Abstract
The present experiment was conducted to evaluate salt tolerance in varieties of Indian mustard (Brassica juncea). Sterilized seeds were grown under natural environment in pots containing soil amended with 1.4 (control), 2.8, 4.2 and 5.6 dSm−1 NaCl and sampled at 30 days after sowing. Growth was recorded in terms of length, fresh mass, dry mass and leaf area of plants, which was drastically reduced in Chapka Rohini, while there was little effect of NaCl treatment on Varuna. In Chapka Rohini, a rise in the level of proline was observed which followed the decline in protein content. The declines in net photosynthesis and other related parameters have been attributed to this decline. From the results, it could be suggested that Varuna is salt-tolerant while Chapka Rohini is the salt-sensitive variety of mustard among the screened genotypes. Photosynthetic capacity is a major factor in conferring the salt-sensitiveness and tolerance in plant varieties.
Introduction
Salinity is the major constrain of crop production in arid and semi-arid regions where soil salt content is naturally high and precipitation can be insufficient for leaching (Zhao et al. Citation2007). The secondary result of salinity is the sodicity in clay soils. Over 6% of the world's land is affected by salinity/sodicity, this accounts for more than 800 million ha of land (Food and Agriculture Organization of the United Nations [FAO] Citation2005). Leaching either through natural or anthropogenic activities wash soluble salts into the subsoil, leaving sodium bound to the negative charges of the clay due to an increase in its concentration.
Downhill gradient of water potential from root zone to soil imposes physiological drought, initially leads to induction of osmotic balance strategy characterized by accelerated build up of proline and other osmolytes stimulated by reactive oxygen species (ROS) signaling. Passive import of excess salt inactivates/dismantles respiratory chain carriers permitting the leakage of electrons thereby excessive generation of ROS in aerial parts. Consequently, the membrane lipids, proteins (Geissler et al. Citation2009) and other cellular biomolecules are oxidized. This not only deteriorates production of high energy intermediates but also perturbs energy-fueled metabolic processes such as photosynthesis (Zobayed et al. Citation2005), and lipid and protein metabolism. The chlorophyll content declines in salt-sensitive plants such as Lycopersicon esculentum (Lapina and Popov Citation1970), Solanum tuberosum (Abdullah and Ahmad Citation1990), Pisum sativum (Hamada and El-Enany Citation1994) and Phaseolus vulgaris (Seemann and Critchley Citation1985). Increased accumulation of NaCl in chloroplasts under salt stress affects growth rate and is often associated with decrease in photosynthetic electron transport activities (Boyer Citation1976; Kirst Citation1989). Salt stress in higher plants inhibits PSII activity (Mishra et al. Citation1991; Masojidek and Hall Citation1992; Belkhodja et al. Citation1994; Everard et al. Citation1994; Singh and Dubey Citation1995; Tiwari et al. Citation1997; Kao et al. Citation2003; Parida et al. Citation2003). One factor responsible for the decrease in the activity of PSII under salt stress is the dissociation of 23 KDa polypeptide bound extrinsically to PSII (Kuabara and Murata Citation1982; Miyao and Murata Citation1983; Murata et al Citation1992). An excessive amount of salt causes a series of metabolic dysfunctions (Booth and Beardall Citation1991) as a consequence of compromised enzyme activity, namely, carbonic anhydrase (Ali et al. Citation2008), Rubisco, phosphenolpyruvate carboxylase (Soussi et al. Citation1998), and the degradation of photosynthetic pigments (Soussi et al. Citation1998), lipid peroxidation (Ashraf et al. Citation2010) etc.
Oil crops are the important parts among human consumables. These are very sensitive to salt levels and soil drought status, experiencing water deficit due to osmotic stress possibly coupled with biochemical perturbations induced by the influx of sodium ions. Some of the leguminous plants like faba bean (Abdel Ghaffar et al. Citation1982), chickpea (Lauchli Citation1984), tepary bean (Coons and Pratt Citation1988), pigeon pea (Subbarao and Johansen Citation1991) and common bean (Moreno-Limón et al. Citation2000) have been reported to show salt tolerance. However, the reports on the salt tolerance in mustard genotypes are meager. Indian mustard is one of the important crops in India, because it is very rich in oil content. It can present several opportunities to study its response towards salinity stress in order to screen the salt-tolerant and salt-susceptible cultivars as well as to analyze its genetic diversity. The promising cultivar lines can be exploited either for direct use in moderately saline soils, or for use in selection and breeding programs to make further advancement in salt tolerance. The present study was designed to screen mustard genotypes for salt tolerance, based on variability in photosynthesis and growth characteristics.
Materials and methods
Plant material and treatments
Healthy seeds of 10 genotypes Brassica juncea were obtained from Chola Beej Bhandar Aligarh. The seeds were surface sterilized with 0.01% mercuric chloride solution and washed 2–3 times with double distilled water. The sterilized seeds were sown in earthen pots of 25×25 cm containing soil amended with 1.4 (control), 2.8, 4.2 and 5.6 dSm−1 NaCl. These pots were placed in net house under natural conditions. Thirty-day-old plants were sampled to assess the following parameters. The experiment was conducted according to simple randomised block design. The analysis of variance (ANOVA) was performed as described by Gomez and Gomez (Citation1984).
Fresh mass, dry mass, length and leaf area per plant
Plants were removed along with soil and dipped in water to dislodge the adhering soil particles without injuring the roots. The roots were cut from plants and blotted. The blotted roots were weighed to record their fresh mass and placed in an oven (80°C for 72 h). The samples were weighed again to record their dry mass. The mass was expressed in milligrams. The length of the roots was measured by metric scale and expressed in centimeter. Leaf area was ascertained by gravimetric method. The leaf area of randomly selected leaves from each treatment was determined by tracing its outline on graph sheet and counting the squares covered by leaf on graph paper.
Photosynthetic attributes and SPAD chlorophyll
The photosynthetic attributes such as net photosynthetic rate (PN), stomatal conductance (gs), transpiration rate (E), water use efficiency (WUE) and internal carbon dioxide concentration (Ci) were measured by infra red gas analyzer (IRGA, LICOR 6400, Lincoln NE, USA) in well expanded leaves between 11 and 12 h in bright sunlight. A SPAD chlorophyll meter (Minolta) was used to assess SPAD value of chlorophyll in fresh leaves.
Nitrate reductase (NR) and carbonic anhydrase (CA) activity
The activity of NR was measured following the method adopted by Jaworski (Citation1971). The fresh leaf samples were cut into small pieces and transferred to plastic vials containing phosphate buffer (pH 7.5) followed by the addition of potassium nitrate and isopropanol solutions. The reaction mixture was incubated at 30°C, for 2 h followed by the addition of N-1-naphthyl ethylenediamine dihydrochloride and sulphanilamide. The absorbance of the color was read at 540 nm and was compared with that of the calibration curve. The activity of NR [n moleNO2 g−1 (FM) s−1] was computed on fresh mass basis.
The activity of CA was determined following the procedure described by Dwivedi and Randhawa (Citation1974). The leaf samples were cut into small pieces and suspended in cystein hydrochloride solution. The samples were incubated at 4°C for 20 min. The pieces were blotted on the filter paper and transferred to the test tubes, containing phosphate buffer (pH 6.8) followed by the addition of alkaline bicarbonate solution and bromothymol blue indicator. The test tube was incubated at 4°C for 20 min. The reaction mixture was titrated against 0.05 N HCl after addition of 0.2 ml of methyl red indicator.
Proline content
The proline content in fresh leaf was determined by adopting the method of Bates et al. (Citation1973). Proline was extracted in sulphosalicylic acid. To the extract, an equal volume of glacial acetic acid and ninhydrin solutions were added. The sample was heated at 100°C, to which 5 ml of toluene was added after cooling in ice bath. The absorbance of toluene layer was read at 528 nm, on a spectrophotometer.
Protein content
The total protein content in the leaves was estimated by adopting the methodology of Lowry et al. (Citation1951). The protein was extracted with 0.1 M NaOH and was reacted with Folin phenol reagent to develop blue color and was read at 600 nm. A calibration curve was plotted by using bovine albumin to calculate % protein content in the samples.
Statistical analysis
Each observation was replicated five times. The values for various parameters of the plants were subjected to statistical analysis following the standard procedure described by Gomez and Gomez (Citation1984). The means were compared by Least Significant Difference (LSD) test to study the significance at 5% level of probability.
Results
Growth attributes
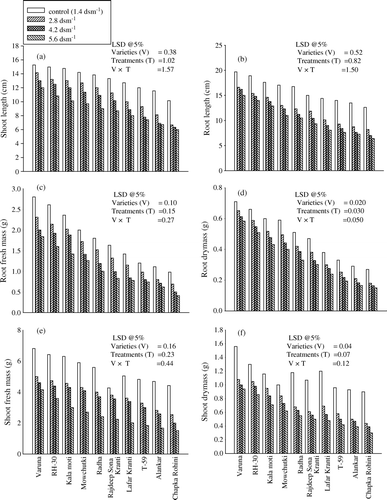
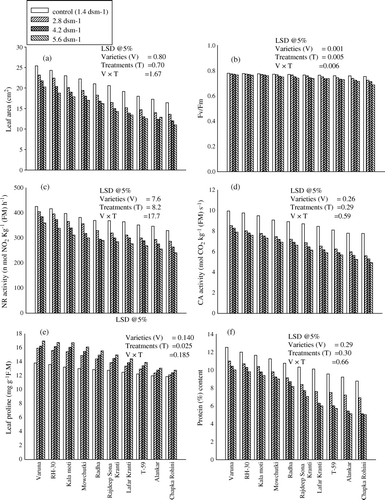
Plants that were raised from the soil amended with different levels of salinity showed significant lower values for all the growth attributes length, fresh and dry mass of root and shoot and leaf area at 30 days after sowing (DAS) (Fig. 3a). The highest level of salinity, i.e., 5.6 dSm−1, caused maximum damage in all the varieties. Among the varieties, Varuna was found to be the most resistant and showed only a 17.74, 39.74 and 20.29% decrease. However, Chapka Rohini experienced a decrease of 44.44, 66.66 and 32.94% in the dry mass of root and shoot and leaf area at highest salinity level (i.e., 5.6 dSm−1), respectively, over their respective controls and will be regarded as most sensitive.
SPAD chlorophyll and photosynthetic attributes
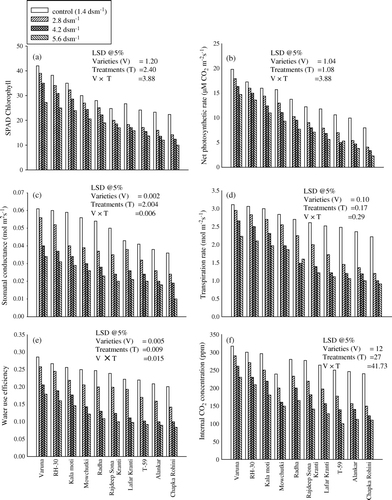
Plants of all the varieties that were raised from the soil amended with different levels of salinity showed a linear decrease in the values of SPAD chlorophyll and photosynthetic attributes (net photosynthetic rate, stomatal conductance, transpiration rate, water use efficiency, internal CO2) and quantum yield of PS II (Fv/Fm) with the increased level of salinity in the soil (, 3b) at 30 DAS. The variety Varuna, RH-30 and Kala Moti exhibited a minimum decrease in response to the highest level of salinity (i.e., 5.6 dSm−1) and decreased the values of SPAD chlorophyll by 35.23%, 34.55% and 31.71%, net photosynthetic rate by 25.69%, 21.20% and 31.25% and the quantum yield of PS II by 1.79%, 1.67% and 1.93%. The response was closely followed by Mowchutki, Radha, Rajdeep Sona Kranti. However, the maximum damage was recorded in Chapka Rohini, at the above-mentioned salinity level.
Nitrate reductase and carbonic anhydrase activities
Data depicted in c and 3d showed that the activity of both the enzymes (NR and CA) in all the varieties differ widely in their response to varied level of salinity. Out of the different salinity levels the lowest (2.8 dSm−1) was least toxic to all the varieties. The varieties Alankar and Chapka Rohini were highly sensitive where the lowest saline level (2.8d Sm−1) caused severe inhibition in the activity of NR and CA. The values of these enzymes were reduced by 15.27 and 23.20% in Alankar and 13.03 and 27.92% in Chapka Rohini below their respective control. Moreover, this degree of damage increased with the increased level of salinity in the soil. The variety Varuna was found to be the most resistant.
Proline content
Proline content of all the varieties increased with an increased level of salinity in the soil. Variety Varuna possessed maximum values for proline content at all the three salinity levels (2.8, 4.2 or 5.6dSm−1) and it was 15.48, 17.58 and 22.86% higher over the control. On the other hand Chapka Rohini possessed the least values at all the above said salinity levels and were 2.10, 4.54 and 7.65% higher over their control plants (e).
Protein content
Enormous variability in the protein content of Brassica juncea genotypes was observed under various levels of salt treatments (f). Protein content of all the varieties showed significant decrease with the increased level of salt in the soil. Variety Chapka Rohini experienced severe damage at different saline level and decreased the protein content by 21.32, 41.73 and 42.87% in response to 2.8, 4.2 and 5.6 dSm−1, over their control plants. Varuna was found to be the most resistant and showed only 20.11% decrease even at the highest level of salinity (5.6 dS m−1). The response was closely followed by RH-30.
Discussion
Photosynthetic system is the backbone of any plant, so if any changes in these attributes occur on being exposed to any type of stress, it can be used as a good stress marker. In the present study we found clear-cut variability among the genotypes of mustard in response to different saline levels in terms of photosynthetic attributes. The order of performance of the genotypes regarding photosynthetic performance is Varuna>RH-30>Kala Moti>Mowchutki>Radha>Rajdeep Sona Kranti>Lafar Kranti>T-59>Alankar>Chapka Rohini. It has been reported that salt damages the photosynthetic machinery at multiple levels, such as pigments biosynthesis (a), stomatal functioning and gaseous exchange, structure and function of thylakoid membrane, electron transport and enzyme activities (Sudhir and Murthy Citation2004). Excess salt causes the closure of stomata, thereby decreasing the partial CO2 pressure (Bethke and Drew Citation1992) as well as internal CO2 concentration (c, 2f) and consequently the activity of CA (d) because its activity is largely regulated by the CO2 concentration (Tiwari et al. Citation2005). CA is the enzyme which catalyzes the reversible hydration of CO2 and maintains it constant supply to RuBPCase, at the level of the grana of the chloroplast (Majeau and Coleman Citation1994). Rubisco is the primary enzyme for carbon fixation which largely regulates the accumulation of photosynthates and energy metabolism. The reported decrease in the activity of CA by salinity is in agreement with other studies (Hayat et al. Citation2007; Ali et al. Citation2008). The damage caused by salt stress can also be attributed to the water stress or a kind of physiological drought generated by NaCl (Hopkins Citation1995) as evident from the decrease in WUE in the present study (e). Likewise, decreases in all these photosynthetic attributes (PN, gs, Ci, E, WUE) in response to salinity has also been reported in Brassica juncea (Hayat et al. Citation2007; Yusuf et al. Citation2008) and Vigna radiata (Hayat et al. Citation2010). Moreover, a linear decrease in the quantum yield of PS II was also observed in genotypes of mustard in the present study, the reason behind this damage is the physiological drought generated by the salinity which may in turn increase the turnover of D2 protein of PS II leading to decreased quantum yield of PS II (b). Similarly, a decrease in the quantum yield of PS II was also observed in Triticum aestivum (Shahbaz et al. Citation2008) and in Vigna radiata (Hayat et al. 2010) on being exposed to salinity.
Plant growth is directly linked with the photosynthetic machinery. The decrease in all the growth attributes (length, fresh and dry mass and leaf area) could be attributed to a reduction in activity of some enzymes (as evident from c and 3d in the present study) besides the delay in cell division induced by decrease in pressure potential (Shafea Citation2003). Another possible cause for this reduction was the synthesis of compounds required for osmotic adjustment (Shafea Citation2003). Kleinkopf and Wallace (Citation1974) and Gale (Citation1975) proposed that growth inhibition under salinity could be partly due to shortage of energy because process involved in transport of salts and repair of salt damage exerted on membrane or proteins are energy consuming. These results are in agreement with those of Ghoulam et al. (Citation2002), who showed that salinity caused a marked reduction in growth parameters of sugar beet plants. Likewise, saline treatment decreased the growth of soybean genotypes (Khan et al. Citation2009), Brassica juncea (Hayat et al. Citation2007; Ali et al. Citation2008; Yusuf et al. Citation2008) and Vigna radiata (Hayat et al. 2010).
All the genotypes of Brassica juncea showed great variability against saline treatment in term of nitrate reductase activity (c). The activity of this enzyme (NR) is determined by several external and internal factors. Campbell (Citation1999) has highlighted at least four factors that include: (i) The availability of substrate (NO3) at the level of cytoplasm, (ii) the level of functional NR (iii) the activity of functional NR, and (iv) the overall metabolic state of the plant. Salinity was found to affect the uptake of nitrate in two different ways: by direct competition of chloride with nitrate, and at the membrane level and/or membrane proteins by changing plasmalemma integrity (Cramer et al. Citation1985). This may have led to restricted nitrate influx, thus decreasing substrate availability. Since nitrate (substrate) is a key regulator of NR (Solomonson and Barber Citation1990), the activity of NR decreased in response to saline stress. Moreover, the degradation/inactivation and reduction in gene expression and NR-protein synthesis in response to NaCl stress (Ferrario et al. Citation1998) may be another cause of lower NR activity. Similarly, salinity decreased the activity of nitrate reductase activity in Vigna radiata (Hayat et al. 2010), Brassica juncea (Yusuf et al. Citation2008) and in chlorella fasca cells (Shafea Citation2003).
The decline in protein content in genotype of Brassica juncea in the present study (f) may be due to increased hydrolysis of protein or it could be due to decreased protein biosynthesis (Irigoyen et al. Citation1992). In the former case, protein synthetic machinery diverted towards the proline accumulation (as evident from e in the present study). Secondly, an enhanced level of proline could be due to its decreased degradation. Comparable observation regarding the increased content of proline have also been reported earlier in response to salt stress in Brassica juncea (Hayat et al. Citation2007; Yusuf et al. Citation2008), Vigna radiata (Hayat et al. 2010) and in soybean (Khan et al. Citation2009).
Conclusion
From the present investigation based on photosynthetic parameters and biochemical markers, it was observed that among the selected varieties of Indian mustard (Varuna, RH-30, Kala moti, Mowchutki, Radha, Rajdeep Sona Kranti, Lafar Kranti, T-59, Alankar, Chapka Rohini), the variety Chapka Rohini was found to be the most sensitive while Varuna was the most resistant to salt stress. Consequently, we suggest that Varuna would be the best Brassica jumcea var., as it is a well known fact that northern rivers precipitate sufficient salt in the upper surface of soil. Irrigation practices further add to soil salinity raising the water table in these zones, adding more salt to the upper and subsoil zone. The coasts of Kuchh, south west and south east India are also highly influenced by the sea-based salinity problem through flooding and estuaries etc. Thus, Varuna could successfully be grown in such areas predisposed to soil salinity with the range of 2.8–5.6 dSm−1 under Indian geo-climatic conditions.
References
- Abdel Ghaffar AS , El-Attar HA , El-Halfaw MH , Abdel-Salam AA. 1982 . Biological nitrogen fixation technology for tropical agriculture . In : Graham PH , Harris SC , Effects of inoculation, nitrogen fertilizer, salinity, and water stress on symbiotic N2 fixation by Vicia faba and Phaseolus vulgaris . Columbia : CIAT , pp 72 – 82 .
- Abdullah , Z and Ahmad , R. 1990 . Effect of pre- and post kinetin treatment on salt tolerance of different potato cultivars growing on saline soils . J Argon Crop Sci. , 165 : 94 – 102 .
- Ali , B , Hayat , S , Fariduddin , Q and Ahmad , A . 2008 . 24-Epibrassinolide protects against the stress generated by salinity and nickel in Brassica juncea . Chemosphere. , 72 : 1387 – 1392 .
- Ashraf , MA , Ashraf , M and Ali , Q . 2010 . Response of two genetically diverse wheat cultivars to salt stress at different growth stages: Leaf lipid peroxidation and phenolic contents . Pak J Bot. , 42 : 559 – 565 .
- Bates , LS , Walden , RT and Tearse , ID . 1973 . Rapid determination of free proline for water stress studies . Plant Soil. , 39 : 205 – 207 .
- Belkhodja , R , Morales , F , Abadila , A , Gomez-Aparisi , J and Abadia , J . 1994 . Chlorophyll fluorescence as a possible tool for salinity tolerance screening in barley (Hordeum vulgare L.) . Plant Physiol. , 138 : 92 – 96 .
- Bethke , PC and Drew , MC . 1992 . Stomatal and nonstomatal components to inhibition of photosynthesis in leaves of Capsicum annuum during progressive exposure to NaCl salinity . Plant Physiol. , 99 : 219 – 226 .
- Booth , WA and Beardall , J . 1991 . Effect of salinity on inorganic carbon utilization and carbonic anhydrase activity in the halotolerant algae, Dunaliella salina (Chlorophyta) . Phycologia. , 30 : 220 – 225 .
- Boyer , JS . 1976 . “ Water deficits and photosynthesis ” . In Water deficit and plant growth , Edited by: Kozlowski , TT . Vol. IV , 153 – 190 . New York : Academic Press .
- Campbell , HW . 1999 . Nitrate reductase structure, function and regulation bridging the gap between biochemistry and physiology . Annu Rev Plant Physiol Plant Mol Biol. , 50 : 277 – 303 .
- Coons , JM and Pratt , RC . 1988 . Physiological and growth responses of Phaseolus vulgaris and P. acutifolius when grown in fields at two levels of salinity . Annu Rep Bean Iprov Coop. , 31 : 88 – 89 .
- Cramer , GR , Lauchli , A and Polito , VS . 1985 . Displacement of Ca2++ by Na+ from the plasmalemma of root cells. A primary response to salt stress? . Plant Physiol. , 79 : 207 – 211 .
- Dwivedi , RS and Randhawa , NS . 1974 . Evaluation of a rapid test of the hidden hunger of zinc in plants . Plant Soil. , 40 : 445 – 451 .
- Everard , JD , Gucci , R , Kann , SC , Flore , JA and Loescher , WH . 1994 . Gas exchange and carbon partitioning in the leaves of celery (Apium graviolense L.) at various level of root zone salinity . Plant Physiol. , 106 : 281 – 292 .
- Ferrario , S , Valadier , M and Foyer , CH. 1998 . Over-expression of nitrate reductase in tobacco delays drought-induced decreases in nitrate reductase activity and mRNA . Plant Physiol. , 117 : 293 – 302 .
- Food and Agriculture Organization of the United Nations (FAO) . 2005 . Global network on integrated soil management for sustainable use of salt-affected soils . Rome : FAO Land and Plant Nutrition Management Service .
- Gale , J. 1975 . “ The combined effect of environmental factors and salinity on plant growth ” . In Plants in saline environments , Edited by: Poljakoff-Mayber , A and Gale , J . 186 – 192 . Berlin : Springer-Verlag .
- Geissler , N , Hussain , S and Koyro , HW . 2009 . Interactive effects of NaCl salinity and elevated atmospheric CO2 concentration on growth, photosynthesis, water relations and chemical composition of the potential cash crop halophyte Aster tripolium L . Environ Exp Bot. , 65 : 220 – 231 .
- Ghoulam , C , Foursy , A and Fares , K . 2002 . Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in the sugar beet cultivars . Environ Exp Bot. , 47 : 39 – 50 .
- Gomez , KA and Gomez , AA . 1984 . Statistical procedures for agriculture research , 2nd ed , New York : John Wiley .
- Hamada , AM. and El-Enany , AE . 1994 . Effect of NaCl salinity on growth, pigment and mineral element contents, and gas exchange of broad bean and pea plants . Biol Plant. , 36 : 75 – 81 .
- Hayat , S , Ali , B , Hassan , SA and Ahmad , A . 2007 . Effect of 28-homobrasinoldie on salinity induced changes in Brassica juncea . Turk J Biol. , 31 : 141 – 146 .
- Hayat S , Hasan SA , Yusuf M , Hayat Q , Ahmad A. 2010 . Effect of 28-homobrassinolide on photosynthesis, fluorescence and antioxidant system in the presence or absence of salinity and temperature in Vigna radiata . Environ Exp Bot . 69 ( 2 ): 105 – 112 .
- Hopkins , WG . 1995 . Introduction to plant physiology , 72 – 73 . New York : John Wiley .
- Irigoyen , JJ , Emerich , DW and Sanchez-Diaz , M . 1992 . Water stress induced changes in concentration of proline and total soluble sugars in nodulated alfalfa (Medicao sativa) plants . Physiol Plant. , 84 : 55 – 64 .
- Jaworski , EG . 1971 . Nitrate reductase assay in intact plant tissues . Biochem Biophys Res Commun. , 43 : 1274 – 1279 .
- Kao , WY , Tsai , TT and Shih , CN . 2003 . Photosynthetic gas exchange and chlorophyll fluorescence of three wild soybean species in response to NaCl treatments . Photosynthetica. , 41 : 415 – 419 .
- Khan F , Siddiqi TO , Mahmooduzzafar , Ahmad A 2009 . Morphological changes and antioxidant defence systems in soybean genotypes as affected by salt stress . J Plant Interact . 4 : 295 – 306 .
- Kirst , GO . 1989 . Salinity tolerance of eukaryotic marine algae . Annul Rev Plant Physiol Plant Mol Biol. , 40 : 21 – 53 .
- Kleinkopf , PA and Wallace , A . 1974 . Physiological basis for salt tolerance in Tamarix romossissimia . Plant Sci Lett. , 3 : 157 – 163 .
- Kuabara , T and Murata , N . 1982 . Inactivation of photosynthetic oxygen evolution and concomitant release of three polypeptids in the photosystem II particles of spinach chloroplasts . Plant Cell Physiol. , 23 : 533 – 539 .
- Lapina , LP and Popov , BA . 1970 . Effect of sodium chloride on photosynthetic apparatus of tomatoes . Fiziol Rast , 17 : 580 – 584 .
- Lauchli , A . 1984 . “ Salinity tolerance in plants strategies for crop improvement ” . In Salt exclusion: An adaptation of legumes for crops and pastures under saline conditions , Edited by: Staples , RC and Toenniessen , GH . 171 – 188 . New York : Wiley .
- Lowry , OH , Rosebrough , NJ , Farr , AL and Randall , RJ . 1951 . Protein measurement with folin phenol reagent . J Biol Chem. , 193 : 265 – 275 .
- Majeau , N and Coleman , JR . 1994 . Correlation of carbonic anhydrase and ribulose-1,5-bisphosphate carboxylase/oxygenase expression in pea . Plant Physiol. , 104 : 1393 – 1399 .
- Masojidek , J and Hall , DO . 1992 . Salinity and drought are amplified by high irradiance in sorghum . Photosynthetica. , 27 : 159 – 171 .
- Mishra , SK , Subrahmanyam , D and Singhal , GS . 1991 . Inter-relationship between salt and light stress on the primary processes of photosynthesis . J Plant Physiol. , 138 : 92 – 96 .
- Miyao , M and Murata , N . 1983 . Partial disintegration and reconstitution of the photosynthetic oxygen evolution system. Binding of 24 kilodalton and 18 kilodalton polypeptides . Biochim Biophys Acta , 725 : 87 – 93 .
- Moreno-Limón , S , Maiti , RK and Forough bakhch , R . 2000 . Genotypic variability in Phaseolus bean cultivars exposed to salinity at the germination stage . Crop Res. , 19 : 487 – 492 .
- Murata , N , Mohanty , PS , Hayashi , H and Papageorgiou , GC . 1992 . Glycine betaine establishes the association of extrinsic proteins with the photosynthetic oxygen evolving complex . FEBS Lett. , 296 : 187 – 189 .
- Parida , AK , Das , AB and Mittra , B . 2003 . Effects of NaCl on the structure, pigment complex composition, and photosynthetic activity of mangrove Bruguiera parviflora chloroplasts . Photosynthetica. , 41 : 191 – 200 .
- Seemann , JR and Critchley , C . 1985 . Effects of salt stress on the growth, ion content, stomatal behavior and photosynthetic capacity of a salt-sensitive species, Phaseolus vulgaris L . Planta. , 164 : 151 – 162 .
- Shafea , AA . 2003 . Growth and nitrate reductase activity of Chlorella fusca as affected by long-term salinity . Biol Plant. , 46 : 423 – 427 .
- Shahbaz , M , Ashraf , M and Athar , HR . 2008 . Does exogenous application of 24-epibrassinolide ameliorate salt induced growth inhibition in wheat (Triticum aestivum L.)? . Plant Growth Regul. , 55 : 51 – 64 .
- Singh , AK and Dubey , RS . 1995 . Change in chlorophyll a and b contents and activities of photosystems 1 an 2 in rice seedlings induced by NaCl . Photosynthetica. , 31 : 489 – 499 .
- Solomonson , LP and Barber , MJ . 1990 . Assimilatory nitrate reductase functional properties and regulation . Ann Rev Plant Physiol Mol Biol. , 41 : 225 – 253 .
- Soussi , M , Ocana , A and Lluch , C . 1998 . Effects of salt stress on growth, photosynthesis and nitrogen fixation in chickpea (Cicer arietinum L.) . J Exp Bot. , 49 : 1329 – 1337 .
- Subbarao GV , Johansen C 1991 . Comparative salinity responses among pigeon pea genotypes and their wild relatives . Crop Sci . 31 : 415 – 418 .
- Sudhir P , Murthy SDS . 2004 . Effects of salt stress on basic processes of photosynthesis . Photosynthetica . 42 : 481 – 486 .
- Tiwari , A , Kumar , P , Singh , S and Ansari , SA . 2005 . Carbonic anhydrase in relation to higher plants . Photosynthetica. , 43 : 1 – 9 .
- Tiwari , BS , Bose , A and Ghosh , B . 1997 . Photosynthesis in rice under a salt stress . Photosynthetica. , 34 : 303 – 306 .
- Yusuf , M , Hasan , SA , Ali , B , Hayat , S , Fariduddin , Q and Ahmad , A . 2008 . Effect of salicylic acid on salinity-induced changes in Brassica juncea . J Integ Plant Biol. , 50 : 1 – 7 .
- Zhao , J , Ren , W , Zhi , D , Wang , L and Xia , G . 2007 . Arabidopsis DREB1A/CBF3 bestowed transgenic tall rescue increased tolerance to drought stress . Plant Cell Rep. , 26 : 1521 – 1528 .
- Zobayed , SMA , Afreen , F and Kozai , T . 2005 . Temperature stress can alter the photosynthetic efficiency and secondary metabolite concentrations in St. John's wort . Plant Physiol Biochem. , 43 : 977 – 984 .