Abstract
Gluconacetobacter diazotrophicus secretes to the culture medium a lysozyme, active against Xanthomonas albilineans. High molecular mass glycoproteins, obtained from sugarcane stalks, initially inhibit the secretion of the enzyme from bacteria to the culture medium, but it is actively secreted later. Alternatively, the production of these plant glycoproteins decreases when G. diazotrophicus is experimentally inoculated into sugarcane stalk segments. Xanthan production is achieved by the pathogen X. albilineans infecting sugarcane stalks but it was nullified by G. diazotrophicus when the endosymbiont is growing in plant tissues together the pathogen.
Introduction
Leaf scald is a major sugarcane disease (Ricaud and Ryan Citation1989) occurring mostly in sugarcane-producing countries and continues spreading. Xanthomonas albilineans is the leaf scald pathogen. It is a systemic, xylem invading, Gram negative gamma Proteobacteria, motile by a single polar flagellum. The pathogen is transmitted by either cuts and bruises caused by infection or stalk cutting implements. There is also evidence for soil, water and aerial transmission (Saumtally et al. Citation1996). The initial characteristic symptom of disease is a whiteish-yellow streak (‘pencil-line’) 1–2 mm wide on the leaf along the main veins. The streaks may later become more enlarged and the affected leaf becomes wilted and necrotic. A common symptom in mature diseased cane is the abnormal formation of side shoots on stalks, and basal side shoots tend to develop more rapidly than those higher up, whereas the opposite occurs in healthy stalks (Rott et al. Citation1997). One of the troublesome aspects of this disease is that many sugarcane cultivars tolerate the pathogen without exhibiting symptoms or symptoms escape detection (Hayward Citation1974; Purseglove Citation1979; Ricaud and Ryan Citation1989).
Vascular bundles of diseased plants exudate a yellowish gum when cut (Purseglove Citation1979). Solas et al. (Citation2003) found that whiteish-yellow streaks develop large bulliform cells and the gum exudate occludes phloem and bundle vessels, and partially entered mesophyll cells. The gum has been identified as a xanthan-like polysaccharide, produced by the pathogen and composed of glucose, mannose and glucuronic acid (Fontaniella et al. Citation2002).
On the other hand, Gluconacetobacter diazotrophicus is a sugarcane, N2-fixing endophyte. It is a Gram negative, aerobic rod bacterium motile within sugarcane by a long flagellum. This diazotroph was found by Cavalcante and Döbereiner (Citation1988), using a semi-solid medium containing high concentrations of sucrose, in roots, stems and leaves of sugarcane. The mechanism through which bacteria enter sugarcane seedlings has not yet been conclusively established. James et al. (Citation1994) suggested that G. diazotrophicus colonizes the root surface only after bacterial consumption of sucrose in the habitat. Then, bacteria would enter the root apoplast (Dong et al. Citation1994) through lateral root junctions and the loose cells of the root cap. This bacterium colonizes the interior of root epidermal cells and the root intercellular spaces (James et al. Citation1994). Moreover, James et al. (Citation1994) propose that G. diazotrophicus could be distributed from the base of the stem to other organs via the stem xylem vessels, since they also detect xylem colonization in the basal region of the stalk.
Since both microorganisms, X. albilineans and G. diazotrophicus can simultaneously invade a sugarcane plant, interactions between bacteria could be achieved. In fact, G. diazotrophicus, isolated from sugarcane stalks, purified and cultured in liquid medium, produces a lysozyme-like bacteriocin that inhibits the growth of X. albilineans (Piñón et al. Citation2002). Also, this sugarcane endophyte infecting sugarcane tissues immobilized in calcium alginate is able to secrete the bacteriocin into the medium that inhibits the growth of X. albilineans (Blanco et al. Citation2005a).
The aim of this work is to study the production of this lysozyme-like bacteriocin by G. diazotrophicus in sugarcane stalks to establish a possible mechanism of biological control of scald leaf.
Material and methods
Bacterial strains and growth conditions
Xanthomonas albilineans, strain NCPPB 887, was cultured on liquid Willbrink medium (Dye Citation1980), the composition of which, per liter, was: Peptone, 5.0 g; sucrose, 20 g; KH2PO4, 0.5 g; MgSO4. 7 H2O, 0.25 g; agar, 15 g, pH, 7.0, or, alternatively, on the same bacterial medium supplied with 2% (w/v) glucose-1-P, at 37°C.
Gluconacetobacter diazotrophicus, strain 166 (CM-INICA) isolated from sugarcane (Blanco et al. Citation2005b), was maintained in an N-poor solid medium (Cavalcante and Döbereiner Citation1988), which contained 10% (w/v) sucrose, pH 5.5, and in the same liquid medium supplied with 1.0 ml of sterile glycoproteins purified from sugarcane juice. Cultures were maintained for 16 days at 30°C. The size of the inoculums added in the experiments was previously standardized as 0.2 units of optical density (approximately 20 mg dry weight). The growth rate of the bacteria was measured by Nephelometry at 710 nm, cell dry weight of several culture aliquots versus their absorbance at 710 nm was measured. To know the dry weight of bacteria, a straight line of cell dry weight of several culture aliquots versus their absorbance at 710 nm, previously measured, was constructed by using different known weights of bacteria.
Micrococcus luteus, strain CECT241 was selected for the standard assay of lysozyme and it is maintained in the salt agar medium (Magaña and Ruiz Citation1967; MacKay et al. Citation1984).
Plant material and preparation of glycoproteins from juice sugarcane
Stalks from 7-month-old plants of S. officinarum cv. Mayarí 55-14, grown in field conditions at the Real Jardín Botánico Alfonso XIII (Complutense University, Madrid), were cut, mechanically crushed, and the crude juice was centrifuged at 5000 g for 15 min at 4°C. The pellet was discarded and the supernatant was filtered through filter paper. This centrifuged juice was then filtered through a Sephadex G-10 column (15×2.5 cm) embedded in 10 mM sodium phosphate buffer, pH 6.8. Elution was also carried out with the same buffer. Fractions (1.0 ml) 1–17 were discarded. Fractions 18–35, containing mid-molecular mass glycoproteins (MMMG) and high-molecular mass glycoproteins (HMMG), were filtered through a Sephadex G-50 column (30×?2.5 cm) pre-equilibrated as above. Fractions 40–60 contained HMMG whereas MMMG eluted in fractions 66–100 (Legaz et al. Citation1998). Eluates were monitored for carbohydrates according to Dubois et al. (Citation1956) and for protein according to Lowry et al. (Citation1951).
Lysozyme production in G. diazotrophicus liquid medium with sugarcane juice glycoproteins
Sugarcane juice glycoproteins were sterilized by filtration through Millipore filters, 0.25 µm pore diameter. Sterile MMMG and HMMG solutions (1.0 ml per flask) were added to two different sets of erlenmeyer flasks containing 100 ml of liquid Potato-P medium. The composition of this medium was: peeled potato, 200 g; sucrose, 100 g; biotin, 1.0 mg, Na2MoO4·2H2O, 20 mg; MnSO4, 23.5 mg; boric acid, 28 mg; ZnSO4, .4 mg (Cavalcante and Döbereiner Citation1988).
A new set without sugarcane glycoproteins was used as a control. Flasks were inoculated with 20 mg dry weight of G. diazotrophicus and cultured at 30°C. Everyday, six flasks (two without added glycoproteins, two containing MMMG and two containing HMMG) were centrifuged at 12,000 g for 15 min at 2°C to remove stimated in supernatants according to Lowry et al. (Citation1951). The specific activity of the secreted bacteriocin against X. albilineans and M. luteus was estimated measuring changes of absorbance at 710 nm.
Sugarcane stalks infection with G. diazotrophicus and X. albilineans and protein and carbohydrate estimation in juice
S. officinarum cv. Mayarí 55-14 stalks from 7-month-old plants were obliquely cut in segments of about 8.0 cm in length and superficially sterilized with 50% (v/v) bleach 7 for 5 min. Later, they were washed with abundant sterilized, distilled water. Ten of these segments were inoculated with 20 mg dry weight of G. diazotrophicus, 10 with 20 mg dry weight of X. albilineans, 10 with the same weight of both microorganisms and five without inoculation (control). All of them were maintained at room temperature for 11 days. Following five days after the first inoculation, five segments previously inoculated with G. diazotrophicus were inoculated with 20 mg dry weight of X. albilineans again and segments previously inoculated with X. albilineans were then inoculated with G. diazotrophicus. After 11 days, segments were washed with distilled water and mechanically crushed. Juices were centrifuged at 10,000 g for 15 min at 2°C. Supernatants were filtered through filter paper and used to separate sugarcane polysaccharides and glycoproteins and to extract bacterial xanthans.
Extraction of xanthans
Extraction of xanthan was carried out by using juices obtained from 7-month-old stalks of cv. Mayarí 55-14 without inoculation, inoculated with G. diazotrophicus and X. albilineans or either G. diazotrophicus or X. albilineans. Separation of xanthan was carried out according to the method described by Galindo and Albiter (Citation1996) with some modifications. Juices from differently-treated stalks were centrifuged at 10,000 ?g for 15 min at 2°C and supernatants (47 ml) were precipitated with continuous shaking by adding 47 ml of iso-propyl alcohol containing 3% (w/v) of KCl. Mixtures were maintained at 4°C for 2 h before centrifugation at the same conditions as above. Supernatants were discarded and pellets containing precipitated xanthans were dried in vacuum.
High viscosity dry residues were washed with 10 ml of iso-propyl alcohol, centrifuged at 10,000 g for 15 min at 2°C and pellets were dried in vacuum. Dry residues were weighted and 0.2 g of dry weight from each dry residue were redissolved in 50 ml of 10 mM sodium phosphate buffer pH 6.8. Residues, redissolved in buffer, were heated at 30°C for 10 min, shaking and filtered through filter paper. Each sample (5.0 ml) was filtered through Sephadex G-10 column embedded in 10 mM sodium phosphate buffer at pH 6.8. Fractions (1.0 ml) 1–27 were discarded. Fractions 27–47 were mixed. Then, aliquots of 6.0 ml of each mixture were filtered through a Sephadex G-50 column, embedded in the same buffer. Fractions 50–77 contained HMMC whereas MMMC eluted in fractions 77–131. Eluted fractions from columns were monitored for carbohydrates according to Dubois et al. (Citation1956).
Acidic hydrolysis and sugar extraction
Fractions obtained from Sephadex G-50 filtration, containing carbohydrates, were hydrolyzed with 6 N HCl at 80°C overnight and were dried under reduced pressure. Each residue was ground with 3 ml of cold 80% (v/v) ethanol, stored for 2 h at 2°C and then, centrifuged at 10,000 g for 15 min at 2°C. After evaporation to dryness, xanthan precipitates were dissolved in 200 µl 10 mM sodium borate buffer, pH 9.2, and used for capillary electrophoresis (CE) analysis.
Capillary electrophoresis
Capillary electrophoresis was performed using a Spectraphoresis 500 system from Spectra-Physics (Fremont, CA, USA). Microbore fused-silica tubing coated with polyimide (Scientific Glass Engineering, Milton Keynes, UK) of 75 µm inner diameter (i.d.) and 190 µm outer diameter (o.d.) with a total length of 70 cm and a separation length of 63 cm were used. The capillary was enclosed in a cassette for easy handling. On-line detection was performed with a variable-wavelength UV-VIS detector of 6 nm band width (Spectra-Physics, Fremont). Detection of saccharides was monitored at 200 nm and electrophoregrams were recorded using a SP 4290 integrator (Spectra-Physics, Fremont).
New capillaries were conditioned according to Legaz and Pedrosa (Citation1993). Saccharide standards used were D-glucose, D-mannose, D-glucuronic acid, D-glucose-1-P, galactitol and D-fructose (all from Sigma Chemical Co., St Louis, MO, USA). Standard saccharides as well as sample solutions were prepared in 10 mM sodium borate buffer, pH 9.2. Voltage was applied so that ions migrated from the anode to the cathode.
Results and discussion
Growth rate of G. diazotrophicus and X. albilineans
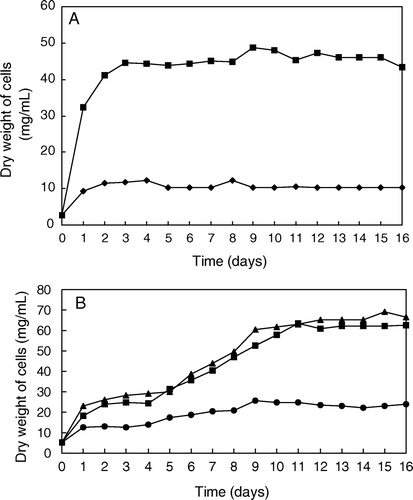
Growth rate of both bacteria was measured by nephelometry following absorbance changes at 710 nm. To know the increase in the dry weight of bacteria, a straight line of cell dry weight versus absorbance at 710 nm was constructed by using different known weights of bacteria. As shown in A, the growth of X. albilineans was enhanced when the medium containing sucrose was supplied with glucose-1-P. The stationary phase of growth was reached after three days of culture at 37°C in both media. The growth rate of G. diazotrophicus was higher than that of X. albilineans after 16 days of culture. However, G. diazotrophicus grew slower (B), since it reached the stationary phase of growth at the ninth day in liquid culture with added MMMG and at the eleventh day on added HMMG, whereas the bacteria in the potato-P liquid medium did not reached the stationary phase after 16 days of culture.
Preparation of sugarcane juice glycoproteins
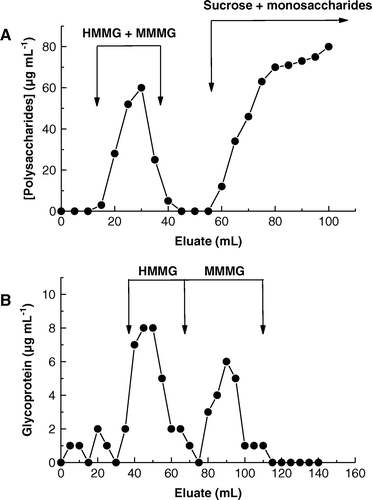
Elution profile of sugarcane juice through a Sephadex G-10 column is shown in A. Two main peaks of carbohydrates were obtained, the first peak being a mixture of MMMG and HMMG (8 ml, 0.24 mg carbohydrate) which were recovered in fractions 18–35, while the second peak was composed of sucrose, the main component of sugarcane juice, and several monosaccharides. Fractions 18–35 were mixed and filtered through a Sephadex G-50 column. Elution profile through Sephadex G-50 column (B) was bimaximal, recovering 0.12 mg of HMMG (fractions 40–60) and 0.11 mg of MMMG (fractions 66–100).
Lysozyme secretion from G. diazotrophicus
Protein secretion from G. diazotrophicus cells was observed to reach its maximum value on the third day of culturing, when the protein concentration reached its maximum value. During the subsequent days, the rate of protein secretion was constant (A). This effect could be observed for the different treatments described in Methods. One of these proteins developed lysozyme activity against X. albilineans (Piñón et al. Citation2002). Another species from the genus Xanthomonas, X. campestris, is sensitive against cell-lytic proteins secreted by soil Pseudomonas which also produce and secrete proteases (Shastry and Prasad Citation2002). Proteases are also secreted by Erwinia amylovora (Zhang et al. Citation1999), a common epiphytic bacterium on sugarcane leaves, although it has never been described inside stalks or leaves.
Figure 3. Time-course of (A) protein secretion from G. diazotrophicus cells to potato-P liquid culture media, and secretion of lysozyme active against (B) X. albilineans and (C) M. luteus. (▴) Liquid medium without additions (control), (▪) liquid medium with HMMG and (•) liquid medium with MMMG. Values are the mean of three replicates. Vertical bars give standard error where larger than the symbols.
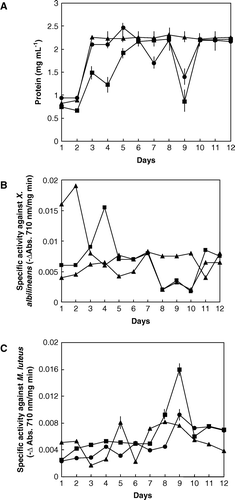
In glycoprotein addition experiments, the secretion of lysozyme from G. diazotrophicus initially decreased but the further addition of HMMG to the culture media increased lysozyme production and secretion. The maximum activity against X. albilineans was reached at the fourth day of culture (B) and at the ninth day of culture when assayed against M. luteus (C), although the growth of G. diazotrophicus was similar in the potato-P medium as well as in the same medium supplied with HMMG. Similar results were obtained by Stepnaya et al. (Citation2001) for the action acidic exopolysaccharides of X. campestris on its own secreted enzymes, such as muramidase, endopeptidase, and neutral phosphatase, as well as egg lysozyme, increasing or decreasing enzyme activity as a function of the time culture or substrates used.
Protein and carbohydrates in juices from stalks inoculated with X. albilineans and G. diazotrophicus
Protein and carbohydrate concentration of fractions eluted from a Sephadex G-50 column loaded with sugarcane juice extracted from the cv. Mayarí 55-14, 7-month-old stalks previously inoculated with G. diazotrophicus or X. albilineans individually and sequentially or, alternatively, at the same time with both microorganisms is shown in . Fractions 50–77 contained high molecular weight, sugarcane carbohydrates (HMMC) whereas medium molecular weight carbohydrates (MMMC) eluted in fractions 77–131.
Figure 4. Elution profiles, by filtration through a Sephadex G50 column, of protein (•) and polysaccharides (▪) from sugarcane juices obtained from (A) non-inoculated stalk segments, (B) inoculated with G. diazotrophicus, (C) inoculated with X. albilineans, (D) firstly with the endosymbiont and later with the pathogen, (E) firstly with the pathogen and later with the endosymbiont, and (F) inoculated simultaneously with both microorganisms.
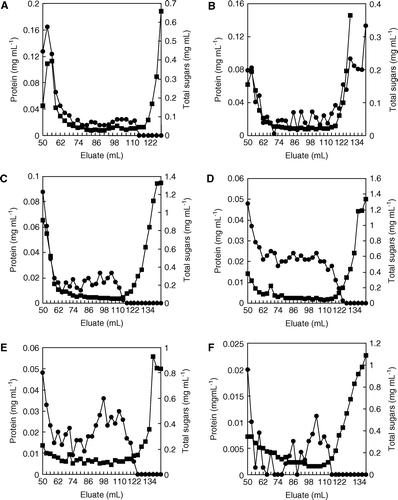
HMMC concentration increased in juices obtained from stalks inoculated with X. albilineans (C), as previously reported by Fontaniella et al. (Citation2003) for diseased plants cultured in field conditions, whereas HMMC concentration from stalks inoculated with G. diazotrophicus (B) decreased by comparing it with those obtained from non-inoculated stalks (A). Since G. diazotrophicus is an endophyte, it may use the plant sugars as their carbon source; however, the possibility of X. albilineans using the plant sugars as carbon source cannot be ruled out; additionally, this bacterium released high amounts of putative bacterial polysaccharides outside the cells (C).
When the inoculation of stalk segments was achieved using both microorganisms at the same time, polysaccharide concentration was lower (F) than that obtained when only X. albilineans infected stalks, but higher than that obtained from stalks inoculated only with G. diazotrophicus. Protein and polysaccharide concentrations were very similar for stalks inoculated with both microorganisms at different times (D, E).
Xanthan extraction
The concentration of total polysaccharides precipitated with iso-propanol and eluted from Sephadex G-50 column is shown in . Fractions 50–77 were identified as xanthan-like polysaccharides (Solas et al. Citation2003). Production of xanthans by X. albilineans infecting sugarcane (C) was supported by the fact that the polysaccharide concentration in juices from infected plants was higher than that found in juices from non-inoculated (A) and G. diazotrophicus inoculated stalks (B). This would indicate that X. albilineans was able to release xanthans when bacteria developed inside sugarcane tissues. However, no polysaccharide release was found when bacteria were cultured in Willbrink liquid medium or Willbrink medium supplied with 2% glucose-1-P (Blanch et al. Citation2008). X. albilineans differed from X. campestris at this point, since the later bacterial species is able to produce and secrete xanthans in liquid media enriched with sucrose, glucose or, even, some plant bagasses, such as that obtained from cassava plants (Woiciechowski et al. Citation2004).
Figure 5. Elution profiles, by filtration through a Sephadex G50 column, of iso-propanol precipitated polysaccharides from sugarcane juices obtained from (A) non-inoculated stalk segments, (B) inoculated with G. diazotrophicus, (C) inoculated with X. albilineans, (D) firstly with the endosymbiont and later with the pathogen, (E) firstly with the pathogen and later with the endosymbiont, and (F) inoculated simultaneously with both microorganisms.
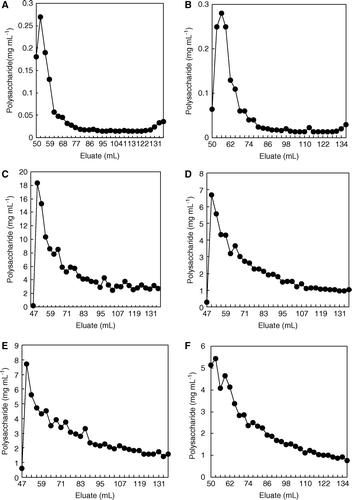
The amount of xanthan extracted from stalks inoculated with both microorganisms was lower than that found from stalks inoculated only with the pathogen. Indeed, the concentration of total polysaccharides precipitated with iso-propyl alcohol was similar when stalks were inoculated with both microorganisms and independent on the sequence of sugarcane stalks inoculation (D–F).
Acidic hydrolysis of polysaccharides and capillary electrophoresis
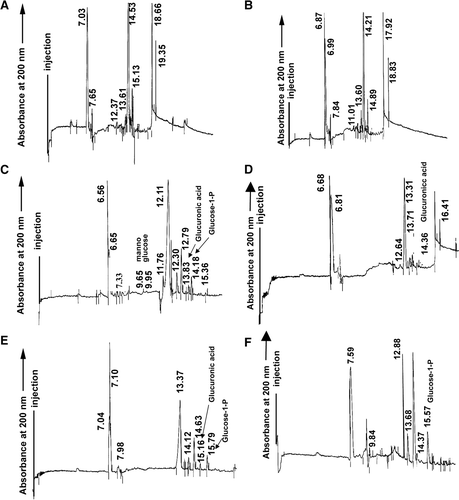
Eluates presumably containing xanthans from the different treatments specified in , this is, from 50–59 ml (A), from 53–62 ml (B), from 50–56 ml (C, D and F), and 50–56 ml, and those from 65–68 ml (E), were mixed and hydrolyzed with 6 N HCl at 80°C for 12 h. All hydrolysates were analyzed by capillary electrophoresis in order to detect glucuronic acid as a proof of their xanthan nature.
As it is shown in C (eluates from juices of stalk segments only inoculated with X. albilineans), a main peak at 6.65 min was identified as sucrose. Cellobiose (7.33 min), mannose (9.65 min), glucose (11.76 min), glucuronic acid (13.83 min), all of them structural units of xanthan, and glucose-1-P (14.18 min), used by the bacterium as a precursor during xanthan production, were also revealed. Galactitol, used as internal standard, migrated at 12.11 min. However, mannose was not found in hydrolyzates of polysaccharides from stalk segments inoculated with X. albilineans before, simultaneously or after to the inoculation with G. diazotrophicus (E, F and D). Also, glucose-1-P and glucuronic acid did not occur in hydrolyzates of polysaccharides from stalk segments inoculated with G. diazotrophicus before or simultaneously to the inoculation with X. albilineans (D) and in stalk segments inoculated with both microorganisms at the same time (F), respectively. These results could indicate that G. diazotrophicus impeded the production of xanthans by the pathogen invading sugarcane tissues, probably through the production of a bacteriocin capable to inhibit the growth of X. albilineans.
Figure 6. Electropherograms of acidic-hydrolyzed xanthans from sugarcane juices obtained from (A) non-inoculated stalk segments, (B) inoculated with G. diazotrophicus, (C) inoculated with X. albilineans, (D) firstly with the endosymbiont and later with the pathogen, (E) firstly with the pathogen and later with the endosymbiont, and (F) inoculated simultaneously with both microorganisms. Numbers near the peaks represent the absolute migration time value. Migration time of standards were sucrose (6.65 min), cellobiose (7.33 min), mannose (9.65 min), glucose (11.76 min), glucuronic acid (13.83 min), glucose-1-P (14.18 min), and galactitol (12.11 min), used as internal standard.
Acknowledgements
This paper has been supported by a Grant from the Dirección General de Investigación Científica y Tecnológica (Ministerio de Ciencia y Tecnología, Spain) BFI2003-06234. Y. Blanco is supported by a FPI grant, AP2001-1415, from the Ministerio de Educación y Ciencia (Spain).
References
- Blanch , M , Legaz , ME and Vicente , C . 2008 . Xanthan production by Xanthomonas albilineans infecting sugarcane stalks . J Plant Physiol. , 165 : 366 – 374 .
- Blanco , Y , Arroyo , M , Legaz , ME and Vicente , C . 2005a . Isolation from Gluconacetobacter diazotrophicus cell walls of specific receptors for sugarcane glycoproteins, which act as recognition factors . J Chromatogr A. , 1093 : 204 – 211 .
- Blanco , Y , Blanch , M , Piñón , D , Legaz , ME and Vicente , C . 2005b . Antagonism of Gluconacetobacter diazotrophicus (a sugarcane endosymbiont) against Xanthomonas albilineans (pathogen) studied in alginate-immobilized sugarcane stalk tissues . J Biosci Bioeng. , 99 : 366 – 371 .
- Cavalcante , VA and Döbereiner , J . 1988 . A new acid-tolerant nitrogen-fixing bacterium associated with sugarcane . Plant Soil , 108 : 23 – 31 .
- Dong , Z , Canny , MJ , McCully , ME , Roboredo , MR , Cabadilla , CF , Ortega , E and Rodes , R . 1994 . A nitrogen-fixing endophyte of sugarcane stems . Plant Physiol. , 105 : 1139 – 1147 .
- Dubois , M , Gilles , KA , Hamilton , JK , Rebers , PA and Smith , F . 1956 . Colorimetric method for determination of sugar and related substances . Anal Chem. , 28 : 350 – 356 .
- Dye , DW . 1980 . “ Xanthomonas ” . In Laboratory guide for identification of plant pathogenic bacteria , Edited by: Schaad , NW . 45 – 49 . St Paul, MN : American Phytopathological Society .
- Fontaniella , B , Millanes , AM , Piñón , D , Rodríguez , CW , Vicente , C and Legaz , ME . 2003 . Effect of leaf scald on the content of sucrose and polysaccharides of two sugarcane cultivars . Food Sci Biotechnol. , 12 : 346 – 350 .
- Fontaniella , B , Rodriguez , CW , Piñón , D , Vicente , C and Legaz , ME . 2002 . Identification of xanthans isolated from sugarcane juices obtained from scalded plants infected by Xanthomonas albilineans . J Chromatogr B. , 770 : 275 – 281 .
- Galindo , E and Albiter , V . 1996 . High-yield recovery of xanthan by precipitation with isopropyl alcohol in a stirred tank . Biotechnol Progr. , 12 : 540 – 547 .
- Hayward , AC . 1974 . Latent infection by bacteria . Annu Rev Phytopathol. , 12 : 87 – 93 .
- James , EK , Reis , VM , Olivares , FL , Baldani , JI and Döbereiner , J . 1994 . Infection of sugar cane by the nitrogen-fixing bacterium Acetobacter diazotrophicus . J Exp Bot. , 45 : 757 – 766 .
- Legaz , ME and Pedrosa , MM . 1993 . Separation of acidic proteins by capillary zone electrophoresis and size-exclusion HPLC: A comparison . J Chromatogr A. , 655 : 21 – 29 .
- Legaz , ME , Pedrosa , MM , de Armas , R , Rodríguez , CW , Rios , V and Vicente , C . 1998 . Separation of soluble glycoproteins from sugarcane juice by capillary electrophoresis . Anal Chim Acta. , 372 : 201 – 208 .
- Lowry , OH , Rosebrough , NH , Farr , AL and Randall , RJ . 1951 . Protein measurement with the Folin phenol reagent . J Biol Chem. , 193 : 265 – 275 .
- MacKay , BJ , Goodman , H , Cox , D , Grossbard , BL , Iacono , VJ and Pollock , JJ . 1984 . Development of an enzyme-linked immunosorbent assay for determination of lysozyme in human parotid and submandibular-sublingual salivas . J Clin Microbiol. , 19 : 844 – 848 .
- Magaña , J and Ruiz , J . 1967 . Mechanisms of regulation of urease biosynthesis in Proteus rettgeri . J Bacteriol. , 93 : 1294 – 1298 .
- Piñón , D , Casas , M , Blanch , M , Fontaniella , B , Blanco , Y , Vicente , C , Solas , MT and Legaz , ME . 2002 . Gluconacetobacter diazotrophicus, a sugar cane endosymbiont, produces a bacteriocin against Xanthomonas albilineans, a sugar cane pathogen . Res Microbiol. , 153 : 345 – 351 .
- Purseglove , JW . 1979 . Tropical crops: Monocotyledons , 127 – 132 . London : Longman Group Ltd .
- Ricaud , C and Ryan , C . 1989 . Leaf scald. Diseases of sugarcane: Major diseases , 39 – 58 . Amsterdam : Elsevier .
- Rott , P , Mohamed , S , Klett , P , Soupa , D , de Saint-Albin , A , Feldmann , P and Letourmy , P . 1997 . Resistance to Leaf Scald Disease is associated with limited colonization of sugarcane and wild relatives by Xanthomonas albilineans . Phytopathol. , 87 : 1202 – 1213 .
- Saumtally , S , Medan , H and Autrey , LJC . 1996 . Evolution of aerial infection of leaf scald caused by Xanthomonas albilineans (Ashby) Dowson in sugarcane . Proc Int Soc Sugar Cane Technol. , 22 : 493 – 497 .
- Shastry , S and Prasad , MS . 2002 . Extracellular protease from Pseudomonas sp (CL 1457) active against Xanthomonas campestris . Proc Biochem. , 37 : 611 – 621 .
- Solas , MT , Piñón , D , Acevedo , R , Fontaniella , B , Legaz , ME and Vicente , C . 2003 . Ultrastructural changes and production of a xanthan-like polysaccharide associated with scald of sugarcane leaves caused by Xanthomonas albilineans . Eur J Plant Pathol. , 109 : 351 – 359 .
- Stepnaya , OA , Ryazanova , LP , Krupyanko , VI and Kulaev , IS . 2001 . Influence of acidic exopolysaccharide of Xanthomonas campestris IBPM 124 on the kinetic parameters of extracellular bacteriolytic enzymes . Biochem. (Moscow) , 66 : 662 – 666 .
- Woiciechowski , AL , Soccol , CR , Rocha , S and Pandey , A . 2004 . Xanthan gum production from cassava bagasse hydrolysate with Xanthomonas campestris using alternative sources of nitrogen . App Biochem Biotechnol. , 118 : 305 – 312 .
- Zhang , YX , Bak , DD , Heid , H and Geider , K . 1999 . Molecular characterization of a protease secreted by Erwinia amylovora . J Mol Biol. , 289 : 1239 – 1251 .