Abstract
To better understand how mammalian herbivory affects tallgrass prairie, I set up field exclosures to test effects of above-ground herbivory (AGH) (Bison bison L. is a potential above-ground herbivore), below-ground herbivory (BGH) (Geomys bursarius and Spermophilus tridecemlineatus are potential below-ground herbivores), and the interaction of the exclusion of both, on tallgrass prairie plant species, plant community parameters, and soil parameters in the Flint Hills of Northeastern Kansas. I found that (1) AGH reduced the cover of some forbs but increased the cover of others while both AGH and BGH decreased the cover of dominant C4 grasses, (2) no treatments significantly affected species richness but ABH reduced total cover and maximum height of the vegetation while BGH reduced species evenness and maximum height, and (3) no treatments affected soil pH or soil nitrogen but BGH reduced soil organic matter, soil phosphorus, and soil potassium. Whereas above-ground mammalian herbivory has significant effects on individual plant populations and plant community structure, it is BGH that mainly affects soil parameters. Results suggest that for prairie patches where both kinds of herbivory are present, these effects may be additive leading to cumulative effects on tallgrass prairie plants both directly, through herbivory, and indirectly, through soil changes.
Introduction
Tallgrass prairies are found throughout the world and are one of the major terrestrial ecosystems of the mid-continental United States (US) where they lie in a broad tract bordering deciduous forest on the east and shortgrass prairies on the west (Risser et al. Citation1981; Knapp et al. Citation1998). The US tallgrass prairie has been intensely studied for decades (Collins and Adams Citation1983; Glenn and Collins Citation1993, Myster Citation2006; Haught and Myster Citation2008; Weatherford and Myster in press) and serves as flagship long-term ecological research (LTER) sites of the National Science Foundation (Konza Prairie: Knapp et al. 1998). Much of that research has focused on the patch dynamics and composition of the vegetation, especially the dominant grasses (Collins and Adams Citation1983; Polley and Collins Citation1984; Collins and Uno Citation1985; Glenn and Collins Citation1993; Howe and Brown Citation1999; Silletti et al. Citation2004).
Mammalian herbivory has long been known as a major mechanism producing these patch dynamics, especially above-ground herbivory (AGH) by large mammals such as the bison (Bison bison L: Walter Citation1973; Knapp et al. Citation1998, Citation1999; Hickman and Hartnett Citation2002; Bakker et al. Citation2006). Bison came to dominate the tallgrass prairie in the early-Holocene (Knapp et al. Citation1999). Later as Native Americans moved in, they viewed the Bison as a gift from the Creator which could be hunted (within limits) only in the larger context of the coexistence of all living things, necessary for the continuation of all (Cornell Citation1990). White men, however, saw the Bison as a resource to be exploited. The resulting near-extinction of the Bison was accompanied by the cultivation of the plains as humans fragmented (then as now) the once boundless prairie.
Above-ground grazing has a long evolutionary history in grasslands and, consequently, multiple effects on ecosystems (Milchunas et al. Citation1988) such as reducing (1) invasion by exotic species, (2) plant root/shoot ratios, (3) root growth, (4) succession rate, and (5) vegetation cover (Milchunas et al. Citation1998; Johnson and Matchett Citation2001), but increasing (1) plant richness by reducing the abundance of dominant grasses (Polley and Collins Citation1984, Knapp et al. Citation1999), (2) the availability of plant resources (especially Nitrogen (N): Fahnestock and Knapp Citation1994), and (3) above-ground net primary productivity (ANPP) for some species (Milchunas et al. Citation1998) at some herbivory levels (Dyer et al. Citation1993), and modifying competitive interactions among plants due to N deposition (Polley and Collins Citation1984).
The majority of herbivory in US grasslands occurs below the soil surface, however (Ingham and Detling Citation1986), where it merits further attention. This not only includes herbivory by arthropods and nematodes (Seastedt et al. Citation1988), but also by mammals (e.g., the pocket gopher Geomys bursarius, the 13-lined ground squirrel Spermophilus tridecemlineatus (Thorne and Andersen Citation1990; McMillian et al. Citation1997) who live in the soil-eating plants, turn the soil over (up to 30% of a prairie ground surface can be turned over every year: Kyle et al. Citation2008), and, consequently, change prairie plant recruitment, composition (Forbis et al. Citation2004), biomass, and species richness (Rogers et al. Citation2001). Soil turnover by itself has been demonstrated to enhance invasive plant establishment (D'Antonio et al. Citation1999) and productivity (Collins & Steinauer Citation1998). Indeed, rodent mound patches typically have a different plant composition (more forbs and annuals, less perennial grasses) than the surrounding prairie (Martinsen et al. Citation1990) due to different environmental conditions including exposure of deeper soil layers. Together these two types of herbivory may have synergistic, interactive effects, for example, when small mammals increase in patches that do not have grazing and then cause large community effects due to their dietary selectivity (Grant et al. Citation1982, Milchunas et al. Citation1998).
Because the combined and interactive effects of above-ground and below-ground herbivory by mammals needs to be further investigated in tallgrass prairie (see Gibson et al. Citation1990), field exclosures were setup in a tallgrass prairie in Kansas to address these specific questions: (1) How are individual prairie plant species affected by above-ground mammalian herbivory, by below-ground mammalian herbivory, and by both acting together? (2) How are the plant community parameters of total cover, species richness, species evenness, and above-ground biomass affected by these treatments? (3) How is soil carbon and nutrient availability affected by these treatments? and (4) What do these plant and soil responses imply about how mammals, both above-ground and below-ground, structure tall grass prairie and influence its patch dynamics?
Materials and methods
The study site was the Konza Prairie Biological Station (39°08'N, 96°62'W), a LTER site funded by the National Science Foundation (Knapp et al. Citation1998). Konza is a 3487 ha native tallgrass prairie preserve located in southeastern Kansas (Kula et al. Citation2005) dominated by C4, warm-season grasses such as Andropogen gerardii (big bluestem), Schizachyrium scoparius (little bluestem), Panicum virgatum (switchgrass), and Sorghastrum nutans (indiangrass). Konza receives 835 mm of precipitation per year with a resulting ANPP in an approximate range of 400–600 g/m2 depending on elevation, season, and year (see Chapter 12 in Knapp et al. Citation1998). This experiment took place on an upland area (watershed N4C, grid coordinate F23: ) with Florence soil composed of shallow, rocky, cherty, silty, clay loam (Fahnestock and Knapp Citation1994). The experimental area is also burned every four years in the spring and during the experiment was grazed by bison (Bison bison L) year-round.
Figure 1. Konza Prairie Biological Station divided into watersheds. The experiment took place in watershed N4C which was grazed by native Bison, grazed by cattle between May and October, and burned in the spring every four years.
In July 2003, I experimentally separated the effects of above-ground and below-ground herbivores on tallgrass prairie by setting out these treatments in the study area: (1) a 3.1×3.1 m control plot without treatment, (2) a 3.1×3.1 m plot enclosed by cattle pens held in place by iron fence posts to exclude above-ground mammals, (3) a 3.1×3.1 m plot enclosed by metal trenching of 40 cm below-ground and 15 cm above-ground to exclude below-ground mammals, and (4) a 3.1× 3.1 m plot having a combination of the previous two treatments to exclude both above-ground and below-ground mammals.
In November 2008, I harvested the experiment by first dividing each 3.1× 3.1 m area into nine 0.9×0.9 m replicate subplots (Collins Citation1987) in the center of each 3.1× 3.1 m plot leaving a 20 cm buffer zone between the subplots and the border of the large plots. Five subplots were then chosen randomly among each group of nine subplots for sampling. Within each five subplots, I identified and scored percent cover of all plants (nomenclature followed Hitchcock Citation1971; Towne Citation2002), and also measured the maximum height of the vegetation as a correlate of the above-ground biomass. In addition, three of the five sampled subplots were randomly selected and a soil sample was taken in each using a 12 cm diameter metal ring to a depth of 10 cm. Soil samples were taken to the Oklahoma State University soil laboratory located next to the campus of Oklahoma State University in Oklahoma City and analyzed for percent organic matter content, and for the plant nutrients extractable phosphorus (P),% total nitrogen (N), and extractable potassium (K). Finally,% total carbon (C) was computed from organic matter dividing by 1.724 (Ray Ridlen, pers. comm.) and C/N ratio was computed by dividing% total carbon by% total N.
A two-way analysis of variance (ANOVA: SAS Citation1985) was used to statistically analyze the data with exclusion of above-ground mammals as one main effect, exclusion of below-ground mammals as the other main effect, and interaction between these two main effects were also investigated. The response variables of the ANOVA for the plant data were (1) total cover, (2) species richness, (3) species evenness (using Pielou's J index: Ludwig and Reynolds Citation1988), and (4) maximum height, and for the soil data (1) pH, (2) P, (3) K, (4)% total N, (5)% total C, and (6) C/N ratio. If ANOVA results were significant, means testing was performed using the Ryan-Elinot-Gabriel-Welsch multiple range test (SAS Citation1985).
Results
The control plots were dominated by Andropogon gerardii, Aster ericoides, Schizachyrium scopariumand Sorghastrum nutans, the plots without AGH were dominated by Andropogon gerardii, Aster ericoides, Schizachyrium scoparium, and Rosa multiflora, the plots without BGH were dominated by Andropogon gerardii, Panicum virgatum, and Schizachyrium scoparium, and the plots without either AGH or BGH were dominated by Andropogon gerardii, Panicum virgatum, Schizachyrium scoparium, and Solidago canadensis (). The dominant C4 grasses Andropogon gerardii and Schizachyrium scoparium were found in all treatments but dominant C4 grass Panicum virgatum was not found in the control plots and the interaction plots, and dominant C4 grass Sorghastrum nutans was found only in the control plots. The dominant herb Aster ericoides was not found in both plots without BGH and the woody plant Rosa multiflora was only found in plots with ABH ().
Table 1. Percent cover for all plant species sampled in the subplots (n=4) summed by treatment with these abbreviations: no above-ground herbivory (AGH), no below-ground herbivory (BGH). Life-forms include grasses (C4, C3), forbs (F), and woody plants (W).
Maximum height was greatest in the plots without any herbivory, but those plots also had the smallest evenness (). Total percent cover was significantly different between plots that did or did not have AGH (), where plots without AGH showed close to twice as much cover (a). Evenness was significant for plots that differed by having or not having BGH (), where having BGH leads to a more even distribution of species (b). Evenness also showed a significant interaction () where plots without ABH were most even (c). Maximum height was significantly different between plots that had AGH vs. those that did not () where not having AGH tripled the height of the vegetation (a). There was also a significant interaction term () where plots without any herbivory had the tallest vegetation (b).
Figure 2. (A) Mean and standard error of the total percent cover of subplots by not having above-ground herbivory (AGH) vs. having AGH. (B) Mean and standard error of the species evenness of subplots by having BGH vs. not having BGH, and (C) Mean and standard error of the species evenness of subplots by AGH×BGH interaction. Bars having different letters were significantly different.
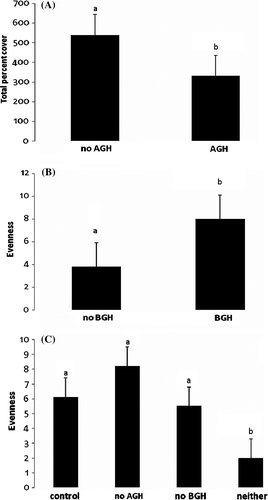
Figure 3. Mean and standard error of the maximum height in meters of subplots by (A) having AGH vs. not having AGH, and (B) AGH×BGH interaction. Bars having different letters were significantly different.
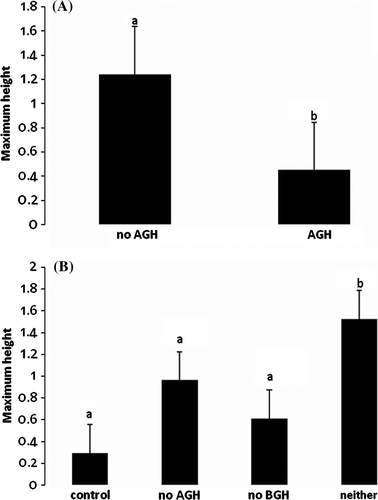
Table 2. Plant community parameters. F statistic summary table for the two-way ANOVA performed with a main effect of above-ground herbivory exclusion, a main effect of below-ground herbivory exclusion, and the interaction effect.
For the soils (), P was also significantly increased by an exclusion of below-ground herbivores (; a) where the additive effect of exclusion of above-ground herbivores also leads to a gain in P (b). Finally, exclusion of below-ground herbivores leads to a significant increase in K (a) and a significant gain in% total carbon (b).
Figure 4. Mean and standard error of P by (A) having BGH vs. not having BGH and (B) AGH ×BGH interaction.
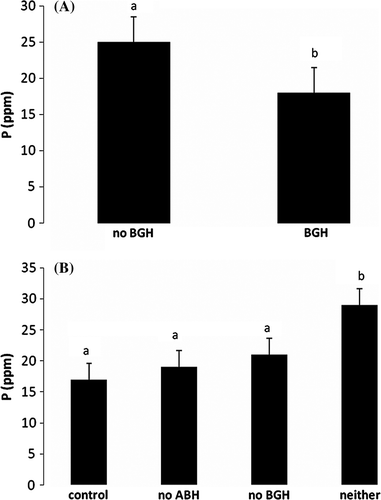
Figure 5. Mean and standard error of (A) K by having BGH vs. not having BGH and (B)% total C by having BGH vs. not having BGH.
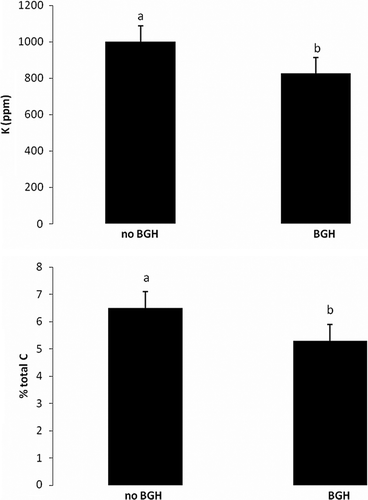
Table 3. Mean±standard error (n=3) of select soil parameters.
Table 4. Soil parameters. F statistic summary table for the two-way ANOVA performed with a main effect of above-ground herbivory exclusion, a main effect of below-ground herbivory exclusion, and the interaction effect.
Discussion
Plant responded individualistically (Gleason Citation1926) to all treatments where the specific plant species sampled in the control plots were similar to other samplings at Konza (Knapp et al. Citation1998). While exclusion of above-ground herbivores increased cover, exclusion of below-ground herbivores only increased cover when applied with exclusion of the above-ground herbivores. These exact same treatment patterns were seen for maximum height of the vegetation. Taken together, these results suggest that above-ground productivity is reduced by AGH, unaffected by BGH by itself, but greatly reduced by both acting together in a synergistic interaction. So these treatments cannot be looked on as the low-to-moderate grazing that has been seen to increase ANPP elsewhere (Risser et al. Citation1981; Dyer et al. Citation1993) and suggest that tallgrass prairie patches that are subjected to both kinds of herbivory may suffer an added reduction in production.
There were no significant effects of the treatments on species richness, but ABH did increase the cover of some forbs while decreased others with a reduced abundance of C4 grasses (Fahnestock and Knapp Citation1994; Hartnett et al. Citation1996; Knapp et al. Citation1998). There were no effects due to below-ground herbivores (Hobbs and Mooney Citation1985). Evenness was the only community parameter that showed a significant effect of BGH (it increased) but both types of herbivory working together significantly decreased it. AGH had no effect on evenness (but see Knapp et al. Citation1998).
Levels of % total N and % total C were within the ranges previously sampled at Konza (Knapp et al. Citation1998). N was low in all plots and unaffected by herbivory treatments. All other soil parameters had an increase when below-ground herbivores were excluded. For P that effect increased when above-ground herbivores were also excluded. Together, results show that AGH affects plant parameters more than BGH, with the effects reversed for soil parameters. Results agreed with other prairie studies in showing that grazing reduced C4 grasses (Penfound Citation1955; Belsky Citation1992, Hartnett et al. Citation1996). However, it may yet be true that differential herbivory can provide a competitive advantage to the least damaged, least palatable species (Clay et al. Citation1993) where the distribution of most prairie species is controlled by herbivory not competition (Fowler Citation2002).
Conclusions
Results suggest how mammalian herbivory can affect plant and soil patch composition. Consequently as individual herbivores move and feed, and as their population numbers change, the patch dynamics of the tallgrass prairie will also change. Managers have manipulated numbers of Bison population structure easily for years and also controlled where they are allowed to roam and feed. By increasing Bison densities there should be a decrease in the dominant C4 grasses and C3 grasses, but an increase in forbs and woody plants. Managing soil mammals is more difficult but fencing and selective use of chemicals may be effective. An increase in those densities should produce patches that are also lower in C4 grasses and C3 grasses but at a lower level then when increasing Bison, with no real changes in forbs and woody plants. Finally, soil patches are most affected by BGH where below-ground mammals may produce patches of reduced C, reduced P, and reduced K.
Acknowledgements
I thank Eva Horn for her help in the field and John Blair and the Konza staff for logistic support in setting up the experiment. I also thank John Blair for commenting on a previous draft of the manuscript.
References
- Bakker , ES , Ritchie , ME , Olff , H , Milchunas , DG and Knops , JHM . 2006 . Herbivore impact on grassland plant diversity depends on habitat productivity and herbivore size . Ecol Let. , 9 : 780 – 788 .
- Belsky , AJ . 1992 . Effects of grazing, competition, disturbance and fire on species composition and diversity in grassland communities . J Veg Sci. , 3 : 187 – 200 .
- Clay , KS , Marks , S and Cheplick , GP . 1993 . Effects of insect herbivory and fungal endophyte infection on competition interactions among grasses . Ecology. , 74 : 1767 – 1777 .
- Collins , SL . 1987 . Interaction of disturbances in tallgrass prairie: a field experiment . Ecology. , 68 : 1243 – 1250 .
- Collins , SL and Adams , DE . 1983 . Succession in grasslands: thirty-two years of change in a central Oklahoma tall-grass prairie . Vegetatio. , 51 : 181 – 190 .
- Collins SL , Steinauer LG. 1998 . Disturbance, diversity and species interactions . In: Knapp AK , Briggs JM , Hartnett DC , Collins SL , Grassland dynamics: long-term ecological research in Tallgrass prairie . Oxford : Oxford University Press . p. 34 – 45
- Collins , SL and Uno , GE . 1985 . Seed predation, seed dispersal and disturbance in grasslands: a comment . Am Nat. , 125 : 866 – 872 .
- Cornell , G . 1990 . Native American perceptions of the environment . NE Indian Quarterly. , 7 : 3 – 13 .
- D'Antonio , CM , Dudley , TD and Mack , M. 1999 . “ Disturbance and biological invasion: direct effects and feedbacks ” . In Ecosystems of the word 16 , Edited by: Walker , LR . 413 – 452 . New York, NY : Elsevier .
- Dyer , MI , Turner , CL and Seastedt , TR . 1993 . Herbivory and its consequences . Ecol Appl. , 3 : 10 – 16 .
- Fahnestock , JT and Knapp , AK . 1994 . Plant responses to selective grazing by bison: interactions between light, herbivory, and water stress . Vegetatio. , 115 : 123 – 131 .
- Forbis , TA , Larmore , J and Addis , E . 2004 . Temporal patterns in seedling establishment on pocket gopher disturbances . Oecologia. , 138 : 112 – 121 .
- Fowler , NL . 2002 . The joint effects of grazing, competition, and topographic position on six savanna grasses . Ecology. , 83 : 2477 – 2488 .
- Gleason , HA . 1926 . The individualistic concept of the plant association . Bull Torrey Bot Club. , 53 : 7 – 26 .
- Glenn , SM and Collins , SL . 1993 . Experimental analysis of patch dynamics in tall-grass prairie plant communities . J Veg Sci. , 4 : 157 – 162 .
- Gibson , PJ , Freeman , CC and Hulbert , LC . 1990 . Effects of small mammal and invertebrate herbivory on plant species richness and abundance in tallgrass prairie . Oecologia. , 84 : 169 – 175 .
- Grant , WE , Birney , EC , French , NR and Smith , DM . 1982 . Structure and productivity of grassland small mammal communities related to grazing-induced changes in vevgeation cover . J Mam. , 63 : 248 – 260 .
- Hartnett , DC , Hickman , KR and Walter , LEF . 1996 . Effects of bison grazing, fire and topography on floristic diversity in tallgrass prairie . J Ran Man. , 49 : 413 – 420 .
- Haught , JA and Myster , RW . 2008 . Effects of species, density, season and prairie-type on seed predation in Oklahoma . Am Midl Nat. , 159 : 482 – 488 .
- Hickman , KR and Hartnett , DC . 2002 . Effects of grazing intensity on growth, reproduction, and abundance of three palatable forbs in Kansas tallgrass prairie . Plant Ecol. , 159 : 23 – 33 .
- Hitchcock , A.S. 1971 . Manual of the grasses of the United States , New York, NY : Dover .
- Hobbs , RJ and Mooney , HA . 1985 . Community and population dynamics of serpentine grassland annuals in relation to gopher disturbance . Oecologia. , 67 : 342 – 351 .
- Howe , HF and Brown , JS . 1999 . Effects of birds and rodents on synthetic tallgrass communities . Ecology. , 80 : 1776 – 1781 .
- Ingham , RE and Detling , JK . 1986 . Effects of defolition nd nematode consumption on growth and leaf gas exchange in Bouteloua curtipendula . Oikos. , 46 : 23 – 28 .
- Johnson , LC and Matchett , JR . 2001 . Fire and grazing regulate belowground processes in tallgrass prairie . Ecology. , 82 : 3377 – 3389 .
- Knapp AK , Briggs JM , Hartnett DC , Collins SL. 1998 . Grassland dynamics: long-term ecological research in Tallgrass prairie . Oxford : Oxford University Press .
- Knapp , AK , Blair , JM , Briggs , JM , Collins , SL , Hartnett , DC , Johnson , LC and Towne , EG . 1999 . The keystone role of Bison in North American tallgrass prairie . BioScience. , 49 : 39 – 50 .
- Kula , AAR , Hartnet , DC and Wilson , GWT . 2005 . Effects of mycorrhizal symbiosis on tallgrass prairie plant-herbivore interactions . Ecol Let. , 8 : 61 – 69 .
- Kyle , CP , Kulmatiski , A and Benrd , KH . 2008 . Influence of pocket gopher mounds on nonnative plant establishment in a shrub-steppe ecosystem . W N Am Nat. , 68 : 374 – 383 .
- Ludwig JA , Reynolds JF 1988 . Statistical ecology: a primer on methods and computing . New York : Wiley & sons .
- Martinsen , GD , Cushman , JH and Whitham , TG . 1990 . Impact of pocket gopher disturbance on plant species diversity in a shortgrass prairie community . Oecologia. , 83 : 132 – 138 .
- McMillian , BR , Kaufman , DW , Kaufman , GA and Matcock , RS . 1997 . Mammals of Konza Prairie: new observations and an updated species list . Pra Nat. , 29 : 263 – 271 .
- Milchunas , DG , Laueuroth , WK and Burke , IC . 1998 . Livestock grazing: animal and plant biodiversity of shortgrass steppe and the relationship to ecosystem function . Oikos. , 83 : 65 – 74 .
- Milchunas , DG , Sala , OE and Laueroth , WK . 1988 . A generalized model of the effects of grazing by large herbivores on grassland community structure . Am Nat. , 132 : 87 – 106 .
- Myster , RW . 2006 . Species-specific effects of grass litter mass and type on emergence of three tall grass prairie species . Ecoscience. , 13 : 95 – 99 .
- Penfound , WT . 1955 . The relation of grazing to plant succession in the tallgrass prairie . Ecology. , 36 : 86 – 94 .
- Polley , HW and Collins , SL . 1984 . Relationships of vegetation and environment in Buffalo wallows . Am Midl Nat. , 112 : 178 – 186 .
- Risser PG , Birney EC , Blocker HD , May SC , Parton WJ , Wiens JA. 1981 . The true prairie ecosystem . Stroudsburg, PA : Hutchinson Ross .
- Rogers , WE , Hartnett , DC and Elder , B . 2001 . Effects of plains pocket gopher (Geomys bursarius) on tallgrass prairie plant community structure . Am Midl Nat. , 145 : 344 – 357 .
- SAS 1985 . User's guide: statistics . Version. 5 ed. Cary, NC : SAS Institute .
- Seastedt , TR , Ramundo , RA and Hayes , DC . 1988 . Maximization of densities of soil animals by foliage herbivory: empirical evidence, graphical and conceptual models . Oikos. , 51 : 243 – 248 .
- Silletti , AM , Knapp , AK and Blair , JM . 2004 . Competition and coexistence in grassland codominants: responses to neighbor removal and resource availability . Can J Bot. , 82 : 450 – 460 .
- Thorne , DH and Anderson , DC . 1990 . Long term soil disturbance pattern by a pocket gopher, Geomys bursarius . J Mam. , 71 : 84 – 89 .
- Towne , EG . 2002 . Vascular plants of Konza Prairie Biological Station: an annotated checklist of species in a Kansas tallgrass prairie . Sida. , 20 : 269 – 294 .
- Walter H. 1973 . Vegetation of the earth and the ecological systems of the geo-biosphere . New York, NY : Springer-Verlag .
- Weatherford JL Myster RW in press Effects of species, water, and nitrogen on competition among three Prairie Grasses . Prairie Naturalist .