Abstract
Cuscuta is a stem holoparasitic plant without leaves or roots, which develops a haustorium and sucks nutrients from host plants. The genus Cuscuta comprises about 200 species, many of which can cause severe problems for certain crops. The parasitic process in Cuscuta begins in finding and attaching to a host plant and then developing a haustorium. The process does not always require any chemical signal, but does require a light signal. Finding a host involves detecting the lower red light:far-red light ratio near a potential host plant by phytochrome. A contact signal is also necessary for haustorium induction. Apparently, cytokinin increase is downstream of the light and contact signal and is critical for haustorium induction. This pathway, however, appears to be slightly different from a standard pathway. The direct connection between Cuscuta and its host involves both the xylem and phloem, and mRNA and proteins can translocate. Several features indicate that Cuscuta is a useful model plant for parasite plant research as well as plant–plant interaction research. These include the simple anatomical structure and seedling development, no chemical requirement for haustorium induction, and the wide range of host plants.
Introduction
Begon et al. (Citation2006) define parasites as organisms that obtain nutrients from a host and cause harm but not immediate death. The term ‘pathogen’ may be applied to any parasite that gives rise to a disease. Symbiotic relationships (living together), in contrast, refer to physical proximity; while mutualistic interactions do not necessarily involve physical relationships. In addition, a mutualistic interaction means a conflict-free relationship.
Some higher plants also have strategies that can be interpreted as being parasitic. These include myco-heterotrophic plants, twining plants, and carnivorous plants.
Myco-heterotrophic plants are typically considered to be mutualistic. Some have no leaves and extremely reduced roots (e.g. Burmannia tenella) (Leake Citation1994). Superficially, certain morphological characteristics resemble those in other parasite plants. Myco-heterotrophic plants have even been misunderstood as root parasites at their early stage (Bidartondo Citation2005). Epiparasitic myco-heterotrophic plants appear to be evolved from mutualistic myco-heterotrophic plants (Merckx et al. Citation2009). However, both groups of myco-heterotrophic plants absorb nutrients and water by way of fungi on the host green plants, without developing haustoria. Host specificity is related to the generalist behavior of arbuscular mycorrhizal fungi and is not always related to the myco-heterotroph itself (Bidartondo et al. Citation2002). One idea is that root parasites evolve from myco-heterotrophic plants, but the taxonomic relationships do not support this (Heide-Jørgensen Citation2008).
Some parasitic plants including Cuscuta twine to host plants, leading to comparisons with twining/climbing plants. Nevertheless, the main strategy of twining/climbing plants is to effectively acquire sunlight; they reduce the sunlight for the plant, and this is therefore not mutualistic. Twining plant/climbing plants have leaves and roots but no haustorium to obtain nutrients. Although certain ivy species develop sucker-like organs, these have no ability to suck nutrients (Heide-Jørgensen Citation2008).
Carnivorous plants evolved independently at least six times and are specialised on insects. Acquiring nutrients from insects (Bauer and Federle Citation2009) resembles the parasitic plant strategy, but with one major difference. Although pitcher development in Nepenthes is energetically less expensive than developing a normal green leaf, photosynthesis is strongly correlated with the uptake of carbon and nitrogen from digested insects (Ellison and Gotelli Citation2009). Although insect nutrients are important for photosynthesis, such nutrients alone are insufficient for survival. In contrast, many parasitic plants especially holoparasitic plants fully rely on host nutrients, and energy from photosynthesis appears to be secondary.
Despite of some similarity to other higher plants, parasitic plants have a unique strategy. The interaction between holoparasitic plants (e.g. Cuscuta) and host plants covers the full range from parasitic or even strong pathogenic interactions to mutualistic interactions (Runyon et al. Citation2008), although the latter appear to be very rare.
Recognition of parasitic plants itself was at a quite early time. According to Heide-Jørgensen (2008), Theophrastus (BC 372–287) is the first person who described Cuscuta in Babylon, as a parasite plant. After the light microscope was introduced into botanic research in the 1800s, parasite plant research mostly focused on the anatomy of the haustorium (Boewig 1898; Kindermann Citation1928). The haustorium of parasite plants is unique and posed an enigma as to how nutrients were absorbed from the host. The next step involved observing the host–parasite plant connection. Hibberd and Jeschke (Citation2001) stated that Solms-Laubach had already reviewed different types of parasite–host plant connection (nutrient absorption from xylem or phloem) in 1867. Despite the recognition of parasite plants at an early stage, studies tended to focus on each specific parasite plant, and no comprehensive study for all parasite plants was available until 1969 (Kuijt Citation1969). Biochemical approaches for parasite–host plant interactions are technically difficult, delaying research. Direct measurement of solute in xylem and phloem is complicated (Jeschke and Hilpert Citation1997), leading to other approaches such as those involving sap-sucking insects (Malone et al. Citation1999). Radiotracer approaches were also used to investigate the carbon and nitrogen flow from host to parasite (Govier et al. Citation1967, Citation1968; Walting and Press Citation2001). More recently, such radiotracers have been replaced by xylem- and phloem-specific fluorescence reagents (e.g. Texas red and Carboxyfluorescein) (Haupt et al. Citation2001; Christensen et al. Citation2003; Birschwilks et al. Citation2007).
Parasite plants have also been studied from the plant physiology/pathology point of view. Indeed, ‘mistletoe’ was recognized as the first plant pathogen by Albertus Magnus around 1200 (Agrios Citation2005). Since then, dodder (e.g. Cuscuta and Cassytha), witchweed (Striga), broomrapes (Orobanche), and mistletoes (Viscum) have been recognized as parasitic higher plants causing agricultural damage (Agrios Citation2005). In the case of bacteria, fungi, nematodes, and virus, the interaction with the plant will cause a clear pathogenic response (Baker et al. Citation1997; Chisholm et al. Citation2006; Jones and Dangl Citation2006). Such plant–pathogen interactions involve ligand-receptor mechanisms for pathogen recognition (elicitor signal transduction) and induction of signal transduction. Nonetheless, parasite plants are clearly plants and have the same plant hormonal system and physiological response. This implies that host plants would not always be able to use the same defense strategy against parasite plants. This consideration gave rise to discussions about comparing parasite plants with herbivores (Pennings and Callaway Citation2002). Although parasite plants have been recognized as weeds that cause agricultural problems, triggering some interest (Prider et al. Citation2009; Vurro et al. Citation2009), parasitization does not always negatively influence the host plant. For example, tomatoes parasitized by Cuscuta altered certain plant hormones (e.g. salicylic acid) and can influence their defense system against insect herbivores (Runyon et al. Citation2008).
Among parasitic plants, there are some advantages to focus research on the holoparasitic plant Cuscuta. Its simple anatomical structure makes Cuscuta suitable for biochemical experiment. For instance, there is no need to separate the plants into several tissues because there are no leaves or roots. Seed germination requires only water, and seedling requires no contact with soil or any chemicals, helping to minimize contamination in biochemical experiments. In particular, the lack of a need to use any chemicals ‘in vitro’ (e.g. plant hormone) makes the system perfectly suited for metabolomics research (Weckwerth Citation2003). Moreover, the wide range of hosts means that Cuscuta is a ‘generalist’, but this is not typical in other host-specific parasitization relationships. Intriguingly, the Cuscuta parasitization strategy is host-unspecific. Hence, it is possible to conduct plant–plant interaction research with a wide range of host plants. Given these characteristics as well as the relevance of Cuscuta as a major plant parasite it is an important system for future research on plant–plant interaction.
However, there are some knowledge gaps. Firstly, many ideas and data obtained from root parasite plants, such as Striga, and stem parasite plants (Cuscuta and mistletoes) were sometimes mixed in earlier reviews. Parasite plants have diversified, and the evolution of Cuscuta appears to be completely different from other root parasite plants (Heide-Jørgensen Citation2008). This underlines the need to review Cuscuta separately. Another point is the haustorium induction of Cuscuta seedlings. There is some discussion for a light signal to induce haustorium as well as chemical taxis to host plants. Although haustorium induction and development is the basis for plant–plant interaction research, some co ntroversy/ambiguous points remain from previous studies. This calls for reviewing previous research that can be useful for understanding the Cuscuta parasitization strategy as well as for the basic understanding of the plant–plant interaction of Cuscuta.
Classification of Cuscuta and types of parasite
The number of parasitic plant species is not small in angiosperms () and parasite plants have evolved multiple times during angiosperm evolution. Cuscuta is classified in the family Convolvulaceae, and the tribe Ipomoeae was relatively close to the genus Cuscutain previous molecular phylogenetics research on nuclear ribosomal internal transcribed spacer sequences and plastid sequences (e.g. rps2) (McNeal et al. Citation2007, Citation2009). The genus Cuscuta consists of 3 subgenera, Monogyna, Cuscuta, and Grammica (McNeal et al. Citation2007), and between 150 and 200 species have been described so far. The subgenus Grammica can be further divided into the sections Eugrammica and Cleistrogrammica (Stefanovic et al. Citation2007).
Table 1. Plant species number in parasitic plant and other higher plants.
In principle, all forms of Cuscuta develop a haustorium, which is a special tissue differentiated from the stem in order to suck nutrients from various host plants. Although it has lost its leaves and roots, Cuscuta is widespread in the world and commonly known as dodder. It causes severe damage to certain crops (e.g. tomato, potato, tobacco), especially in the USA.
There are several types of parasitic plants, for instance holoparasitic or hemiparasitic forms, root parasites or stem parasites. Hemiparasitic plants are defined as having chlorophyll and photosynthesis, holoparasitic plants as lacking these features (Heide-Jørgensen Citation2008). Stem parasites absorb nutrients and water from host stems, whereas root parasites do from host roots.
In general, the root/leaf can be reduced or lost in parasite plants, although this differs between species. For example, all Cuscuta and Cassytha have no leaves or roots, but Olax still has functional roots and many mistletoes have leaves (Heide-Jørgensen Citation2008).
In Cuscuta, the green color in the seedling stage indicates the presence of chlorophyll, but the genus is typically classified as holoparasitic. Cuscuta seedlings normally live less than 3 weeks before becoming parasitic. The seedlings cannot absorb water by themselves (due to lack of roots), and ultimately cannot survive without parasitization. In addition, Cuscuta apparently has a poor CO2 affinity based on the lower amount of Rubisco and chlorophyll (Hibberd et al. Citation1998). In contrast, normal hemiparasite plants can survive without a host plant, although the growth rate declines drastically without parasitization. The green color of Cuscuta can turn into orange or purple after parasitization, underlining that that plant gains energy from a host and does not need to photosynthesize. Despite of the presence of chlorophyll, it is justified to classify Cuscuta as holoparasitic because without parasitization it cannot produce offspring. The haustorium of Cuscuta enters the host stem and connects with the host xylem and phloem (Kuijt Citation1969), indicating clear stem parasitism.
Hibberd and Jeschke (Citation2001) described the connection between the parasite and host. With regard to the xylem, this connection involves lumen–lumen links or parasite xylem parenchyma. The parasite extracts water, inorganic ions, organic acids, and amino acids from the xylem flux. In the phloem connection, specialized cells known as haustorium transfer cells or companion cells absorb water, sugars, and amino acids from the host. In some cases, interspecific plasmodesmata or interspecific sieve plates are involved. Xylem feeders tend to be hemiparasites, and the current direction is mostly from host to parasite due to the larger flux of xylem. As Cuscuta has no roots and no effective photosynthesis system, most of the nutrients apparently come from the host phloem. This makes Cuscuta a phloem feeder, and Haupt et al. (2001) used fluorescent proteins to show a symplasmic connection with companion cells of phloem. A lower phloem flux current here caused a reciprocal interaction between host and parasite. In certain cases, Cuscuta can be a mediator of virus infection for the host plant. Apoplasmic and symplasmic connections are found case by case. The presence of a plasmodesmata connection between Cuscuta and a host plant was shown by Birschwilks et al. (Citation2006).
Parasite strategy of Cuscuta
The steps in the life cycle of parasite plants include: (1) seed germination; (2) early development of the seedling; (3) the search for a host plant, haustorium induction and invasion of the host, haustorium maturation; and (4) interaction with the host plant (Stewart and Press Citation1990; Yoder Citation1999).
Several types of seed germination are known in parasite plants (Press et al. Citation1990): (1) seeds contain sufficient nutrients inside (Cuscuta, Cassytha, some Scrophulariaceae); (2) seeds are covered with a fruit and eaten by birds. Consequently, the seeds are dispersed by birds and germinate on host plants (Viscus, Santalaceae); and (3) Seed germination requires chemicals from the host plant root (Orobanchaeceae) (Press et al. Citation1990). The size of Cuscuta seeds varies but they appear to contain sufficient nutrients.
Early seedlings of parasites differ between species because hemiparasites can conduct photosynthesis, and they can have leaves and roots. In the case of Cuscuta, early seedlings have an unbranched, string-like shape; no leaf or root is present. Seedlings grow upward for several days, then start to rotate until finding a point of attachment. Photosynthesis activity appears to be quite low due to a low CO2 affinity (unpublished data). This makes it necessary for Cuscuta to find a host plant within a few weeks. Otherwise, the seedling dies.
Searching for a host plant is the first important step for parasitization. Since some root parasites require chemical cues for haustorium induction (Estabrook and Yoder Citation1998), it has quite commonly been accepted that many stem parasites also require chemical cues for the search and for haustorium induction. In a recent article about Cuscuta chemical taxis, Runyon et al. (Citation2006) state that some volatile chemical cue is important. Although this idea is attractive and widely accepted, it still needs further discussion. The authors stated that Cuscuta campestris seedlings moved to host plants in the dark, due to the detection of volatile substances from host plants. However, Cuscuta tend also to rotate randomly in the field, and the position and the direction of the apical part show a random distribution (). Indeed, this rotation makes it possible for seedling to attach to host plants nearby. A further aspect is the variety of host plants for Cuscuta. Cuscuta can change from one host to another and back. If the plant needs special volatile chemicals to search for a host, it is difficult to explain why it can parasitize so many different plants except there is a strong overlap between the volatile compositions of the various plants. Furthermore, Cuscuta can do self-parasitization (), and can also move toward acryl rod without any volatile chemicals and induced haustorium in vitro (). This is additional evidence that special chemicals are not necessarily required for the plant–plant interaction, and suggests that there might be at least two mechanisms for contacting the host plants.
Figure 1. Random rotation of Cuscuta campestris seedling with time course in the field. Clock at back indicates time change from (a) to (f). Seedling indicated by orange arrow clearly showed random rotation. White arrow indicates Cuscutta seedling twine each other. Host plant nearby did not influence their rotation activity.
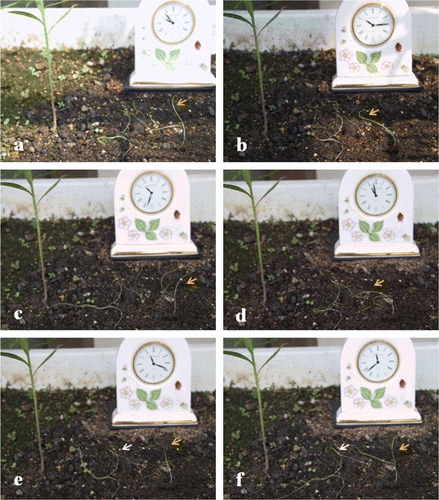
Figure 2. (a) Self-parasitization of Cuscuta japonica. (b) Cross-section of parasite part. Haustorium intrude another one (Furuhashi et al. Citation1995).
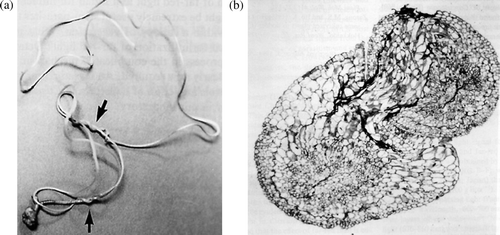
Figure 3. Cuscuta japonica seedlings twining on acryl rod. Without adding any chemical substances, haustorium was induced. Arrow indicates induced haustorium parts.

Twisting to reach host plant is a common phenomenon in the plant kingdom, for example, in vines and climbing plants. However, there is no report that twining vines or climbing plants (e.g. Ipomoea) twist to find other plants based on particular chemical signals. Since most climbing plants can twist even against ‘plastic materials,’ chemical taxis might not be critical for Cuscuta ().
The word ‘haustorium’ originates from the Latin term ‘haurire,’ which means ‘drink,’ one of the most important characteristics of parasite plants. This calls for scrutinizing the mechanism of haustorium induction. There are no hypotheses or data for Cuscuta haustorium evolution. The haustorium appears to have evolved as a specialized tissue, and it is not comparable to the root meristem/lateral shoot meristem/leaf meristem.
Although chemical cues are well-known in the induction of some root parasite haustoria, no reports are available about chemical cues for Cuscuta haustorium induction. The haustorium of Sriga is developed prior to attachment to the host. In Cuscuta the haustorium develops after the contact signal, but it is not induced without a light cue (e.g. far-red (FR) light) (Tada et al. Citation1996). It remains uncertain whether a haustorium induced by light and a contact signal requires another chemical cue for maturation.
One report describes that a haustorium can be induced on the apical part of Cuscuta without host attachment (Heide-Jørgensen Citation2008), but this is based on field studies only. Cuscuta haustorium development progresses sequentially, and it continues even after detachment from a host plant or other object when sufficient stimuli are given (Tada et al. Citation1996).
It is quite commonly accepted that many higher plants avoid dense canopy conditions by detecting the change of red light:far-red light (R:FR) ratio by phytochrome (Pedmale et al. Citation2010). In fact, Cucumber (Cucumis sativus) de-ethiolated seedling experiments showed that seedlings bend away from a FR light source, which is comparable to a patchy canopy environment (Ballaré et al. Citation1992).
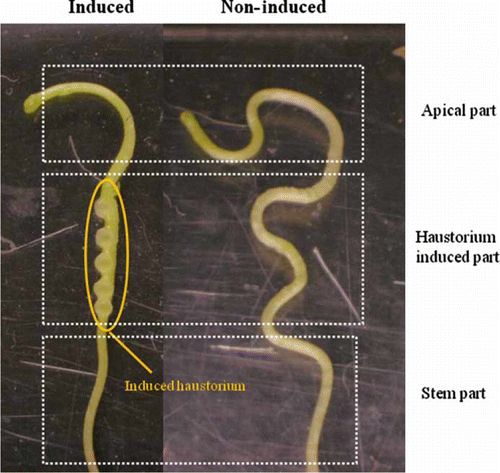
Cuscuta conspicuously showed opposite behavior to a FR light source, and similar behavior as negative phototropism/skototropism was reported only from some tropical vines, e.g. Monstera gigantean (Strong and Ray Citation1975). A photomorphogenic response, hook opening and circumnutating, and induction of twining by blue light and FR light in Cuscuta were described in the past (Lane and Kasperbauer Citation1965) and later confirmed (Orr et al. Citation1996a, Citationb). Lane and Kasperbauer already proposed a potential role of phytochrome in 1965 because of evidence that the R:FR ratio change in light is correlated with the phytochrome (e.g. phytochrome B) Pr and Pfr ratio. These studies, however, did not deal with haustorium induction. Furuhashi et al. first reported in 1995 that a light cue is necessary for Cuscuta (Cuscuta japonica) haustorium induction in self-parasitization experiments in vitro (Furuhashi et al. Citation1995). They also found that the blue + FR light was much more effective for haustorium induction than blue light only, although twining was almost the same for both light types. Moreover, the FR light effect for twining and haustorium induction was strongly enhanced when Cuscuta was treated with blue light prior to FR light. These data pointed to a strong cross-talk between blue and FR light signal transduction. Tada et al. (Citation1996) used acryl rods instead of host plants, and confirmed the effectiveness of FR light for induction as well as FR/red (R) light reversibility, clarifying that both the light signal and the contact signal are required for induction (). Haidar et al. (Citation1997) reported contradictory results for Cuscuta indecora and Cuscuta campestris: seedlings treated with 1 min R light and 1–2 days incubation in the dark showed enhanced haustorium induction, but 2 min FR light did not induce a haustorium. Nevertheless, 1–2 min of R or FR light treatment is no doubt too short, and the subsequent incubation in the dark would probably cause a dark reversion effect of phytochrome. This reverse effect is induced only by brief irradiation and does not occur at longer irradiation. Indeed, Haidar et al. (Citation1997) also showed that longer irradiation (1–2 days) with R light negatively affected haustorium induction but that FR light slightly induced it. Those authors also stated that Cuscuta seedlings treated solely with 100 µM m–2 s–1 FR light showed less induction compared with blue light alone. Note, however, that they used 100 µM m–2 s–1 of FR light, an intensity that may have been ineffective. The result showed that a high irradiance effect of FR light was not required for Cuscuta haustorium induction.
Figure 4. Haustorium induced and non-induced Cuscuta japonica seedling. Only FR light or only contact signal did not induce haustorium. Haustorium was developed between apical and stem part.
The Pfr/Ptotal ratio is around 0.45 in plants growing under blue light, and this ratio is less than the mixture of R and FR light (0.5; Haidar et al. Citation1998). This suggests that haustorium induction by blue light might be triggered by phytochrome. Haidar (Citation2003) indicated a potential role of cryptochrome as a blue light receptor by using the phytochrome inhibitor Gabaculine; this compound inhibits only the phytochrome effect. The author reported that the FR effect in induction was decreased by Gabaculine but that the blue light effect was not influenced. Accordingly, the blue light effect did not involve lower absorption by phytochrome, and the blue light receptor must have been present and played some role. The fact was consistent with previous results that combination of blue and FR light is the most effective light cue for haustorium induction. Although the presence of a blue light receptor is highly likely and cryptochrome is a candidate, no blue light receptor has yet been cloned from Cuscuta and there are no substantial data to conclude that the blue light receptor for haustorium induction is cryptochrome. Li et al. (Citation2009) conducted another study on the blue light effect on Cuscuta. Their proteomics approach used Cuscuta australis and 2D gel electrophoresis: the abundance of some proteins from seedlings was increased under blue versus white light. These included PKS1 homologue (phytochrome signaling component), phytochrome C-like protein, and pectinesterase family proteins. Nevertheless, white light contains both blue and R light but not much FR light. The seedlings did not twine under white light, indicating that the R light effect was larger and white light might not be a good control for this comparison. Although the data are interesting, those proteins said to be increased by blue light might be rather interpreted as a protein concentration decrease in the R light effect. Moreover, in their figure, Cuscuta seedlings appear to develop haustoria. Since haustorium induction requires both the light and the contact signal, a protein concentration increase under blue light would also partly reflect the contact signal. A protein change related to a light signal is not conclusive and requires future studies.
Light flux density can also be a factor for parasitization. Cuscuta develop haustoria much better in shady sites than under strong direct sunlight (based on observations in the field and unpublished data). This indicates that the light signal for induction does not require a large light flux density. Indeed, previous experimental data suggested about 5–10 µM m–2 s–1 of blue or FR light were sufficiently intensive, and it appears that Cuscuta prefers even weaker light flux density for induction. Furthermore, Haidar et al. (Citation1997) showed a light intensity saturation effect upon induction. From an evolutionary perspective, this preference is understandable because the plants need to be close to the host, and light there would be partially blocked and the R:FR ratio lower. In the Haidar (Citation2003) experiments, the effect of high light intensity (mixture of R and FR lights at 200 µM m–2 s–1) on haustorium induction was less than that of lower intensity (40 µM m–2 s–1). This indicates a greater influence of the red signal at higher light intensity. The total intensity of natural sunlight can vary from full sunlight (approximately 2400 µM m–2 s–1) to shade (e.g. 50% of full sunlight) conditions (Clua et al. Citation2006). The percentage of each light is not always the same as blue (400–500 nm; 29.16% of PAR 400–700 nm), R (600–700 nm; 35.64% of PAR 400–700 nm), and FR lights (700–750 nm; 17% intensity compared to total PAR 400–700 nm) (Deitzer Citation1994).
The R:FR ratio for the signal must be another important factor because R light tends to inhibit, but FR light promotes haustorium induction. Haidar and Orr (Citation1999) reported a R:FR ratio between 0.02 and 1.0 (0.1 was the best) for haustorium induction in Cuscuta planiflora.
As Haidar and Orr (Citation1999) pointed out, such conditions would exist in the proximity of other plants. In the case of Cuscuta, it is reasonable to develop haustoria when and where the FR signal is increased. Thus, Cuscuta might detect the reflection of FR from nearby host plants. Many higher plants avoid shade or the canopy of other plants in order to get more light by detecting a reduction in the R:FR ratio with phytochrome (Ballaré Citation2009). The mechanism of shade avoidance is probably similar in higher green plants; Cuscuta may well have improved this system to recognize the proximity of potential hosts. Indeed, the R:FR ratio both in shade and under canopies is less than 1, and the response to shade improves sharply at R:FR < 0.5 (Smith Citation2000).
The signal transduction mechanism of avoiding canopy shade and haustorium induction do not appear to be similar. The R:FR signal caused by canopy shade induces auxin and gibberellin production, but haustorium induction by Cuscuta seedlings did not significantly alter auxin concentrations (Löffler et al. Citation1999). The response to canopy shade increases auxin and degrades cytokinin, leading to inhibition of radial growth and attenuation of leaf development (Carabelli et al. Citation2008). In contrast, Haidar et al. (Citation1998) reported that cytokinin enhances Cuscuta haustorium development but that auxin (as well as Abscisic acid (ABA) and ethylene) inhibits it under blue light. Cuscuta has no roots, the site of cytokinin production. This indicates that the cytokinin level might be lower than in other higher green plants and might modify hormonal signal transduction.
In Cuscuta the relationship between FR signal and plant hormones implies that the haustorium would be completely different from that in the leaf meristem or shoot apical meristem. This, in turn, suggests that the Cuscuta haustorium is not a simple homolog or analog of the leaf/shoot apical meristem.
As both light and contact signal are prerequisites for haustorium induction, it is promising to examine current knowledge on mechanical sensing/thigmomorphogenesis. Such studies, however, are rare in plants compared with research on light signal (e.g. photo signal transduction and phototropism). Firstly, the response to touch or any mechanical stress varies from several seconds (e.g. Mimosa leaf touch response) to long-term continuous thigmomorphogenesis (Monshausen and Gilroy Citation2009). The contact signal for the Cuscuta haustorium is consistent attachment to a host, comparable with long-term thigmomorphogenesis. In general, quick responses tend to be called mechanosensing, and mechanical stress or touch are known to be induced by transient Ca2 +>, pH change, and reactive oxygen species (ROS) production (Peyronnet et al. Citation2008; Hofmann Citation2009). These factors as well as plant hormonal changes are also observed in thigmomorphogenesis (Chehab et al. Citation2009). Any mechanical signal should principally alter plasmamembrane tension, and this change can be caused by either mechanical stimulation or osmotic stresses (Peyronnet et al. Citation2008). The signal transduction pathway of mechanoresponses and osmotic stress responses are different (Monshausen and Gilroy Citation2009), although there is some overlap; for example, AtMSL (mechanosensitive channel) is involved in communicating both signals (Peyronnet et al. Citation2008).
Recent thigmomorphogenesis research on Carica papaya showed that mechanical stimulation increases hypocotyls diameter and lignine content, but decreases leaf width, stem length, root fresh weight, and the accumulation of chlorophyll and anthocyanine (Porter et al. Citation2009). That study also reports hypertrophic outgrowth associated with the periderm and suberin. Since hypertrophic outgrowth induced by mechanical stimulation is comparable to Cuscuta haustorium induction, this analogy is interesting. Chehab et al. (Citation2009) reviewed the relationship between plant hormones and thigmomorphogenesis. Mechanical stimulation can influence at least two different signal transduction pathways (Monshausen and Gilroy Citation2009). One is the Ca2 +> ion-dependent pathway. Mechanical stimulation causes plasmamembrane tension, and potential Ca2 +> ion sensors (e.g. TCH and CML) interact with pinoids such as Ser/Thr protein kinase (PID). Since PID can regulate the PIN family of auxin regulators, auxin should be recognized downstream of mechanical sensing and/o r thigmomorphogenesis. Apparently, the auxin concentration drops and reverses auxin-prompted shoot elongation. A previous study did not support an auxin drop with Cuscuta haustorium induction (Löffler et al. Citation1999). Another type of signal transduction is kinase dependent. Receptor-like wall-associated kinases (WAKs) respond to mechanical stimulation, and this can be influenced by pectinesterase and Ca2 +>. We currently do not know which type is related to Cuscuta haustorium induction.
Overall, mechanical stress or stimulation negatively affects plants, inhibiting growth and decreasing pigments (e.g. anthocyanine). The fact that the Cuscuta stem attached to a host can change color into pale is somewhat similar to previous studies.
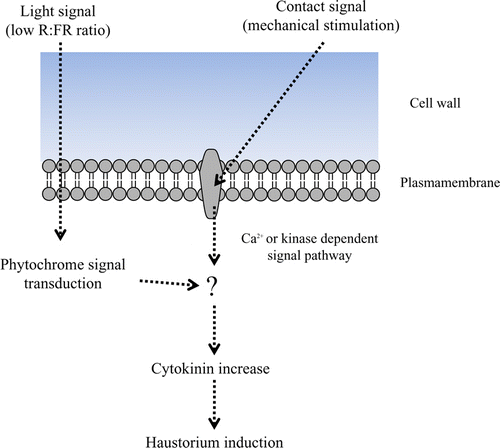
Since cytokinin can induce haustoria under dark conditions (Haidar et al. Citation1998), cytokinin can be envisioned as being downstream of haustorium induction signal transduction and as being controlled by light and contact signals (). Otherwise, the cytokine increase is spontaneous, but there are no substantial data to support this under natural conditions. Since Cuscuta initially find the host plant after detecting the FR light conditions, the light signal effect for induction might be earlier than the contact signal in nature.
Figure 5. Hypothetical Cuscuta haustorium induction signal pathway. FR light signal by way of phytochrome signal transduction and contact signal transduction are integrated. This leads to cytokinin increase, and eventually the haustorium is developed.
After haustorium induction following host attachment, Cuscuta seedlings need to invade the host tissue in order to reach the phloem. The presence and activity of pectinesterase, polyphenoloxidase, and polygalacturonase in Cuscuta campestris has been confirmed (Nun and Mayer Citation1999; Nun et al. Citation1999). The increase of pectinesterase by blue light (probably by haustorium induction) is consistent with this (Li et al. Citation2009). The factor that activates these enzymes is present both in Cuscuta and the host plant (Nun et al. Citation1999); thus, the mechanism that helps Cuscuta avoid causing self-damage is still unknown (Nun and Mayer Citation1999).
Pectinesterase is present in bacteria and fungi, and plays an important role for plant pathogens to penetrate or degrade plant cell walls (e.g. soft roots, vascular wilts, and leaf-spot disease) (Wood Citation1960). The role of this enzyme appears to be quite similar in Cuscuta and in bacteria/fungi as plant pathogens.
Pectinesterase itself is ubiquitous in the plant kingdom and is involved in seed germination, cell adhesion, and stem elongation with increasing acidic pectin and decreasing cell wall pH. These could be influenced by plant hormones (e.g. auxin and gibberellic acid) (Micheli Citation2001). Although cell-to-cell interactions or macromolecule movement (including viruses) through plasmodesmata would be implicated with this enzyme activity, no substantial data are available regarding plant–plant interaction between Cuscuta and the host plant.
Plant–plant interaction
Plant–plant interactions start after hyphae have reached the phloem and xylem of the host plant. Considering the ubiquitous presence of Cuscuta and the translocation of various substances during parasitization, Cuscuta is a key plant for studying host–parasite plant interactions.
Many parasite plants, e.g. Viscum, have a connection with host xylem (Popp and Richter Citation1998). In xylem, the strong water current is unidirectional from root to apex, namely from host to parasite. In contrast, a weaker current is bidirectional in phloem. In any case, Cuscuta connect their phloem with the host phloem directly: probably any suitable size of metabolites, proteins, or macromolecules would be taken, without special selection (). Plant–plant interactions between Cuscuta and host plants have recently been studied. These efforts revealed the translocation of mRNA and proteins between host and parasite. As outlined in a recent review, microarray experiments confirmed the presence of host plant mRNA in Cuscuta (Westwood et al. Citation2009). Translocation of proteins from host (Arabidopsis) to Cuscuta has also been studied; the sieve size was predicted to be 27–36 kDa based on GFP fused protein experiments (Birschwilks et al. Citation2007). However, plasmodesmata connections would not allow molecules over 800 Da to permeate without causing dilation (Haywood et al. Citation2002). Viral Movement protein (MP) protein or non-cell-autonomous protein (NCAP) can induce microchannel dilation, but no information in this regard is known from Cuscuta.
Figure 6. Scheme of chemical translocation between Cuscuta and host plant. Sieve of Cuscuta haustorium allows translocation of metabolites and proteins less than 30 kDa.
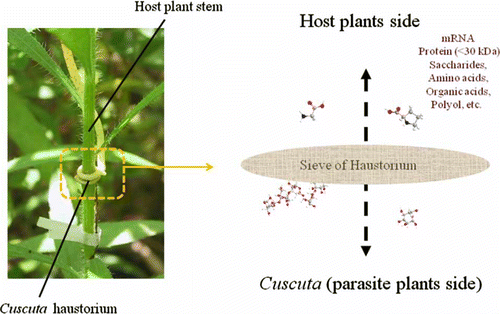
Cuscuta is thought to mediate a flowering signal inhibitor from one host plant to another (Heide-Jørgensen Citation2008). As predicted, the sieve size in the phloem is 27–36 kDa. Accordingly, one of the important candidates of florigen named FT, which is expressed in phloem (Corbesier and Coupland Citation2006) and whose amino acids are less than 190 aa (Kobayashi et al. Citation1999), would be able to move between host and Cuscuta. Nonetheless, there are many factors that influence the FT pathway (Corbesier and Coupland Citation2006), among them sucrose, the plant hormone (e.g. gibberellin, cytokinin, salicylic acid) pathways (Wada et al. Citation2010), and the circadian clock pathway. As salicylic acid is related to stress-induced flowering, there is a possibility that a pathological response of the host plant to Cuscuta might induce flowering.
Unspecific movement of metabolites and macromolecules between parasite and host implies a problematic relationship. One example involves parasite–host incompatibility. Incompatibility types can be classified into biochemical defenses and mechanical defenses (e.g. hypertrophy formation or lignification). In a previous Cuscuta study, antipathogenic assay revealed that naphthoquinone is one of the antipathogenic/antimicrobial substances produced by the host plant (Ancistrocladus heyneanus). These substances trigger a subsequent hypersensitive reaction and lignification by the host plant. This was interpreted as a biochemical incompatibility to Cuscuta (Bringmann et al. Citation1999).
These incompatible responses are similar to pathogenic responses. As such, some plant–plant interactions more closely resemble pathogenic than symbiotic interactions. Most pathogens involved in plant diseases involve elicitor-derived (e.g. β-glucan) signal transduction. In the case of Cuscuta parasitization, certain glycans in the host cell wall degraded by Cuscuta may be elicitors and cause incompatibility, but data on this issue are rare. Ethylene inhibits growth of Cuscuta at the non-parasitic stage, but exogenously supplied ethylene has no effect on the parasitic phase (Haidar et al. Citation1998). This means that the ethylene increase caused by the host pathogenic response would not block Cuscuta parasitization.
From the parasite perspective, it would be important to degrade unpleasant substances taken up from the host. Viscum, for example, apparently degrades sorbitol absorbed from the host (Richter and Popp Citation1992; Wanek and Richter Citation1993). To date, no studies suggest that Cuscuta degrades host metabolites or macromolecules, although the parasite probably has abilities similar to Viscum.
General discussion
Cuscuta evolved as parasites by losing their leaves and roots: they grow upward and their stem deteriorates after germination. Their continuous growth and ability to successively change hosts makes the occurrence of co-evolution between Cuscuta and specific hosts unlikely. No other mutant form lacking leaves and/or no roots has been reported among the higher plants. Only lateral root loss in Oryza (Wang et al. Citation2006) and juvenile leaf loss in Arabidopsis (Hamada et al. Citation2000) were reported, but this does not represent complete leaf or root loss. This implies a uniqueness of the Cuscuta evolutionary strategy. It also suggests difficulties to interpret the system based solely on mutant research involving model plants. No explanations for the leaf and root loss in Cuscuta have been advanced, and no fossil data are available to suggest an intermediate situation.
To date, there is no clear idea how Cuscuta evolved. The ancestral form might be a kind of twining plant because Cuscuta is phylogenetically close to the tribe Ipomoea. There is a report that Ipomoea hederacea preferentially climbed to some colored structure (e.g. green and yellow), indicating Ipomoea can possibly use light reflectance to search for structure for climbing (Price and Wilcut Citation2007). Photoreceptor (e.g. phytochrome) was also identified from Ipomoea (Lariguet and Dunand Citation2005), and it is conceivable that an ancestral form of Cuscuta could have a similar host detection mechanism. A strong contact signal by twining to a host might have led to reduced chlorophyll, root, and leaf development due to mechanical stress. At that stage of evolution, certain individuals that could develop hyphae-like tissue to suck nutrients would have been superior competitors. Because the haustorium is a specialized tissue and not homologous to leaves or roots, its evolution could occur parallel with the reduction or loss of the latter two.
Although light and contact signals are necessary for haustorium induction, both the FR light and contact signal pathways leading to haustorium induction are not well-known (a, b). For example, the FR light signal pathway for shade avoidance up-regulates auxin but down-regulates cytokinin. In Cuscuta, however, the FR signal can increase cytokinin due to the increase of cytokinin in the haustorium (Haidar et al. Citation1998). The contact signal normally inhibits growth of other higher plants, but enhances Cuscuta haustorium development. Hence, the essential evolutionary change must have been an improvement downstream of FR light and the contact signal pathway, an evolutionary change that is linked to the unique parasite strategy of Cuscuta. A direct connection to the phloem might require the evolution of special enzymes in order to degrade unpleasant metabolites or macromolecules. Data from current model plants cannot be directly applied to the Cuscuta parasitization mechanism and parasite strategy. Other types of approaches are required to elucidate a wide range of biological phenomena. At the same time, Cuscuta research suggests that new aspects of light signal transduction and thigmomorphogenesis can be found by investigating other plants. Further detail research for clarifying light and thigmomorphogenesis signal transduction in Cuscuta is required.
Figure 7. Differences of cytokinin pathways in higher plants and Cuscuta. (a) Comparison of hypothetical FR light signal transduction pathway between other higher plants (Carabelli et al. Citation2008; Balare 2009[AQ unlisted]) and Cuscuta. Cytokinin increase in haustorium induction looks opposite output from other higher plants. (b) Comparison of hypothetical contact signal transduction pathway between other higher plants (Monshausen and Gilroy Citation2009) and Cuscuta.
![Figure 7. Differences of cytokinin pathways in higher plants and Cuscuta. (a) Comparison of hypothetical FR light signal transduction pathway between other higher plants (Carabelli et al. Citation2008; Balare 2009[AQ unlisted]) and Cuscuta. Cytokinin increase in haustorium induction looks opposite output from other higher plants. (b) Comparison of hypothetical contact signal transduction pathway between other higher plants (Monshausen and Gilroy Citation2009) and Cuscuta.](/cms/asset/fd096089-83d4-4228-925b-2a96e713431e/tjpi_a_541945_o_f0007g.gif)
Some past research on Cuscuta was based solely on external observations. In contrast, current molecular biological research is laboratory based under specified conditions. These different research approaches are sometimes completely separated. In particular, molecular plant biology has focused only on a few model plants (e.g. Arabidopsis). These models are suitable to understand common biological characteristics, but not unique phenomena. Indeed, no model plant is available that parasitizes other plants. A current scientific issue is how to utilize molecular biological data that are mainly obtained from model plants to understand other plants. This involves comparing research on Cuscuta with model plant research. Moreover, modern experimental data should be integrated into this endeavor because our ultimate goal is also to explain natural phenomena occurring in the field.
Acknowledgements
Prof. M. Popp, Prof. A. Richter, and Dr D. Engelmeier provided useful comments at an early stage of the manuscript. Dr M. Stachowitsch and Dr Höhenwarter improved English.
References
- Agrios GN . 2005 . Plant pathology. , 5th ed . Burlington, MA : Elsevier . p. 922 .
- Baker , B , Zambryski , P , Staskawicz , B and Dinesh-Kumar , SP . 1997 . Signaling in plant-microbe interactions . Science. , 276 : 726 – 733 .
- Ballaré , CL . 2009 . Illuminated behaviour: phytochrome as a key regulator of light foraging and plant anti-herbivore defence . Plant Cell Environ. , 32 : 713 – 725 .
- Ballaré , CL , Scopel , AL , Radosevich , SR and Kendrick , RE . 1992 . Phytochrome-mediated phototropism in de-etiolated seedling . Plant Physiol. , 100 : 170 – 177 .
- Bauer , U and Federle , W . 2009 . The insect-trapping rim of Nepenthes pitchers surface structure and function . Plant Signal Behav. , 4 ( 11 ) : 1019 – 1023 .
- Begon , M Townsend , CR Harper , JL 2006 . Ecology “from individuals to ecosystems”. , 4th ed Malden, MA : Blackwell 738
- Bidartondo , MI . 2005 . The evolutionary ecology of myco-heterotrophy . New Phytol. , 167 : 335 – 352 .
- Bidartondo , MI , Redecker , D , Hijri , I , Wiemken , A , Bruns , TD , Dominguez , L , Sérsic , A , Leake , JR and Read , DJ . 2002 . Epiparasitic plants specialized on arbuscular mycorrhizal fungi . Nature. , 419 : 389 – 392 .
- Birschwilks , M , Haupt , S , Hofius , D and Neumann , S . 2006 . Transfer of phloem-mobile substances from the host plants to the holoparasite Cuscuta sp . J Exp Bot. , 57 ( 4 ) : 911 – 921 .
- Birschwilks , M , Sauer , N , Scheel , D and Neumann , S . 2007 . Arabidopsis thaliana is a susceptible host plant for the holoparasite Cuscuta spec . Planta. , 226 : 1231 – 1241 .
- Boewig BS 1898 . The histology and development of Cassytha filiformis , L. Boston, MA : Ginn & Company. Pennsylvania University , New series 5, contribution from the botanical laboratory , p. 399 – 418 .
- Bringmann , G , Schlauer , J , Rückert , M , Wiesen , B , Ehrenfeld , K , Proksch , P and Czygan , FC . 1999 . Host-derived acetogenins involved in the incompatible parasitic relationship between Cuscuta reflexa (Convolvulaceae) and Ancistrocladus heyneanus (Ancistrocladaceae) . Plant Biol. , 1 : 581 – 584 .
- Carabelli , M , Possenti , M , Sessa , G , Ciolfi , A , Sassi , M , Morelli , G and Ruberti , I . 2008 . A novel regulatory circuit underlying plant response to canopy shade . Plant Signal Behav. , 3 ( 2 ) : 137 – 139 .
- Chehab , EW , Eich , E and Braam , J . 2009 . Thigmomorphogenesis: a complex plant response to mechano-stimulation . J Exp Bot. , 60 ( 1 ) : 43 – 56 .
- Chisholm , ST , Coaker , G , Day , B and Staskawicz , BJ . 2006 . Host-microbe interactions: shaping the evolution of the plant immune response . Cell. , 124 : 803 – 814 .
- Christensen , NM , Dörr , I , Hansen , M , van der Kooij , TAW and Schulz , A . 2003 . Development of Cuscuta species on a partially incompatible host: induction of xylem transfer cells . Protoplasma. , 220 : 131 – 142 .
- Clua , A , Fernandez , G , Ferro , L and Dietrich , M . 2006 . Photosynthetic photon flux density during seed development of narrowleaf birdsfoot trefoil (Lotus glaber) influences seed production and subsequent dormancy and germination . Lotus Newsl. , 36 ( 2 ) : 54 – 57 .
- Corbesier , L and Coupland , G . 2006 . The quest for florigen: a review of recent progress . J Exp Bot. , 57 ( 13 ) : 3395 – 3403 .
- Deitzer G. 1994 . Spectral comparisons of sunlight and different lamps . In: Tibbitts TW , International lighting in controlled environments workshop NASA-CP-95-3309; Mar 27–30; University of Wisconsin. Kennedy Space Center FL : NASA Conference Publication .
- Ellison , AM and Gotelli , NJ . 2009 . Energetics and the evolution of carnivorous plants – Darwin's ‘most wonderful plants in the world’ . J Exp Bot. , 60 ( 1 ) : 19 – 42 .
- Estabrook , EM and Yoder , JI . 1998 . Plant-plant communications: rhizosphere signaling between parasitic angiosperms and their hosts . Plant Physiol. , 116 : 1 – 7 .
- Furuhashi , K , Kanno , M and Morita , T . 1995 . Photocontrol of parasitism in a parasitic flowering plant, Cuscuta japonica Chois, cultured in vitro . Plant Cell and Physiol. , 36 ( 3 ) : 533 – 536 .
- Govier , RN , Brown , JGS and Pate , JS . 1968 . Hemiparastitic nutrition in angiosperms II. Root haustoria and leaf glands of Odontites verna (Bell.) Dum. And their relevance to the abstraction of solute from the host . New Phytol. , 67 : 963 – 972 .
- Govier , RN , Nelson , MD and Pate , JS . 1967 . Hemiparastitic nutrition in angiosperms I. The transfer of organic compounds from host to Odontites verna (Bell.) Dum. (Scrophulariaceae) . New Phytol. , 66 : 285 – 297 .
- Haidar , MA . 2003 . Characterization of the interaction between cryptochromes and phytochromes in blue light-induced coiling and prehaustorium development of dodder (Cuscuta campestris) seedling . Ann Appl Biol. , 143 : 57 – 62 .
- Haidar , MA and Orr , GL . 1999 . The response of Cuscutap laniflora seedlings to red and far-red, blue light and end-of-day irradiations . Ann Appl Biol. , 134 : 117 – 120 .
- Haidar , MA , Orr , GL and Westra , P . 1997 . Effects of light and mechanical stimulation on coiling and prehaustoria formation in Cuscuta spp . Weed Res. , 37 : 219 – 228 .
- Haidar MA Orr GL Westra P. The response of dodder (Cuscuta spp.) seedlings to phytohormones under various light regimes Ann Appl Biol 1998 132 331 – 338
- Hamada , S , Onouchi , H , Tanaka , H , Kudo , M , Liu , YG , Shibata , G , Machida , C and Machida , Y . 2000 . Mutation of WUSCHEL gene of Arabidopsis thaliana result in the development of shoots without juvenile leaves . Plant J. , 24 ( 1 ) : 91 – 101 .
- Haupt , S , Oparka , KJ , Sauer , N and Neumann , S . 2001 . Macromolecular trafficking between Nicotiana tabacum and the holoparasite Cuscuta reflexa . J Exp Bot. , 52 ( 354 ) : 173 – 177 .
- Haywood V Kragler F Lucas WJ Plasmodesmata: pathways for protein and ribonucleoprotein signaling Plant Cell 2002 14 S303 – S325
- Heide-Jørgensen HS 2008 Leiden, , the Netherlands Brill Parasitic flowering plants p. 421
- Hibberd , JM , Bungard , RA , Press , MC , Jeschke , WD , Scholes , JD and Quick , WP . 1998 . Localization of photosynthetic metabolism in the parasitic angiosperm Cuscuta reflexa . Planta. , 205 : 506 – 513 .
- Hibberd , JM and Jeschke , WD . 2001 . Solute flux into parasitic plants . J Exp Bot. , 52 ( 363 ) : 2043 – 2049 .
- Hofmann NR 2009 Early signaling events in mechanosensing Plant Cell 21 2191
- Jeschke , WD and Hilpert , A . 1997 . Sink-stimulated photosynthesis and sink-dependent increase in nitrate uptake: nitrogen and carbon relations of the parasitic association Cuscuta reflexa–Ricinus communis . Plant Cell Environ. , 20 : 47 – 56 .
- Jones , JDG and Dangl , JL . 2006 . The plant immune system . Nature. , 444 : 323 – 329 .
- Kindermann , A . 1928 . Haustorialstudien an Cuscuta Arten . Planta. , 5 : 769 – 783 .
- Kobayashi , Y , Kaya , H , Goto , K , Iwabuchi , M and Araki , T . 1999 . A pair of related genes with antagonistic roles in mediating flowering signals . Science. , 286 : 1960 – 1962 .
- Kuijt , J . 1969 . The biology of parasitic flowering plants , Berkeley, CA : University of California Press .
- Lane , HC and Kasperbauer , MJ . 1965 . Photomorphogenic responses of dodder seedlings . Plant Physiol. , 40 : 109 – 116 .
- Lariguet , P and Dunand , C . 2005 . Plant photoreceptors: phylogenetic overview . J Mol Evol. , 61 : 559 – 569 .
- Leake , JR . 1994 . Tansley review no. 69 the biology of myco-heterotrophic (‘saprophytic’) plants . New Phytol. , 127 : 171 – 216 .
- Li , D , Wang , L , Yang , X , Zhang , G and Chen , L . 2009 . Proteomic analysis of blue light-induced twining response in Cuscuta australis . Plant Mol Biol. , 72 ( 1–2 ) : 205 – 213 .
- Löffler , C , Czygan , FC and Proksch , P . 1999 . Role of indole-3-acetic acid in the interaction of the phanerogamic parasite Cuscuta and host plants . Plant Biol. , 1 : 613 – 617 .
- Malone , M , Watson , R and Pritchrad , J . 1999 . The spittlebug Philaenus spumarius feeds from mature xylem at the full hydraulic tension of the transpiration stream . New Phytol. , 143 : 261 – 271 .
- McNeal , JR , Arumugunathan , K , Kuehl , JV , Boore , JL and dePamphilis , CW . 2007 . Systematics and plastid genome evolution of the cryptically photosynthetic parasitic plant genus Cuscuta (Convolvulaceae) . BMC Biol. , 5 ( 55 ) : 1 – 19 .
- McNeal , JR , Kuehl , JV , Boore , JL , Mack , JL and dePamphilis , CW . 2009 . Parallel loss of plastid introns and their maturase in the genus Cuscuta . PLoS ONE. , 4 ( 6 ) : 1 – 8 .
- Merckx , V , Bidartondo , MI and Hynson , NA . 2009 . Myco-heterotrophy: when fungi host plants . Ann Bot. , 104 : 1255 – 1261 .
- Micheli , F . 2001 . Pectin methylesterases: cell wall enzymes with important roles in plant physiology . Trends Plant Sci. , 6 ( 9 ) : 414 – 419 .
- Monshausen , GB and Gilroy , S . 2009 . Feeling green: mechanosensing in plants . Trends Cell Biol. , 19 ( 5 ) : 228 – 235 .
- Nun , NB and Mayer , AM . 1999 . Culture of pectin methylesterase and polyphenoloxidase in Cuscuta campestris . Phytochemistry. , 50 : 719 – 727 .
- Nun , NB , Mor , A and Mayer , AM. 1999 . A cofactor requirement for polygalacturonase from Cuscuta campestris . Phytochemistry. , 52 : 1217 – 1221 .
- Orr , GL , Haidar , MA and Orr , DA . 1996a . Small seed dodder (Cuscuta planiflora) phototropism toward far-red when in white light . Weed Sci. , 44 : 233 – 240 .
- Orr , GL , Haidar , MA and Orr , DA . 1996b . Small seed dodder (Cuscuta planiflora) gravitropism in red light and red plus far-red . Weed Sci. , 44 : 795 – 796 .
- Pedmale UV , Celaya RB , Liscum E. 2010 . Phototropism: mechanism and outcomes . In: The Arabidopsis book . Rockville, MD : American Society of Plant Biologists , 1 – 26 .
- Pennings , S and Callaway , RM . 2002 . Parasitic plants: parallels and contrasts with herbivores . Oecologia. , 131 : 479 – 489 .
- Peyronnet , R , Haswell , ES , Barbier-Brygoo , H and Frachisse , JM . 2008 . AtMSL9 and AtMSL10: sensors of plasma membrane tension in Arabidopsis roots . Curr Biol. , 18 : 730 – 734 .
- Popp , M and Richter , A . 1998 . Ecophysiology of xylem-tapping mistletoes . Ecol Veg Sci. , 59 : 659 – 674 .
- Porter , BW , Zhu , YJ , Webb , DT and Christopher , DA . 2009 . Novel thigmomorphogenetic responses in Carica papaya: touch decreases anthocyanin levels and stimulates petiole cork outgrowths . Ann Bot. , 103 : 847 – 858 .
- Press , MC , Graves , JD and Stewart , GR . 1990 . Physiology of the interaction of angiosperm parasites and their higher plant hosts . Plant Cell Environ. , 13 : 91 – 104 .
- Price , AJ and Wilcut , JW . 2007 . Response of ivyleaf morningglory (Ipomoea hederacea) to neighboring plants and objects . Weed Technol. , 21 ( 4 ) : 922 – 927 .
- Prider , J , Watling , J and Facelli , JM . 2009 . Impacts of a native parasitic plant on an introduced and a native host species: implications for the control of an invasive weed . Ann Bot. , 103 : 107 – 115 .
- Richter , A and Popp , M . 1992 . The physiological importance of accumulation of cyclitols in Viscum album L . New Phytol. , 121 : 431 – 438 .
- Runyon , JB , Mescher , MC and Moraes , CD . 2006 . Volatile chemical cues guide host location and host selection by parasitic plants . Science. , 313 : 1964 – 1967 .
- Runyon , JB , Mescher , MC and Moraes , CMD . 2008 . Parasitism by Cuscuta pentagona attenuates host plant defenses against insect herbivores . Plant Physiol. , 146 : 987 – 995 .
- Smith , H . 2000 . Phytochromes and light signal perception by plants – an emerging synthesis . Nature. , 407 : 585 – 591 .
- Stefanovic , S , Kuzmina , M and Costea , M . 2007 . Delimitation of major lineages within Cuscuta subgenus Grammica (Convolvulaceae) using plastid and nuclear DNA sequences . Am J Bot. , 94 ( 4 ) : 568 – 589 .
- Stewart , GR and Press , MC . 1990 . The physiology and biochemistry of parasitic angiosperms . Annu Rev Plant Physiol Plant Mol Biol. , 41 : 127 – 151 .
- Strong DR Jr , Ray TS Jr. 1975 . Host tree location behavior of a tropical vine (Monstera gigantea) by skototropism . Science . 190 : 804 – 806 .
- Tada , Y , Sugai , M and Furuhashi , K . 1996 . Haustoria of Cuscuta japonica, a holoparasitic flowering plant, are induced by cooperative effect of far-red light and tactile stimuli . Plant Cell Physiol. , 37 ( 8 ) : 1049 – 1053 .
- Vurro , M , Boari , A , Evidente , A , Andolfi , A and Zermane , N . 2009 . Natural metabolites for parasitic weed management . Pest Manag Sci. , 65 : 566 – 571 .
- Wada , KC , Yamada , M , Shiraya , T and Takeno , K . 2010 . Salicylic acid and the flowering gene FLOWERINGLOCUST homolog are involved in poor-nutrition stress-induced flowering of Pharbitis nil . J Plant Physiol. , 167 : 447 – 452 .
- Walting , JP and Press , MC . 2001 . Impacts of infection by parasitic angiosperms on host photosynthesis . Plant Biol. , 3 : 244 – 250 .
- Wanek , W and Richter , A . 1993 . l-idiol: NAD + 5-oxidoreductase in Viscum album: utilization of host-derived sorbitol . Plant Physiol Biochem. , 31 ( 2 ) : 205 – 211 .
- Wang , H , Taketa , S , Miyao , A , Hirochika , H and Ichii , M . 2006 . Isolation of a novel lateral-rootless mutant in rice (Oryza sativa L.) with reduced sensitivity to auxin . Plant Sci. , 170 : 70 – 77 .
- Weckwerth , W . 2003 . Metabolomics in systems biology . Annu Rev Plant Biol. , 54 : 669 – 689 .
- Westwood , JH , Roney , JK , Khatibi , PA and Stromberg , VK . 2009 . RNA translocation between parasitic plants and their hosts . Pest Manag Sci. , 65 : 533 – 539 .
- Wikström , N , Savolainen , V and Chase , MW . 2001 . Evolution of the angiosperms: calibrating the family tree . Proc R Soc Lond B. , 268 : 2211 – 2220 .
- Wood , RKS . 1960 . Pectic and cellulolytic enzymes in plant disease . Annu Rev Plant Physiol. , 11 : 299 – 322 .
- Yoder , JI . 1999 . Parasitic plant responses to host plant signals: a model for subterranean plant–plant interactions . Curr Opin Plant Biol. , 2 : 65 – 70 .