Abstract
The present study investigates the interactive mechanism involved during the uptake of heavy metals and stress tolerance in Brassica juncea L. seedlings under the influence of Mn(II) in binary combinations with Cr(VI), Ni(II), Co(II) and Cu(II) in terms of changes in antioxidative enzymes-superoxide dismutase (SOD), guaiacol peroxidase (GPX), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR). The order of uptake of heavy metals by the seedlings in single metal solutions was observed to be, Mn>Cu>Co>Cr>Ni. As revealed by multiple regression and path analysis, manganese (Mn) in binary combination with other heavy metals mutually decreased the uptake of each other. Mn and other metals were antagonistic to each other for the activities of SOD, GPX, APX and GR, and decreased the effect of each other through interaction, whereas antagonism between these metals increased the activity of CAT.
Abbreviations
| APX | = |
ascorbate peroxidase |
| CAT | = |
catalase |
| DHAR | = |
dehydroascorbatereductase |
| GR | = |
glutathione reductase |
| MDHAR | = |
monodehydroascorbatereductase |
| ROS | = |
reactive oxygen species |
| SOD | = |
superoxide dismutase |
Introduction
Phytoremediation is emerging as a cost-effective, ecofriendly ‘green clean’ technology for the abatement of heavy metal pollution at hazardous waste sites (Ebbs and Kochian Citation1998; Lai and Chen Citation2009). However, the understanding of the physiological responses of phytoremediators to the heavy metals is a prerequisite in the restoration of heavy metal polluted sites (Schnoor Citation2000; Chaney et al. Citation2007). The tolerance potential of plants to heavy metals depends upon various physiological and molecular mechanisms (Khan et al. Citation2009). A common feature of heavy metal stress is the increased production of reactive oxygen species (ROS) such as superoxide anion radical (·O2−), hydrogen peroxide (H2O2), hydroxyl radical (·OH), singlet oxygen (1O2) etc., in plant tissues (DiToppi and Gabbrielli Citation1999; Arora et al. Citation2002). ROS are partially reduced forms of atmospheric oxygen which are also generated in plant cells during normal metabolic processes (Fridovich Citation1986; Alscher Citation1989; Dat et al. Citation1998). Heavy metal stress enhances the production of ROS up to 30-fold, leading to oxidative stress (Mittler Citation2002). These species react with lipids, proteins, pigments and nucleic acids and cause lipid peroxidation, membrane damage and inactivation of enzymes, thus affecting the cell viability (Halliwell Citation1994; Blokhina et al. Citation2002). In response to heavy metal stress, plants employ various defense mechanisms which result in avoidance, exclusion and/or detoxification of heavy metal ions (Hall Citation2002).
The synchronous action of various antioxidative enzymes such as CAT, SOD, APX and the thiol-regulated enzymes (DHAR, MDHAR and GR) of the ascorbate-glutathione pathway is a predominant mechanism of ROS quenching (Paridha et al. Citation2004; Shanker et al. Citation2004). Low molecular weight antioxidant metabolites such as ascorbic acid and reduced glutathione and organic ligands rich in cysteine and non-protein thiols also provide protection against ROS (Cobbett Citation2000; Chen et al. Citation2001). By evoking antioxidative enzyme induction as a general adaptive response, hyperaccumulator plants used for phytoremediation have adapted themselves well against oxidative stress caused by heavy metals (Van and Clijsters Citation1999; Narang et al. Citation2008; Ansari et al. Citation2009). Several studies have been carried out on the defense mechanism of plants under oxidative stress (Mizuno et al. Citation1988; Hegedüs et al. Citation2001; Lee et al. Citation2001; Cao et al. Citation2004; Wang et al. Citation2004a,Citationb). The coexisting heavy metals at multi-elemental contaminated sites have synergistic, additive or antagonistic effects in the plants (Rosko and Rachlin Citation1977; Martin-Prevel et al. Citation1987; Symeonidis and Karataglis Citation1992 ; Siedlecka Citation1995; Krupa et al. Citation2002). The knowledge of detoxification mechanism is of critical importance from a practical standpoint of optimizing the mechanism of phytoremediation through improvement in cellular defense mechanism to combat heavy metal stress in hyperaccumulator plants (Aravind and Prasad Citation2005). In view of this, the present study was undertaken to investigate the interactive effects of manganese (Mn) in combination with other heavy metals on the growth, metal uptake and antioxidative mechanism of Brassica juncea.
Material and methods
Heavy metal treatments
Certified seeds of B. juncea L. cv ‘PBR-91’ were surface sterilized with 0.01% HgCl2. For the study of heavy metal uptake, the concentrations of heavy metals used were: 0, 25, 50 and 100 mg l−1. The seedlings did not survive in solutions containing chromium (Cr), nickel (Ni), cobalt (Co) and copper (Cu) at concentrations higher than 100 mg l−1.
Therefore, for the study on antioxidative enzymes, the following treatments were selected:
-
Single metal treatments–0 and 100 mg l−1 of each metal (Cr, Mn, Ni, Co and Cu).
-
Binary treatments–Mn treatments in binary combinations with other metals, all at 100 mg l−1.
Seeds were germinated on Whatman No. 1 filter paper, lined inside 9 cm diameter sterilized Petri plates moistened with aqueous solutions of heavy metals in single and binary combinations, pH 6.5. Solutions were prepared using analytical reagent (AR) grade chemicals, K2CrO4, NiSO4·6H2O, CoCl2·6H2O, CuSO4·5H2O and ZnSO4·7H2O. Chemicals used in the study were purchased from Sigma-Aldrich, Qualigens, Loba-Chemi, SD Fine Chem and Central Drug House, India. Seedlings grown in double distilled water served as the controls. The Petri dishes were kept in a growth chamber maintained at 25±0.5°C, 16:8 h dark-light photoperiod (1700 Lux), for a growth period of seven days.
Metal analysis and enzyme assays
After seven days, seedlings were harvested, thoroughly washed with distilled water, dried at 80°C for 24 h and digested in a digestion mixture (H2SO4: HNO3: HClO4 in 1: 5: 1 ratio) (Allen et al. Citation1976). The concentrations of metals in the solutions were determined using atomic absorption spectrophotometer (Model AA 6200, Shimadzu, Japan).
One gram of fresh seedlings was homogenized in 3 ml of 100 mM potassium phosphate buffer, pH 7.0, containing 1% (w/v) insoluble polyvinylpyrrolidone (Polyclar-AT, Sigma-Aldrich) in an ice chilled pestle and mortar. The homogenates were centrifuged at 15,000 g for 20 min at 5°C and the supernatants were used for assaying the activities of antioxidative enzymes.
Superoxide dismutase (EC 1.15.1.1)
Superoxide dismutase was estimated after Kono (Citation1978). The method is based on the inhibitory effects of SOD on the reduction of nitroblue tetrazolium (NBT) by superoxide radicals, generated by the autooxidation of hydroxylamine hydrochloride. The reduction of NBT was followed by an absorbance increase at 540 nm. The reaction mixture containing 1.8 ml sodium carbonate buffer (pH 6.0), 750 µl NBT and 150 µl triton X-100 was taken in the test cuvette and the reaction was initiated by the addition of 150 µl hydroxylamine hydrochloride. 70 µl of the enzyme extract was added after 2 min and the percentage inhibition in the rate of NBT reduction was recorded as increase in the absorbance at 540 nm.
Hydroxylamine hydrochloride is auto-oxidized to nitrite by superoxide radicals. The addition of NBT induces an increase in the absorbance at 540 nm due to the production of blue formazon. With the addition of superoxide enzyme, superoxide radicals get trapped, and hence there is an inhibition of reduction of NBT to blue formazon formation. The percentage inhibition of NBT reduction was calculated as:
Guaiacol peroxidase (EC 1.11.1.7)
The activity of GPX was estimated according to the method given by Putter (Citation1974). GPX catalyzes the decomposition of H2O2 of a large number of organic compounds, such as phenols, aromatic amines, hydroquinones etc., and in particular pyrogallol, guaiacol etc. These cause the reduction of H2O2 to water and oxygen using guaiacol as a substrate.
The reaction is given as:
Catalase (EC 1.11.1.6)
Catalase activity was determined as per the method of Aebi (Citation1974). Catalase catalyzes the decomposition of H2O2 to water and oxygen. The rate of decomposition of H2O2 was followed by decrease in absorbance at 240 nm of the reaction mixture.
Catalase activity can be measured by following either the decomposition of H2O2 or the liberation of O2. H2O2 shows a continual increase in absorption with decreasing wavelength in the UV range. The difference in extinction per unit time is the measure of catalase activity. The reaction mixture was prepared using 1.5 ml potassium buffer (100 mM, pH 7.0), 1.2 ml H2O2 (150 mM) and 300 µl of enzyme extract. The decrease in absorption per min was recorded at 240 nm spectrophotometrically. Enzyme activity was determined using extinction coefficient 6.93×10−3 mM−1cm−1. One unit of enzyme activity was calculated as the amount of enzyme required to release half the peroxide oxygen from H2O2. Unit activity and specific activity were calculated as for GPX.
Ascorbate peroxidase (EC 1.11.1.11)
The activity of ascorbate peroxidase was estimated according to the method proposed by Nakano and Asada (Citation1981). Ascorbate peroxidase is very specific and one of the most important enzymes in plants. It catalyzes the reduction of H2O2 using the substrate ascorbate.
One mole of H2O2 oxidizes one mole of ascorbate to produce one mole of dehydroascorbate. The rate of oxidation of ascorbate was followed by decrease in absorbance at 290 nm. Three ml of reaction mixture consisted of 1.5 ml phosphate buffer (100 mM, pH 7.0), 300 µl of 5 mM ascorbate, 600 µl of 0.5 mM H2O2, and 600 µl enzyme extract. The decrease in absorbance was recorded at 290 nm. One unit of enzyme activity was determined as the amount of enzyme required to oxidize 1 µM of ascorbate min−1 g−1 fw. Unit and specific activities were calculated as for GPX with an extinction coefficient of 2.8 mM−1cm−1.
3.2.2.8 Glutathione reductase (EC 1.8.1.7)
Glutathione reductase activity was measured using the method proposed by Carlberg and Mannervik (Citation1975). Glutathione reductase catalyzes the reduction of glutathione disulphide (GSSG) to sulfhydryl form (GSH) involving the oxidation of NADPH. The reaction was carried in phosphate buffer (50 mM, pH 7.6).
Statistical analysis
The data were analyzed for descriptive statistics, ANOVA, Tukey's multiple comparison tests, multiple regression analysis and path analysis (Sokal and Rholf Citation1981; Bailey Citation1995) using self-coded software in MS-Excel. The binary interaction models developed using multiple regression technique were:
Table 1. Binary metal interactions in terms of β-regression coefficients.
Results
Metal uptake
Mn showed maximum uptake, 0.445 mg g−1 dw, whereas Ni was found to be the metal least accumulated (0.135 mg g−1 dw) at 100 mg l−1 treatment (). In all binary treatments, both the metal ions mutually significantly inhibited the uptake of each other, except for Mn 25: Co 25, Mn 100: Co 100, Mn 100: Co 50, and Co 25: Mn 100. Supplementation of 100 mg l−1 Cr with Mn (25, 50 and 100 mg l−1) reduced the Cr uptake by 66.7%, 62.2% and 68.3%, respectively. Similar trends were observed in binary combinations of Mn with Ni, Co and Cu. Multiple regression interaction models () revealed significant correlations between all the binary combinations of Mn and the uptake of respective metal ions. In (Mn+Cr) and (Mn+Ni), both the ions independently as well as their interaction, inhibited the uptake of each other. In (Mn+Co) and (Mn+Cu), although Mn facilitated the uptake of its coexisting ions, both Co and Cu retarded the uptake of Mn by the seedlings. The mixed interactions occurring between these ions decreased uptake of Mn, whereas the decreased uptake of both Co and Cu was due to the antagonistic interactions. Path analysis () shows that in all the binary combinations of Mn, the uptake of all the metals was increased due to their own direct effects, maximum being caused by Co (0.99). However, Mn and the corresponding metals in binary combinations had indirect negative interactive effects on the uptake of each other, maximum being due to interaction between Cu and Mn where the uptake of Cu is decreased by Mn by a path coefficient of -0.58. Similarly, Mn directly reduces the uptake of other metals, maximum (-0.61) being due to the direct effect of Mn on Cu uptake. Except for the direct effect of Co on Mn uptake and Cu on Mn uptake, all the metals decreased the uptake of each other both through direct and indirect effects, maximum indirect effect (-0.92) being due to Cu on Mn uptake.
Table 2. Metal uptake (mg•g−1 dw) by the seedlings of B. juncea grown in water cultures containing different binary combinations of Mn with other metals.
Table 3. Multiple regression interaction model for metal uptake (Y) in the seedlings of B. juncea grown in water cultures containing binary combinations of Mn with other heavy metals (X1+X2) mg•l−1.
Table 4. Path analysis of direct and indirect effects of heavy metals on the uptake of each other in B. juncea seedlings grown in water culture containing binary combinations (X1+X2) mg•l−1 of Mn with other heavy metals.
Antioxidative enzymes
The corresponding data obtained from the enzyme assays of single metal stressed seedlings showed that there is significant enhancement in the activities of antioxidative enzymes, except for CAT (). Maximum increase in the activities of all the enzymes was observed in the seedlings treated with Mn 100 mg•l−1 alone. The data further revealed that binary combination of metals also increased the activities of antioxidative enzymes. It was found that Mn+Cu combination increased the SOD and GPX activity to 20.8 and 56.7 mM UA/mg protein, respectively. Similarly APX and GR activities were further increased under the combined influence of Mn+Cr and Mn+Ni, respectively. In the treatment (Mn100+Cr100), maximum APX activity was observed to be 9.5 mM UA/mg protein, and the maximum APX activity (10.9 mM UA/mg protein) was observed in the seedlings treated with (Mn100+Ni100) mg•l−1. Binary interaction model () for the relative activities of different antioxidative enzymes as a function of two metals in binary combination derived using multiple regression analysis revealed that there are significant correlations between various enzymatic activities and the metals in binary combinations. Regarding SOD, GPX, APX and GR, it was observed that both the metals independently increased the enzymatic activities as indicated by the positive values of β-regression coefficients, but the interactive effects, caused by the antagonistic interactions occurring between the coexisting metal ions, were negative on the activity of these enzymes. Catalase, however, was decreased under the influence of all the metals eliminating the effects of metals in combination. The interactive effects of metals in binary combinations with Mn were positive.
Figure 1. Specific activities (mM UA/mg protein) (Mean±SD) of antioxidative enzymes in the seedlings of B. juncea grown in binary combinations of Mn with other heavy metals (mg l−1).
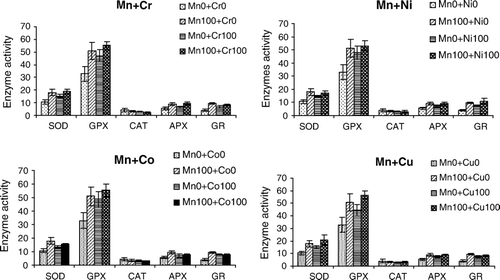
Table 5. β-regression coefficients for the activities of different antioxidative enzymes (Y) in the seedlings of B. juncea grown in water cultures containing binary combinations of Mn (X1) with other heavy metals (X2).
Discussion
The present study revealed antagonistic effects of metals on the uptake of each other. This may be due to the competitive inhibition of heavy metal uptake by the seedlings. It was evident that the uptake of heavy metals induced a strong antioxidative response in B. juncea seedlings by increasing the activities of antioxidative enzymes, except for catalase. Pandey et al. (Citation2005) and Shanker et al. (Citation2004) reported increase in SOD activity in B. juncea and Vigna radiata under Cr (VI) stress. Gao et al. (Citation2008) and Posmyk et al. (Citation2009) also reported that the SOD activity in Jatropha curcas and red cabbage seedlings increased concomitantly with increasing Cu concentrations in the medium. Wang et al. (Citation2004b) attributed Cu-induced increase in SOD activity to Cu being a redox active metal capable of generating harmful free radicals, such as hydroxyl, peroxyl and alkoxyl radicals. The present study did not reveal any significant change in the catalase activity. Wang et al. (Citation2004a) observed significant increase in APX, GPX and SOD, but CAT activity decreased significantly in B. juncea seedlings treated with Cu2+. Sharma et al. (Citation2008) also observed reduced CAT activity in B. juncea seedlings under Ni stress. In sunflower roots Cu increased GPX, but SOD and CAT were reduced (Jouili and El Ferjani Citation2003, Citation2004). A decrease in CAT activity was also observed in oat leaves after exposure to the toxic Cu concentrations by Luna et al. (Citation1994). Decrease in CAT activity in plants on metal exposure could be attributed to metal ions binding or replacing some components like Fe2+ in the enzyme or that the increased H2O2 level causes inactivation of the enzyme (Mazhoudi et al. Citation1997). Also, it may be possible that catalase is more sensitive to metal ions as these readily bind to thiol groups, thereby inactivating the thiol containing enzyme. Feierabend et al. (Citation1992) has already shown that, under stress conditions, inactivation of CAT is linked to H2O2 accumulation.
Ascorbate-glutathione cycle in chloroplast is the main component of the defense system for scavenging H2O2 that ultimately converts H2O2 to H2O and O2. This cycle mainly involves APX, POX and GR. It was also reported that in many plant species, excessive uptake of heavy metals, such as Ni, cadmium (Cd) and lead (Pb) induced a strong increase in POX activity (Mazhoudi et al. Citation1997; Baccouch et al. Citation2001). APX plays a crucial role in ascorbate-glutathione cycle, scavenging H2O2 using ascorbate as a substrate. The induction of APX activity due to Cr in plants is also reported in Vigna radiata (Shanker et al. Citation2004), and B. juncea (Diwan et al. Citation2008); due to Cu in B. juncea (Wang et al. Citation2004b); due to Mn in Cucumis sativus (Shi et al. Citation2006).
Enhancement in the GPX activity as observed in metal stressed B. juncea seedlings is also coherent with the findings previous workers. Cr induced increase in GPX activity was observed in the leaves of Amaranthas viridis (Liu et al. Citation2008). Increase in GPX activity in has been reported in different plants due to Cu (Jouili and El Ferjani Citation2003, Citation2004; Wang et al. Citation2004b; Posmyk et al. Citation2009), Mn (Demirevska-Kepova et al. Citation2004), Hg (Cho and Park Citation2000; Narang et al. Citation2008) and Pb (Ruley et al. Citation2004).
Since APX eliminates H2O2 by converting ascorbate to dehydroascorbate, the increased production of dehydroascorbate is recycled back to ascorbate with the help of GR, thereby catalyzing this last rate limiting step of glutathione cycle. Increased activity of GR under metal stress is reported by many researchers, which is also in agreement with our findings. In the present study, maximum enhancement in the activity of GR (9.56 mM UA mg−1 protein) was observed at the single metal treatment of 100 mg l−1 Mn. Moreover, the binary combination (Mn100+Ni100) caused maximum increase (10.9 mM UA mg−1 protein) in the enzymatic activity. This result is in accordance with the finding of several workers (Prasad et al. Citation1999; Dixit et al. Citation2001; Shi et al. Citation2006; Srivastava et al. Citation2006; Tanyolac et al. Citation2007; Diwan et al. Citation2008; Khan et al. Citation2009). This increased activity of GR under metal stress can be attributed to the fact that it provides GSH for the synthesis of phytochelatins (Baisak et al. Citation1994).
The results of the present study suggest that B. juncea seedlings try to counteract high concentration of ROS produced under metal toxicity through a coordinated increase in the activities of enzymes involved in their detoxification. Enhanced enzyme activities under single metal stress in the current study are in broad agreement with many other earlier reports, as it has been shown that these enzymes are triggered by ROS following exposure to metal-induced oxidative stress. However, investigations focusing on the adaptive physiological and biochemical mechanisms of the interactions between different metals are rather scanty. The perusal of literature regarding the antioxidative defense system in plants showed that little information is available with respect to multiple metal stress. In our earlier experiments on the interactive effects of Cr and Ni on the growth and uptake potential of B. juncea seedlings, it was observed that Mn in combination with Cr and Ni caused positive interactive effect on the root-shoot length and dry weight of the seedlings owing to antagonistic interactions occurring between them leading to mutual amelioration of their toxicities which is manifested through better seedling growth as compared to the seedlings grown in single treatments of Cr and Ni, as well as in other binary combinations such as, (Cr+Ni), (Cr+Co), (Cr+Cu), (Ni+Co) and (Ni+Cu) (Kaur et al. Citation2009a, Kaur et al. Citation2010). This improved growth in binary combinations involving Mn could be due to increased tolerance of B. juncea by the enhanced activation of antioxidative enzymes, as observed in the present investigation. Moreover, this increase in the activities of antioxidative enzymes under binary metal stress was also observed in binary combinations of zinc (Zn), in our previous experiment (Kaur et al. Citation2009b). It was observed that of all the binary combinations tested, Zn+Co and Zn+Ni were most effective in increasing the activities of GPX and GR, respectively, whereas Zn+Cu and Zn+Cr increased the activities of APX and SOD, respectively. The increase in the activities of antioxidative enzymes under binary metal stress was attributed to the accelerated operation of the defence mechanism of the plant that confers greater stress tolerance towards combined toxicities of the heavy metals.
Conclusion
The present study establishes that Mn suppresses the toxicities of Cu, Co, Cr and Ni, due to the antagonistic interactions between them, thereby improving seedling growth in B. juncea. Furthermore, B. juncea counteracts the high concentrations of ROS produced under metal toxicity through a coordinated increase in the activities of antioxidative enzymes required for their detoxification.
Acknowledgements
Thanks are due to the Ministry of Environment and Forests, Government of India, for financial assistance (sanction # 19-87/2000-RE).
References
- Aebi , H . 1974 . “ Catalase ” . In Methods of enzyme analysis , Edited by: Bergmeyer , HU . 673 – 684 . Weinheim : Verlag Chemie .
- Allen , SE , Grimshaw , HM , Parkinson , JA , Quarmby , C and Roberts , JD . 1976 . “ Chemical analysis ” . In Methods in plant ecology , Edited by: Chapman , SB . 424 – 426 . Oxford : Blackwell Scientific Publications .
- Alscher , RG . 1989 . Biosynthesis and antioxidant function of glutathione in plants . Physiol Planta. , 77 : 457 – 464 .
- Ansari , KH , Ahmed , A , Umar , S and Iqbal , M . 2009 . Mercury induced changes in growth variables and antioxidative enzyme activities in Indian mustard . J Plant Inter. , 4 : 131 – 136 .
- Aravind , P and Prasad , MNV . 2005 . Cd-Zn interactions in a hydroponic system using C. demersum L. Adaptive ecophysiology, biochemistry and molecular toxicology . Braz J Pl Physiol. , 17 : 3 – 20 .
- Arora , A , Sairam , RK and Srivastava , GC . 2002 . Oxidative stress and antioxidative system in plants . Curr Sci. , 82 : 1227 – 1238 .
- Baccouch , S , Chaoui , A and El Ferjani , E . 2001 . Nickel toxicity induces oxidative damage in Zea mays roots . J Pl Nutr. , 24 : 1085 – 1095 .
- Baisak , R , Rana , D , Archarya , PBS and Kar , M . 1994 . Alterations in the activities of active oxygen scavenging enzymes of wheat leaves subjected to water stress . Plant Cell Physiol. , 35 : 495 – 499 .
- Bailey , NTJ . 1995 . Statistical methods in biology , 255 Cambridge, , UK : Cambridge University Press .
- Bala , R and Thukral , AK . 2008 . Interactions between chromium and plant growth regulators on the growth of Spirodela polyrrhiza (L.) Schleiden . Terres Aq Environ Toxicol. , 2 : 14 – 18 .
- Bala R , Thukral AK . 2010 . Phytoremediation of Cr(VI) by Spirodela polyrrhiza (L.) Schleiden employing reducing and chelating agents . Inter J Phytorem . (in press) .
- Blokhina , O , Violainen , E and Fagerstedt , KV . 2002 . Antioxidants, oxidative damage and oxygen deprivation stress: A review . Annals of Bot. , 91 : 179 – 194 .
- Cao , X , Ma , LQ and Tu , C. 2004 . Antioxidative responses to arsenic in the arsenic-hyperaccumulator Chinese brake fern (Pteris vittata L.) . Environ Poll. , 128 : 317 – 325 .
- Carlberg , I and Mannervik , B . 1975 . Purification of the flavoenzyme glutathione reductase from rat liver . J Bio Chem. , 250 : 5475 – 5480 .
- Chaney , RL , Angle , JS , Broadhurst , CL , Peters , CA , Tappero , RV and Sparks , DL . 2007 . Improved understanding of hyperaccumulation yields commercial phytoextraction and phytomining technologies . J Environ Qual. , 36 : 1429 – 1443 .
- Chen , NC , Kanazawa , S , Horiguchi , T and Chen , NC . 2001 . Effect of chromium on some enzyme activities in the wheat rhizosphere . Soil Microorg. , 55 : 3 – 10 .
- Cho , UH and Park , JO . 2000 . Mercury induced oxidative stress in tomato seedlings . Plant Sci. , 156 : 1 – 9 .
- Cobbett , CS . 2000 . Phytochelatins biosynthesis and function in heavy metals detoxification . Curr Opinion Plant Bio. , 3 : 211 – 216 .
- Dat , JF , Lopez-Delogado , H , Foyer , CH and Scott , IM . 1998 . Parallel changes in H2O2 and catalase during the thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings . J Plant Physiol. , 116 : 1351 – 1375 .
- Demirevska-Kepova , K , Simova , SL , Stoyanova , Z , Holzer , R and Feller , U . 2004 . Biochemical changes in barley plants after excessive supply of copper and manganese . Environ Exper Bot. , 52 : 253 – 266 .
- DiToppi , LS and Gabbrielli , R . 1999 . Response to cadmium in higher plants . Environ Exper Bot. , 41 : 105 – 130 .
- Diwan , H , Ahmed , A and Iqbal , M . 2008 . Genotypic variation in the phytoremediation potential of Indian mustard for chromium . Environ Manag. , 41 : 734 – 736 .
- Dixit , V , Pandey , V and Shyam , R . 2001 . Differential and oxidative responses to cadmium in roots and leaves of pea (Pisum sativum L. cv. Azad) . J Exp Bot. , 52 : 1101 – 1109 .
- Ebbs , SD and Kochian , LV . 1998 . Phytoextraction of zinc by oat (Avena sativa), barley (Hordeum vulgare), and Indian mustard (Brassica juncea) . Environ Sci Tech. , 32 : 802 – 806 .
- Feierabend , J , Schann , C and Hertwig , B . 1992 . Photoinactivation of catalase occurs under both high and low temperature stress conditions and accompanies photosystem II . J Plant Physiol. , 100 : 1554 – 1561 .
- Fridovich , I . 1986 . Superoxide dismutase . Advances in Enzymol. , 58 : 62 – 97 .
- Gao , S , Yan , R , Cao , M , Yang , WS and Wang , FC . 2008 . Effects of copper on growth, antioxidant enzymes and phenylalanine ammonia-lyase activities in Jatropha curcas L. seedlings . Plant Soil Environ. , 54 : 117 – 122 .
- Hall , JL . 2002 . Cellular mechanisms for heavy metal detoxification and tolerance . J Exp Bot. , 53 : 1 – 11 .
- Halliwell , B . 1994 . Free radicals and antioxidants, personal view . Nutr Reviews. , 52 : 253 – 265 .
- Hegedüs , A , Erdei , S and Horváth , G . 2001 . Comparative studies of H2O2 detoxifying enzymes in green and greening barley seedlings under cadmium stress . Plant Sci. , 160 : 1085 – 1093 .
- Jouili , H and El Ferjani , E . 2003 . Changes in antioxidant and lignifying enzyme activities in sunflower roots (Helianthus annuus L.) stressed with copper excess . CRB. , 326 : 639 – 644 .
- Jouili , H and El Ferjani , E . 2004 . Effect of copper excess on superoxide dismutase, catalase and peroxidase activities in sunflower seedlings (Helianthus annuus L.) . Acta Physiol Planta. , 26 : 29 – 35 .
- Kaur , R , Bhardwaj , R and Thukral , AK . 2009a . Uptake of heavy metals, and antioxidative enzymes in Brassica juncea L. seedlings as affected by Zn in binary combinations with other heavy metals . Plant Stress. , 3 : 17 – 25 .
- Kaur , R , Bhardwaj , R and Thukral , AK . 2009b . Interactive effects of Cr(VI) with other heavy metals on the growth and metal uptake potential of Brassica juncea L. seedlings . Terres Aq Environ Toxicol. , 3 : 16 – 27 .
- Kaur , R , Bhardwaj , R and Thukral , AK . 2010 . Interactive effects of Ni in binary combinations with other heavy metals on the growth and metal uptake in Brassica juncea . Can J Pure Appl Sci. , 4 : 1011 – 1019 .
- Khan , I , Ahmad , A and Iqbal , M . 2009 . Modulation of antioxidant defence system for arsenic detoxification in Indian mustard . Ecotoxicol Environ Safety. , 72 : 626 – 634 .
- Kono , Y . 1978 . Generation of superoxide radical during autooxidation of hydroxylamine and an assay for superoxide dismutase . Arch Biochem Biophys , 186 : 189 – 195 .
- Krupa , Z , Siedlecka , A , Skorzynska-Polit , E and Maksymeic , W. 2002 . “ Heavy metal interactions with plant nutritents ” . In Physiology and biochemistry of metal toxicity and tolerance in plants , Edited by: Prasad , MNV and Strazalka , K . 287 – 301 . Dordrecht : Kluwer Academic Publishers .
- Lai , H and Chen , Z . 2009 . In-situ selection of suitable plants for the phytoremediation of multi-metal-contaminated sites in central Taiwan . Inter J Phytorem. , 11 : 235 – 250 .
- Lee , DH , Kim , YS and Lee , CB . 2001 . The inductive responses of the antioxidant enzymes by salt stress in the rice (Oryza sativa L.) . J Plant Physiol. , 158 : 737 – 745 .
- Liu , D , Zou , J , Wang , M and Jiang , W . 2008 . Hexavalent chromium uptake and its effects on mineral uptake, antioxidant defense system and photosynthesis in Amaranthus viridis L . Bioresource Tech. , 99 : 2628 – 2636 .
- Luna , CM , González , CA and Trippi , VS . 1994 . Oxidative damage caused by an excess of copper in oat leaves . Plant Cell Physiol. , 35 : 11 – 15 .
- Martin-Prevel , P , Gagnard , J and Gautier , P . 1987 . Plant analysis: A guide to the nutrient requirements of temperate and tropical crops , 722 New York : Lavoisier Publishing Inc .
- Mazhoudi , S , Chaoui , A , Ghorbal , MH and El Ferjani , E . 1997 . Response of antioxidant enzymes to excess copper in tomato (Lycopersicon esculentum, Mill.) . Plant Sci. , 127 : 129 – 137 .
- Mittler , R . 2002 . Oxidative stress, antioxidants and stress tolerance . Trends Plant Sci. , 7 : 405 – 410 .
- Mizuno , M , Kamei , M and Tsuchida , H . 1988 . Ascorbate peroxidase and catalase cooperate for protection against hydrogen peroxide generated in potato tubers during low-temperature storage . Biochem Mol Bio Inter. , 44 : 717 – 726 .
- Nakano , Y and Asada , K . 1981 . Hydrogen peroxide is scavenged by ascorbate specific-peroxidase in spinach chloroplasts . Plant Cell Physiol. , 22 : 867 – 880 .
- Narang , U , Thukral , AK , Bhardwaj , R and Garg , SK . 2008 . Role of antioxidative defence system in Eichhornia crassipes (Mart.) Solms . Can J Pure Appl Sci. , 2 : 537 – 545 .
- Pandey , V , Dixit , V and Shyam , R . 2005 . Antioxidative responses in relation to growth of mustard (Brassica juncea cv. Pusa Jaikisan) plants exposed to hexavalent chromium . Chemosphere. , 61 : 40 – 47 .
- Paridha , AK , Das , AB and Mohanty , P . 2004 . Investigations on the antioxidative defense responses to NaCl stress in a mangrove, Bruguiera parviflora: Differential regulations of isoforms of some antioxidative enzymes . Plant Growth Regul. , 42 : 1 – 14 .
- Posmyk , MM , Kontek , R and Janas , KM . 2009 . Antioxidant enzymes activity and phenolic compounds content in red cabbage seedlings exposed to copper stress . Ecotoxicol Environ Saf. , 72 : 596 – 602 .
- Prasad , KVSK , Saradhi , PP and Sharmila , P . 1999 . Concerted action of antioxidant enzymes and curtailed growth under zinc toxicity in Brassica juncea . Environ Exper Bot. , 42 : 1 – 10 .
- Putter J. 1974 . Peroxidase. Methods of enzymatic analysis . Bergmeyer Hu , Weinhan : Verlag Chemie . pp 685 – 690 .
- Rosko , JJ and Rachlin , JW . 1977 . The effect of cadmium, copper, mercury, zinc and lead on cell division, grown and chlorophyll a content of the chlorophyte Chlorella vulgaris . Bull Torr Bot Club. , 104 : 226 – 275 .
- Ruley , AT , Sharma , NC and Sahi , SV . 2004 . Antioxidant defense system in a lead accumulating plant, Sesbania drummondii . Plant Physiol Biochem. , 42 : 899 – 906 .
- Schnoor , JL . 2000 . “ Phytostabilization of metals using hybrid poplar trees ” . In Phytoremediation of toxic metals, using plants to clean-up the environment , Edited by: Raskin , I and Ensley , YBD . 133 – 150 . New York : John Wiley & Sons .
- Shanker , AK , Djanaguiraman , M , Sudhagar , R , Chandrashekar , CN and Pathmanabhan , G . 2004 . Differential antioxidative response of ascorbate glutathione pathway enzymes and metabolites to chromium speciation stress in green grass (Vigna radiata (L.) Wilczek. cv CO4) roots . Plant Sci , 166 : 1035 – 1043 .
- Sharma , P , Bhardwaj , R , Arora , HK and Arora , N . 2008 . 28-homobrassinolide-regulated effects of nickel on metal uptake, protein content and antioxidative defence system in Brassica juncea L . Bio Planta. , 52 : 767 – 770 .
- Shi , QH , Zhu , ZJ , Qian , QQ and Yu , JQ . 2006 . Effect of excess manganese on the antioxidant system in Cucumis sativus L. under two light intensities . Environ Exp Bot. , 58 : 197 – 205 .
- Siedlecka , A . 1995 . Some aspects of interactions between heavy metals and plant mineral nutrients . Acta Soc Bot Pol. , 64 : 265 – 272 .
- Sokal , RR and Rholf , FJ . 1981 . Biometry: The principles and practice of statistics in biological research , San Francisco : WH Freeman and Co .
- Srivastava , S , Mishra , S , Tripathi , RD , Dwivedi , S and Gupta , DK . 2006 . Copper-induced oxidative stress and responses of antioxidants and phytochelatins in Hydrilla verticillata (L.f.) Royle . Aq Toxicol. , 80 : 405 – 415 .
- Symeonidis , L and Karataglis , S. 1992 . Interactive effects of cadmium, lead and zinc on root growth of two metal tolerant genotypes of Holcus lanatus L . Biometals. , 5 : 173 – 178 .
- Tanyolac , D , Ekmekci , Y and Unalan , S . 2007 . Changes in photochemical and antioxidant enzyme activities in maize (Zea mays L.) leaves exposed to excess copper . Chemosphere. , 67 : 89 – 98 .
- Van , AF and Clijsters , H . 1999 . Effect of the metals on enzyme activity in plants . Plant Cell Environ. , 13 : 195 – 206 .
- Wang , H , Shan , XQ , Wen , B , Zhang , S and Wang , ZJ . 2004a . Response of antioxidative enzymes of accumulation of copper in a copper hyperaccumulator of Commelina communis . Arch Environ Contam Toxicol. , 47 : 185 – 192 .
- Wang , SH , Yang , ZM , Yang , H , Lu , B , Li , SQ and Lu , YP . 2004b . Copper induced stress and antioxidative responses in roots of Brassica juncea L . Bot Bull Acta Sin. , 45 : 203 – 212 .