This work has been supported by grants from Agencia Española de Cooperación con iberoamérica A/018020/08 (AECI) and Ministerio de Educación y Ciencia (Spain) BFU2006-14263. We gratefully acknowledge the excellent technical assistance of Mrs Raquel Alonso and Mr David Varela.
References
- Agnitori VP. 1990 . Diseases of sugarcane and sugar beet . Oxford : Oxford and IBH Publishing Co. Pvt. Ltd .
- Alexander , KC and Ramakrishnan , K . 1980 . Infection of the bud, establishment in the host and production of whips in sugarcane smut (Ustilago scitaminea) of sugarcane . Proc Intern Soc Sugarcane Technol. , 17 : 1452 – 1455 .
- Barber , MS , McConnell , VS and DeCaux , BS . 2000 . Antimicrobial intermediates of the general phenylpropanoid and lignin specific pathways . Phytochemistry , 54 : 53 – 56 .
- Bostock , RM , Wilcox , SM and Wand , G . 1999 . Adaskaveg. Suppression of Monilinia fruticola cutinase production by peach fruit surface phenolic acids . Physiol Mol Plant Phatol. , 54 : 37 – 50 .
- Chona , BL . 1943 . Sugarcane smut and its control . Indian Farm. , 4 : 401 – 404 .
- de Armas , R , Santiago , R , Legaz , ME and Vicente , C . 2007 . Levels of phenolic compounds and enzyme activity can be used to screen for resistance of sugarcane to smut (Ustilago scitaminea) . Austral Plant Pathol. , 36 : 32 – 38 .
- Douds , DD , Nagahashi , G and Abney , GD . 1996 . The differential effects of cell wall-associated phenolics, cell walls, and cytosolic phenolics of host and non-host roots on the growth of two species of AM fungi . New Phytol. , 133 : 289 – 294 .
- Faulds , CB and Williamson , G . 1999 . The role of hydroxycinnamates in the plants cell wall . J Sci Food Agric. , 79 : 393 – 395 .
- Froeliger , EH and Leong , SA . 1991 . The a mating type genes of Ustilago maydis are idiomorphs . Gene. , 100 : 113 – 122 .
- Harrison , HF , Peterson , JK , Snook , ME , Bohar , JR and Jackson , DM . 2003 . Quantity and potential biological activity of caffeic acid in sweet potato [Ipomoea batatas (L.) Lam.] storage root periderm . J Agric Food Chem. , 51 : 2943 – 2948 .
- Izadi , MB and Moosawi-Jorf , A . 2007 . Isolation and identification of yeast-like and mycelial colonies of Ustilago scitaminea using specific primers . Asian J Plant Sci. , 6 : 1137 – 1142 .
- Kawazu , K , Zhang , H and Kanzaki , H . 1996 . Accumulation of benzoic acid in suspension cultured cells of Pinus thunbergii in response to phenylacetic acid administration . Biosci Biotechnol Biochem. , 60 : 1410 – 1412 .
- Keen , NT and Littlefield , LJ . 1979 . The possible association of phytoalexins with resistance gene expression in flax to Melampsora lini . Physiol Plant Pathol. , 14 : 265 – 280 .
- Link , KP , Dickson , AD and Walker , JC . 1929 . Further observations on the ocurrence of protocatechuic acid in pigmented onion scales and its relation to disease resistence in onions . J Biol Chem. , 84 : 719 – 725 .
- Lloyd , HL and Naidoo , G . 1981 . A qualitative semi-automated technique for the assessment of smut colonization of sugarcane stalk tissue prior the whip formation . Sugarcane Pathol Newsl. , 26 : 48 – 51 .
- Martínez , M , Medina , I , Naranjo , S , Rodríguez , CW , de Armas , R , Piñón , D , Vicente , C and Legaz , ME . 2000 . Changes of some chemical parameters involved in sucrose recovery from sugarcane juices, related to the susceptibility or resistance of sugarcane plants to smut (Ustilago scitaminea) . Int Sugar J. , 102 : 445 – 448 .
- McMartin , A . 1945 . Sugarcane smut: Reappearance in Natal . S Afr Sugar J. , 29 : 55 – 57 .
- Moosawi-Jorf , SA and Izadi , MB . 2007 . In vitro detection of yeast-like and mycelial colonies of Ustilago scitaminea in tissue-cultured plantlets of sugarcane using polymerase chain reaction . J Appl Sci. , 7 : 3768 – 3773 .
- Moosawi-Jorf , SA , Teymour-Zadeh , M and Izadi , MB . 2006 . Sexual incompatibility system in Iranian isolates of Ustilago scitaminea, the casual agent of sugarcane smut . Proc 17th Iranian Plant Protect Congr. , 2 : 256
- Müller , KO and Börger , H . 1940 . Experimentelle untersuchungen uber die Phytophthora-resistenz der kartoffel . Arb Biol Reichsasnstalt Landw Forstw Berlin. , 23 : 189 – 231 .
- Pederson , CT , Safir , GR , Siqueira , JO and Parent , S . 1991 . Effect of phenolic compounds on asparagus mycorrhiza . Soil Biol Biochem. , 23 : 491 – 494 .
- Ralph , S , Park , JY , Bohlman , NJ and Mansfield , SD . 2006 . Dirigent proteins in conifer defense: gene discovery, phylogeny, and differential wound-and insect-induced expression of a family of DIR and DIR-like genes in spruce (Picea spp.) . Plant Mol Biol. , 60 : 21 – 40 .
- Ravn , H , Andary , C , Kovacs , G and Molgaard , P . 1989 . Caffeic acid esters as in vitro inhibitors of plant pathogenic bacteria and fungi . Biochem Syst Ecol. , 17 : 175 – 184 .
- Rice , EL . 1995 . Biological control of weeds and plant disease-advances in applied allelopathy , Norman, , USA : University of Oklahoma Press .
- Santiago , R , de Armas , R , Legaz , ME and Vicente , C . 2008a . Separation from Ustilago scitaminea of different elicitors which modify the pattern of phenolic accumulation in sugarcane leaves . J Plant Pathol. , 90 : 87 – 96 .
- Santiago , R , de Armas , R , Fontaniella , B , Vicente , C and Legaz , ME . 2009 . Changes in soluble and cell-bound hydroxycinnamic and hydroxybenzoic acids in sugarcane cultivars inoculated with Sporisorium scitaminea sporidia . Eur J Plant Pathol. , 124 : 439 – 450 .
- Santiago R , Fontaniella B , Millanes AM , Vicente C , Legaz ME . 2008b . Biochemical responses of plants to invaders . Trivandrum: Research Sign Post. Chapter 9, Biochemical responses of sugarcane plants to smut (Ustilago scitaminea) . 125 138 .
- Schenck S. 1999 . Molecular aspects of the sugarcane smut disease pathogen, Ustilago scitaminea . In: Rao GP , Bergamin-Filho A , Magarey RC , Austrey LJC Sugarcane pathology . New York : Science Publishers Inc., USA . 131 139 .
- Singh , N , Somai , BM and Pillay , D . 2004 . Smut disease assesment by PCR and microscopy in inoculated tissue cultured sugarcane cultivars . Plant Sci. , 167 : 987 – 994 .
- Singh , M , Tamma , RV and Nigg , HN . 1989 . HPLC identification of allelopathic compounds from Lantana camara . J Chem Ecol. , 15 : 81 – 87 .
- Suryaprakash , P , Kumar , RP and Prakash , V . 2000 . Thermodynamics of interaction of caffeic acid and quinic acid with multisubunit proteins . Int J Biol Macromol. , 27 : 219 – 228 .
- Thaung , MM . 2005 . Rust, smuts and their allies in Burma . Austral Mycol. , 24 : 29 – 46 .
- Vanky , K . 1999 . The new classificatory system for smut fungi and two new genera . Mycotaxon , 91 : 35 – 49 .
- Waller , JM . 1970 . Sugarcane smut (Ustilago scitaminea) in Kenya: Infection and resistance . Trans British Mycol Soc. , 54 : 405 – 414 .