Abstract
The endophytic flora of Wollemia nobilis was investigated in search for alternative paclitaxel producers. On one hand, metabolic profiling of the obtained specimens using an immunoenzymatic technique was carried out. On the other, we aimed at revealing the genetic background of presumed paclitaxel biosynthesis in the isolates.
We found an indication of endophytic taxane production in the extracts of two strains, Phomopsis sp. and Cladosporium langeronii. A PCR-based screening for taxadiene synthase, a gene unique to the formation of the taxane skeleton, confirmed the molecular blueprint for paclitaxel biosynthesis to be an inherent characteristic of the latter. Although this result makes C. langeronii an interesting candidate for further study, we postulate that proclaiming it ‘a fungus factory for paclitaxel’, as has been done for several other endophytes in the past, might still be premature.
Introduction
Paclitaxel – the first billion dollar blockbuster drug of plant origin (Croteau Citation2005) is a highly functionalized diterpenoid, primarily obtained from the inner bark of Taxus brevifolia (Wani et al. Citation1971). While the search for alternative sources of this compound brought quite an abundance of reports on paclitaxel-producing endophytes in the 1990s (Stierle et al. Citation1993; Strobel et al. Citation1996; Li et al. Citation1998a, Citationb; Noh et al. Citation1999), no conclusive follow-up data concerning fungal metabolite profile or genetic background of the biosynthetic pathway leading to paclitaxel is available, and the world's market still relies on yew-derived supply of the diterpene.
Wollemia nobilis, an ancient conifer thought to have been extinct for millions of years, was discovered only in 1994 in a remote Triassic sandstone gorge of the Wollemi National Park in the Blue Mountains, north-west of Sydney (Hill Citation1996). This relictual pine was found to harbor a paclitaxel-producing endophyte Pestalotiopsis guepini (Strobel et al. Citation1997). Thanks to the unprecedented conservation programme undertaken by the Australian government (NSW Department of Environment and Conservation Citation2006) making Wollemi pine commercially available as a potted plant, the great opportunity of studying this extraordinary conifer worldwide is now at hand.
In the present study we investigate the endophytic flora of Wollemia nobilis in search for new paclitaxel-synthesizing specimens. In parallel to utilizing an immunoenzymatic detection technique to confirm taxane presence in fungal cultures, as well as investigating the presumed cytotoxic activity of the extracts therefrom, we also addressed the question of molecular blueprint for paclitaxel production being an inherent genetic trait of the endophytic isolates. The route leading to paclitaxel in planta requires 19 enzymatic steps (Walker and Croteau Citation2001) and taxadiene synthase (TXS, EC 4.2.3.17) is considered to be a catalyst of the committed one (Wildung and Croteau Citation1996), i.e., the slow bioconversion of the parent olefin (geranylgeranyl pyrophosphate, GGPP) to taxadiene – the tricyclic taxane core. Thus, our PCR-based screen for txs as a target gene not only bears clear implications for paclitaxel biosynthetic pathway elucidation in the endophytes, but might also shed some light on the controversial hypothesis of horizontal gene transfer (Rosewich and Kistler Citation2000; Kurland et al. Citation2003). Namely, if one accepts the notion that the origin for fungal paclitaxel production arose through lateral transfer of genetic information from Taxus spp., and accounts for the wide means of dispersal possessed by fungi, it is conceivable that fungal paclitaxel synthesizers may be found in many plants worldwide, including the relictual Wollemi pine. Furthermore, we hope to gain more insight into the fine-tuned equilibrium of plant-microbe interactions (Staniek et al. Citation2008; Hines and Zahn Citation2009) under evolutionary, environmental, physiological and genetic control.
Materials and methods
Chemicals
Paclitaxel (≥95%, HPLC) reference compound and Potato Dextrose Agar medium were purchased from Sigma-Aldrich (St Louis, MO, USA). Glucose, peptone and Gamborg B5 medium were supplied by Duchefa (Haarlem, The Netherlands). All other chemicals and organic solvents were delivered by Merck (Darmstadt, Germany).
Plant material
Taxus baccata L. was purchased at a local market in Groningen and authenticated at the Botanical Garden ‘De Kruidhof’, Buitenpost, The Netherlands. A voucher specimen (gro-ASOK-01) is deposited in our department. A juvenile (one-year-old, 30 cm in length) Wollemia nobilis W. G. Jones, K. D. Hill & J. M. Allan, bearing the registered Wollemi pineTM logo was purchased in 2008 from Arboretum Kalmthout, Belgium – a representative of Wollemi Australia exclusively licensed by the Royal Botanic Gardens Sydney (RBGS) through NSW National Parks and Wildlife Service (NPWS) to propagate and market the Wollemi pine in Australia and internationally. The conifer was additionally authenticated at the Botanical Garden ‘De Kruidhof’, Buitenpost, The Netherlands. A voucher specimen (gro-ASOK-02) is deposited in our department.
Isolation, cultivation and identification of fungal endophytes
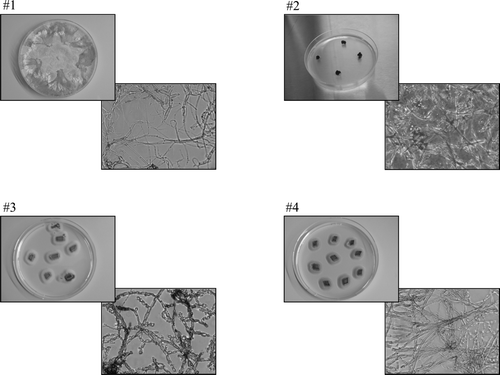
Strains #1, 3 and 4 were isolated from the surface-treated (70% ethanol for 10 min) Wollemi pine needles by hyphal tip transfer method (Strobel et al. Citation1997). Strain #2 was isolated from the surface-treated (70% ethanol for 10 sec, 15% NaOCl:H2O, 1:1 for 3 min, 3×sterile water for 2 min) needles while attempting to generate plant callus cultures on B5 medium (Gamborg et al. Citation1968) containing 0.5 mg/l 2,4-dichlorophenoxyacetic acid under a day/night regime (16/8 h; 2500 lux) (van Uden et al. Citation1990).
All strains were reintroduced onto Potato Dextrose Agar (PDA), and subsequently transferred into Sabouraud-Dextrose broth (peptone 10 g/l, glucose 20 g/l, pH 5.6). A slightly modified S7 medium (Stierle et al. Citation1993) comprising: glucose 1 g/l, fructose 3 g/l, sucrose 6 g/l, peptone 1 g/l, sodium acetate 1 g/l, yeast extract 250 mg/l, thiamine 1 mg/l, biotin 1 mg/l, pyridoxal 1 mg/l, Ca(NO3)2 6.5 mg/l, phenylalanine 5 mg/l, MgSO4 3.6 mg/l, CuSO4 1 mg/l, ZnSO4 2.5 mg/l, MnCl2 5 mg/l, FeCl3 2 mg/l, benzoic acid 100 mg/l, 1M KH2PO4 buffer (pH 6.8) 1 ml/l was the experimental medium. Growth incubation was conducted at room temperature with moderate rotation speed (100 rpm) under a day/night regime (16/8 h; 1200 lux). Subculturing was performed by medium refreshment of a one-week-old fungal suspension in a 1:100 ratio. The volume of experimental cultures was 1 litre.
Strain identification was performed by Centraalbureau voor Schimmelcultures (CBS), Institute of the Royal Netherlands Academy of Arts and Sciences, Utrecht, The Netherlands, according to standard procedures:
#1: Phomopsis sp. (Uecker Citation1988 )
Genus identification was based on the sequence of the ITS (internal transcribed spacer) of the nuclear ribosomal DNA. The gene sequence was obtained by PCR using primers V9G (de Hoog and van den Ende Citation1998)+LS266 (Masclaux et al. Citation1995) and ITS1+ITS4 (White et al. Citation1990). The same primers were used in the sequencing reactions. A contig was formed using the sequenced DNA fragments. The cultures remained sterile and species identification based on DNA sequences was not possible by lack of appropriate reference sequences.
#2: Cladosporium langeronii (Fonseca, Leão & Nogueira) Vuill. (Zalar et al. Citation2007 )
Identification was based on the sequence of the ITS of the nuclear ribosomal DNA. The sequence was determined using the primers V9G and LS266 for both PCR and sequencing reactions.
#3: Acremonium-like anamorph
The anamorph produced in our subcultures was structurally very simple, and similar forms are known to occur in various species of Acremonium, but also in Verticillium and related Hypocrealean fungi. The sequence of the ITS of the nuclear ribosomal DNA was determined using the primers V9G and LS266 for both PCR and sequencing reaction. The (partial) sequence of the β-tubulin was determined using the primers Bt10 (O'Donnell and Cigelnik Citation1997) and BT2b (Glass and Donaldson Citation1995) for both PCR and sequencing reactions. This fungus belongs to the Sordariomycetes, but no sufficiently close matches were found for the ITS rDNA and β-tubulin genes with reference strains to more precisely assess its phylogenetic position.
#4: Lecythophora anamorph of Coniochaeta velutina (Fuckel) Cooke (Weber Citation2002 )
Identification was based on the sequence of the ITS of the nuclear ribosomal DNA. The sequence was determined using the primers ITS1 and ITS4 for both PCR and sequencing reactions. This sequence matched with the ITS sequence of Coniochaeta velutina, however, the cultures only developed the anamorphic state.
Extract preparation
Taxus, the original paclitaxel producer, was employed as a reference organism providing for positive controls of the experimental procedures. Crude and purified extracts of dried Taxus baccata needles were prepared as described previously (Grothaus et al. Citation1995).
After Büchner-filtration, the fungal mycelia were macerated and extracted with CH2Cl2:MeOH (1:1), while the filtrates were extracted with CH2Cl2. As the separation proved difficult in case of specimen #3, the entire culture broth was extracted with CH2Cl2. The solvent was removed from the organic extracts by rotary evaporation under reduced pressure at ~ 40°C (Strobel et al. Citation1994).
Immunodetection
CIEIA – a competitive inhibition enzyme immunoassay system for the quantitative detection of taxanes in biological matrices (Grothaus et al. Citation1993) was performed using a commercially available kit (Hawaii Biotech, Inc., Aeia, Hawaii). The extracts (10 mg dry residue) were dissolved in a CIEIA compatible solvent containing 20% methanol.
DNA isolation and PCR
Efficient extraction of plant and fungal DNA was accomplished using DNeasy® Plant Mini Kit (Qiagen Inc., USA) according to the manufacturer's protocol. The genomic DNA was used as a template for temperature gradient PCR reactions conducted with Phusion TM DNA Polymerase (2U/µl) (Finnzyme OY, Finland), in the Eppendorf Mastercycler gradient thermocycler. Thermal conditions used were: 98°C for 1 min (initial denaturation), 35×[98°C for 10 sec (denaturation), 20 sec (annealing) in temperature gradient, 72°C (extension) for the period of time dependent on the length of the amplified gene fragment and enzyme efficiency], 72°C for 10 min (final extension). Taxadiene synthase specific primers: ctxs_fwd (5′-caaacccatgtcgaattgagaag-3′) and ctxs_rev (5′-caagtttgcatacactctggaatct-3′) (Zhou et al. Citation2007) were purchased from Operon Biotechnologies, Germany.
PCR products were purified with QIAquick® PCR Purification Kit & QIAquick® Gel Extraction Kit (Qiagen Inc., USA). Plasmid DNA purifications were performed using QIAprep® Spin Miniprep Kit (Qiagen Inc., USA).
Molecular cloning and sequence analysis
A-tailed Phusion amplification products were ligated into pGEM®-T & pGEM®-T Easy Vector Systems (Promega Corporation, USA). The obtained plasmid vectors were transformed into XL1-blue competent Escherichia coli strain (Stratagene, USA). Endonuclease digestions of DNA inserts were performed using restriction enzymes from New England BioLabs Inc., USA. Sequence analysis was performed by Macrogen Ltd, Seoul, Korea.
Cytotoxic activity tests
Cytotoxic activity of the extracts was investigated against a human ovarian cancer cell-line OVCAR-3 (ATTC®) by means of MTS assay (CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay, Promega Corporation, USA).
The cancer cells were routinely cultured in DMEM (Invitrogen®), supplemented with 10% of heat inactivated fetal bovine serum (Invitrogen®) and suitable antibiotics: 1% penicillin/streptomycin (Invitrogen®), at 37°C in the presence of 5% CO2. The addition of paclitaxel reference, plant and fungal extracts, dissolved in 1% DMSO sterilized by filtration (0.2 µm) and diluted with cell culture medium, in consecutive 2-fold dilutions was preceded by seeding the test cells into 96-well plates (5000 cells/well) and 24 h incubation. Cell viability was assessed 72 h later by means of the MTS assay, following the instructions of the manufacturer. All assays were performed in triplicate.
Microscopy
Microscopic observations and imaging were performed by means of the Olympus CKX41 inverted microscope coupled with the Altra 20 Soft Imaging System.
Results and discussion
Four distinct isolates of endophytic fungi were recovered from the sampled Wollemia nobilis needles ().
Figure 1. Macro- and micromorphological (Olympus CKX41SV, ×400) characteristics of endophytic isolates.
Each fungus was grown in the experimental medium, extracted as described, and assayed for paclitaxel. As current chromatographic methods for the determination of taxanes in biological matrices are considered to be relatively insensitive, an immunoenzymatic system (CIEIA) was employed in order to gain insight into the presence of taxane compounds in the endophytic extracts. The reactivity of this assay towards paclitaxel was 0.5 ng/ml. The average taxane concentration values in crude and purified extracts of Taxus baccata needles, providing for a positive control of taxoid presence verification, amounted to 270 µg/ml and 20 µg/ml, respectively, which constitutes 0.05% of dry weight and corresponds to taxane concentrations in Taxus needles as previously reported (Grothaus et al. Citation1995). Of the fungi isolated, specimens #1 and #2 were the most interesting. Organic extracts of their culture medium filtrates provided evidence of paclitaxel at a concentration of 10.1 (±2.7) ng/ml and 19.4 (±0.4) ng/ml, respectively, and of 13.4 (±1.0) ng/ml in the mycelial extract for the isolate #2 alone.
The presumed cytotoxic effect of the fungal extracts was further investigated. To ensure for the positive control of proliferation arrest, the ovarian cancer cells were treated with paclitaxel, as well as with the purified extract of Taxus baccata needles. The IC50of paclitaxel in the cell line tested was approximately 50 nM as found previously (Kolfschoten et al. Citation2002) and corresponded to the amount of the drug in the reference plant material. We found a clear cytotoxic effect of the fungal extracts (), but it is uncertain whether these effects are to be ascribed to paclitaxel due to poor solubility and minute amounts of the drug detected.
Table 1. Cytotoxic effect of endophytic extracts.
To further address the question of paclitaxel biosynthesis being an inherent characteristic of the fungal isolates, our research continued at a genomic level, with polymerase chain reaction being the basic tool of investigation. Thus, the genomic DNA of all fungal specimens served as a template in hope for efficient annealing with the specific starters proposed by Zhou et al. (2007). While the PCR-based screen yielded a number of potentially interesting amplification products, the genetic capacity to synthesize the tricyclic taxane core was ultimately confirmed only for the isolate #2 (). High similarity of the amplicon obtained with its plant counterparts (96% sequence identity within the conserved region) () gives unequivocal evidence for the presence of taxadiene synthase in the fungal genome and allows us to ascribe the biosynthesis of paclitaxel ab initio to be a genuine feature of C. langeronii. This makes the isolate an interesting candidate for further in-depth study involving the confirmation of txs enzyme activity followed by fermentation up-scaling and optimization as well as genetic engineering.
Figure 2. PCR analysis of the presence of taxadiene synthase (~ 1.0 kb) in Cladosporium langeronii. Taxus baccata needles were a source of plant DNA samples, providing for a positive control of txs presence verification (M – GeneRulerTM 1 kb DNA Ladder, Fermentas; G – annealing temperature gradient: 45–65°C).
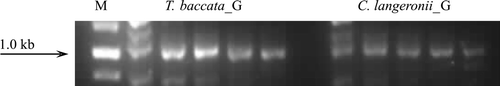
Figure 3. Taxadiene synthase sequence alignment (http://blast.ncbi.nlm.nih.gov). Query: T. baccata txs gene, accession no. AJ320538, Sbjct: C. langeronii txs sequence obtained in course of research.

While our study failed to re-establish the previously reported endophytic paclitaxel producer, Pestalotiopsis guepini harbored by Wollemia nobilis in its natural habitat (Strobel et al. Citation1997), it recovered interesting isolates bearing indications of presumed taxane biosynthesis, be it on a metabolic (Phomopsis sp. & Cladosporium langeronii) or on a genomic level (C. langeronii). It also allows us to speculate on the origins of fungal paclitaxel and seems to emphasize the impact of environmental factors on its prevalence. On one hand, fungi may be an independently evolved system for paclitaxel production. However, the fact that the biosynthesis of this highly functionalized and unique diterpenoid requires approximately 20 genes (Walker and Croteau Citation2001) makes this rather unlikely. Therefore, it seems plausible that a horizontal transfer of genetic information shaped the evolutionary trajectory of taxonomically unrelated, yet co-existing, species and further influenced the dispersal of endophytic taxane synthesizers in a given ecosystem. Recent reports on paclitaxel-producing fungi harbored not only by Taxus, but also by other species (Kumaran et al. Citation2009; Kumaran and Hur Citation2009), seem to be in accord with that notion thus suggesting that alternative taxane synthesizers may be found in many plants worldwide, including a Wollemia nobilis specimen cultivated ex situ.
Regardless of the aforementioned fundamental speculations, practical aspects should be decisively invoked yet again. As we discussed elsewhere (Staniek et al. Citation2009), a specific plant environment might be required for the sustainable synthesis of the drug. Indeed, while a recent publication (Kumaran and Hur Citation2009) reports three strains of Phomopsis isolated from three different gymnosperms to be paclitaxel producers, the highest yields are ascribed to the one harbored by Taxus cuspidata – the original yew synthesizer of the valuable antineoplastic agent. It is also noteworthy that the overwhelming majority of reports dealing with taxane-producing endophytes describe the fungal symbionts shortly after removal from their plant hosts, not taking full account of the possible impact and nature of the interdependencies between the naturally co-existing species (Staniek et al. Citation2008; Hines and Zahn Citation2009). But one, very recent paper communicates an endeavour to re-establish the co-habitat by proposing a promising co-culture system for Taxus chinensis var. mairei and its endophyte Fusarium mairei (Li et al. Citation2009).
As the highly desirable search for reliable and economically feasible paclitaxel sources seems to have revived the interest in endophytes as potential taxane bio-factories, we still contend that such reports should be viewed as extraordinary claims that demand ultimate justification, namely: Unambiguous evidence for molecular blueprint underlying the postulated microbial paclitaxel biosynthesis, suggesting a means for its induction and manipulation, as well as taking heed of the interactions between plant hosts and their fungal inhabitants.
Acknowledgments
Thanks are due to Jan-Willem Zwart, Botanical Garden ‘De Kruidhof’, Buitenpost, The Netherlands, for providing the plant material, as well as the Identification Team of CBS, Utrecht, The Netherlands, for identifying the endophytic strains.
References
- Croteau , R . 2005 . Taxol biosynthesis and molecular genetics , The Netherlands : Terpnet, Wageningen .
- de Hoog , GS and van den Ende , A . 1998 . Molecular diagnostics of clinical strains of filamentous Basidomycetes . Mycoses. , 41 : 183 – 189 .
- Gamborg , OL , Miller , RA and Ojima , V . 1968 . Nutrient requirements of suspension cultures of soybean root cells . Exp Cell Res. , 50 : 151 – 158 .
- Glass , NL and Donaldson , GC . 1995 . Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes . Appl Environ Microbiol. , 61 : 1323 – 1330 .
- Grothaus , PG , Bignami , GS , O'Malley , S , Harada , KE , Byrnes , JB , Waller , DF , Raybould , TJ , McGuire , MT and Alvarado , B . 1995 . Taxane-specific monoclonal antibodies: Measurement of taxol, baccatin III, and ‘total taxanes’ in Taxus brevifolia extracts by enzyme immunoassay . J Nat Prod. , 58 : 1003 – 1014 .
- Grothaus , PG , Raybould , TJ , Bignami , GS , Lazo , CB and Byrnes , JB . 1993 . An enzyme immunoassay for the determination of taxol and taxanes in Taxus sp. tissues and human plasma . J Immunol Meth. , 158 : 5 – 15 .
- Hill , KD . 1996 . The Wollemi pine: Discovering a living fossil . Nat Res. , 32 : 20 – 25 .
- Hines , PJ and Zahn , LM . 2009 . What's bugging plants? Introduction to special issue . Science. , 324 : 741
- Kolfschoten , GM , Hulscher , TM , Duynam , MC , Pinedo , HM and Boven , E . 2002 . Variation in the kinetics of caspase-3 activation, Bcl-2 phosphorylation and apoptotic morphology in unselected human ovarian cancer cell lines as a response to docetaxel . Biochem Pharmacol. , 63 : 733 – 743 .
- Kumaran , RS and Hur , B-K . 2009 . Screening of species of the endophytic fungus Phomopsis for the production of the anticancer drug taxol . Biotechnol Appl Biochem. , 54 : 21 – 30 .
- Kumaran , RS , Muthumary , J and Hur , B-K . 2009 . Isolation and identification of an anticancer drug, taxol from Phyllosticta tabernaemontanae, a leaf spot fungus of an angiosperm, Wrightia tinctoria . J Microbiol. , 47 : 40 – 49 .
- Kurland , CG , Canback , B and Berg , OG . 2003 . Horizontal gene transfer: A critical view . PNAS , 100 : 9658 – 9662 .
- Li , JY , Sidhu , RS , Bollon , A and Strobel , GA . 1998a . Stimulation of taxol production in liquid cultures of Pestalotiopsis microspora . Mycol Res. , 102 : 461 – 464 .
- Li , JY , Sidhu , RS , Ford , EJ , Long , DM , Hess , WM and Strobel , GA . 1998b . The induction of taxol production in the endophytic fungus Periconia sp. from Torreya grandifolia . J Ind Microbiol Biotech. , 20 : 259 – 264 .
- Li , Y-C , Tao , W-Y and Cheng , L . 2009 . Paclitaxel production using co-culture of Taxus suspension cells and paclitaxel-producing endophytic fungi in a co-bioreactor . Appl Microbiol Biotechnol. , 83 : 233 – 239 .
- Masclaux , F , Gueho , E , de Hoog , GS and Christen , R . 1995 . Phylogenetic relationship of human-pathogenic Cladosporium (Xylohypha) species inferred from partial LS rRNA sequences . J Med Vet Mycol. , 33 : 327 – 338 .
- Noh , M , Yang , J , Kim , K , Yoon , Y , Kang , K , Han , H , Him , S and Park , H . 1999 . Isolation of a novel microorganism Pestalotia heterocornis, producing paclitaxel . Biotech Bioeng. , 64 : 620 – 623 .
- NSW Department of Environment and Conservation . 2006 . Wollemi Pine (Wollemia nobilis) Recovery Plan . NSW Department of Environment and Conservation, Hurstville NSW. DEC 2006/519, ISBN 1 920 887 63 6
- O'Donnell , K and Cigelnik , E . 1997 . Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous . Mol Phylogenet Evol. , 7 : 103 – 116 .
- Rosewich , UL and Kistler , HC . 2000 . Role of horizontal gene transfer in the evolution of fungi . Annu Rev Phytopathol. , 38 : 325 – 363 .
- Staniek , A , Woerdenbag , HJ and Kayser , O . 2008 . Endophytes: Exploiting biodiversity for the improvement of natural product-based drug discovery . J Plant Interact. , 3 : 75 – 93 .
- Staniek , A , Woerdenbag , HJ and Kayser , O . 2009 . Taxomyces andreanae: A presumed paclitaxel producer demystified? . Planta Med. , 75 : 1561 – 1566 .
- Stierle , A , Strobel , GA and Stierle , DB . 1993 . Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew . Science. , 260 : 214 – 216 .
- Strobel , GA , Hess , WM , Ford , E , Sidhu , RS and Yang , X . 1996 . Taxol from fungal endophytes and the issue of biodiversity . J Ind Microbiol. , 17 : 417 – 423 .
- Strobel , GA , Hess , WM , Li , JY , Ford , E , Sears , J , Sidhu , RS and Summerell , B . 1997 . Pestalotiopsis guepinii, a taxol-producing endophyte of the Wollemi pine, Wollemia nobilis . Austr J Bot. , 45 : 1073 – 1082 .
- Strobel GA , Stierle A , Stierle DB . 1994 . Taxol production by Taxomyces andreanae . US Patent 5322779 .
- Uecker , FA . 1988 . A world list of Phomopsis names with notes on nomenclature, morphology, and biology . Mycol Mem. , 13 : 1 – 323 .
- van Uden , W , Pras , N and Maingré , TM . 1990 . The accumulation of podophyllotoxin-β-D-glucoside by cell suspension cultures derived from the conifer Callitris drummondii . Plant Cell Rep. , 9 : 257 – 260 .
- Walker , K and Croteau , R . 2001 . Taxol biosynthetic genes . Phytochemistry. , 58 : 1 – 7 .
- Wani , MC , Taylor , HL , Wall , ME , Coggon , P and McPhail , AT . 1971 . Plant antitumor agents IV. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia . J Am Chem Soc. , 93 : 2325 – 2327 .
- Weber , E . 2002 . The Lecythophora-Coniochaeta complex I. Morphological studies on Lecythophora species isolated from Picea abies . Nova Hedvigia. , 74 : 159 – 185 .
- White TJ , Burns T , Lee S , Taylor J. 1990 . Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics . In: Innis MA , Gelfand GH , Sninsky JJ , White TJ PCR protocols: A guide to methods and applications . San Diego, CA : Academic Press . 315 322 .
- Wildung , MR and Croteau , R . 1996 . A cDNA clone for taxadiene synthase, the diterpene cyclase that catalyzes the committed step of taxol biosynthesis . J Biol Chem. , 271 : 9201 – 9204 .
- Zalar , P , de Hoog , GS , Schroers , H-J , Crous , PW , Groenewald , JZ and Gunde-Cimerman , N . 2007 . Phylogeny and ecology of the ubiquitous saprobe Cladosporium sphaerospermum, with descriptions of seven new species from hypersaline environments . Stud Mycol. , 58 : 157 – 183 .
- Zhou , X , Wang , Z , Jiang , K , Wei , Y , Lin , J , Sun , X and Tang , K . 2007 . Screening of taxol-producing endophytic fungi from Taxus chinensis var. mairei . Prikl Biokhim Mikrobiol. , 43 : 490 – 494 .