Abstract
Artemisinin is a well-known antimalarial drug isolated from the Artemisia annua plant. The biosynthesis of this well-known molecule has been reinvestigated by using [1-13C]acetate, [2-13C]acetate, and [1,6-13C2]glucose. The 13C peak enrichment in artemisinin was observed in six and nine carbon atoms from [1-13C]acetate and [2-13C]acetate, respectively. The 13C NMR spectra of 13C-enriched artemisinin suggested that the mevalonic acid (MVA) pathway is the predominant route to biosynthesis of this sesquiterpene. On the other hand, the peak enrichment of five carbons of 13C-artemisinin including carbon atoms originating from methyls of dimethylallyl group of geranyl pyrophosphate (GPP) and farnesyl pyrophosphate (FPP) was observed from [1,6-13C2]glucose. This suggested that GPP which is supposed to be biosynthesized in plastids travels from plastids to cytosol through the plastidial wall and combines with isopentenyl pyrophosphate (IPP) to form the (E,E)-FPP which finally cyclizes and oxidizes to artemisinin. In this way the DXP pathway also contributes to the biosynthesis of this sesquiterpene.
Introduction
Artemisinin (21) is a sesquiterpene lactone bearing an endoperoxide ring. This is a unique feature found in this sesquiterpene and also considered to be responsible for the effective treatment of malaria caused by the multidrug-resistant Plasmodium falciparum (Liu et al. Citation1979; WHO Citation1981; Klayman et al. Citation1984; Klayman Citation1985). It is derived from the glandular secretory trichomes present in the leaves and flowers of the Artemisia annua plant. The increasing trends of malaria throughout the world and the resistance of existing drugs have increased the demand of this naturally occurring sesquiterpene. Researchers are putting maximum effort for the last 25 years to obtain large amounts of artemisinin through synthesis or by enhancing the yields in the plant through biotechnological methods (Delabays et al. Citation2001; Sy and Brown Citation2002; Ferreira et al. Citation2005; Ro et al. Citation2006; Putalun et al. Citation2007). In order to achieve higher targets through in vivo and in vitro systems, the knowledge of biosynthetic pathway from the earliest precursors to artemisinin is essential. A lot of publications have come up over the last 25 years giving information on the biosynthesis of artemisinin (21) (Akhila et al. Citation1987; Schramek et al. Citation2010) artemisinic acid (14) (Akhila et al. Citation1990; Covello et al. Citation2007), and amorphadiene (Bouwmeester et al. Citation1999) by using 14C and 13C-labeled precursors.
The complete pathway to artemisinin can be divided into three steps: (1) biosynthesis of farnesyl pyrophosphate (FPP, 9) from CO2, acetate, glucose, and isopentenylpyrophosphate (IPP, 6)/dimethylallylpyrophosphate (DMAPP, 7) (C5 units) through the mevalonic acid (MVA) or non-MVA pathways; (2) cyclization of FPP (9) to amorphadiene (13); and (3) biosynthesis of artemisinic acid (14), dihydroartemisinic acid (15), and artemisinin (21) from amorphadiene (13). The biosynthesis of artemisinic acid (14) (Akhila et al. Citation1990) and artemisinin (21) (Akhila et al. Citation1987) was studied using 14C and 3H-labeled precursors in which the biosynthetic intermediates were predicted. The mechanism of hydrogen shifts during the cyclization, and oxidations steps were also explored. In one of the reports the mechanism of cyclization of FPP (9) by amorpha-4,11-diene synthase (ADS) involving isomerization of FPP (9) to (R)-nerolidyl diphosphate (NPP, 10), ionization of NPP (10), and C-1,C-6 ring closure to generate a bisabolyl cation, followed by a 1,3-hydride shift, 1,10-ring closure to generate the amorphane skeleton, and deprotonation at either C-12 or C-13 to afford the final product amorphadiene has been discussed (Picaud et al. Citation2005). ADS uses FPP as substrate, and the isomerization to NPP has been found to occur at the enzyme's active site. The amorphadiene synthase and germacrene A synthase have been isolated and characterized, and this is the most important step in the biosynthesis of isoprenoid compounds and artemisinin in A. annua. (Bouwmeester et al. Citation1999; Mercke et al. Citation2000; Bertea et al. Citation2006). 13C and 2H-labeled sesquiterpenes like dihydroartemisinic acid (15) and dihydro-epi-deoxyarteannuin have been used during the last decade to study the biosynthetic mechanism of conversion of artemisinic acid (14) to artemisinin (21) under in vivo conditions in A. annua. (Brown and Sy Citation2004, Citation2007a, Citationb).
Recently the focus of research on biosynthesis of artemisinin (21) has shifted towards the site of synthesis of intermediate compounds along with artemisinin (21) and location of relevant enzymes in different compartments of the cell, ultimately giving information on the existence of MVA or DXP pathways (Olsson et al. Citation2009). A recent approach in this direction has revealed that artemisinin (21) was predominantly biosynthesized from (E,E) FPP (9) whose central isoprenoid unit had been obtained via the non-mevalonate pathway. The isotopologue data confirm the previously proposed mechanism for the cyclization of (E,E)-FPP to amorphadiene (13) and its oxidative conversion to artemisinin (21). The experiments were performed on live plants by using an atmosphere of 13CO2 (Schramek et al. Citation2010).
It has been amicably demonstrated now in bacteria and phototrophic eukaryotes that in addition to MVA pathway the DXP path also exists during the formation of IPP (6) and DMAPP (7) which are the building blocks of all the terpene compounds (Rohmer et al. Citation1993, Citation1996; Eisenreich et al. Citation1997, Citation1998, Citation2001; Lichtenthaler Citation1999; Rohmer Citation1999; Hoeffler et al. Citation2002). It has also been established through different piece of works that the mevalonate pathway is responsible for the biosynthesis of sesqui- and triterpenes in the cytoplasm, whereas the DXP pathway operates in plastids for the biosynthesis of mono-, di-, and tetraterpenes along with carotenoids (Arigoni et al. Citation1997). Thus, both the mechanisms are compartmentalized through the walls of the cell organs. This compartmental separation is not absolute because there have been examples when MVA has been found to be incorporated into monoterpenoidal and diterpenoidal moieties which are supposed to be formed in plastids (Schwarz Citation1994; Lichtenthaler et al. Citation1997). There are also reports that both the pathways exist in higher plants, and a ‘crosstalk’ between the two pathways can be explained by the exchange of metabolic intermediates between the cytoplasm and the plastids (Arigoni et al. Citation1997; Hemmerlin et al. Citation2003; Schuhr et al. Citation2003; Towler and Weathers Citation2007). In a recent report (Schramek et al. Citation2010) geranyl pyrophosphate (GPP) (8) was shown to cross over the plastidial wall into cytosol and become part of FPP (9) which was finally converted to artemisinin (21).
In order to re-establish the above facts by using more precise precursors, we decided to conduct the experiments by feeding [1-13C]acetate, [2-13C]acetate, and [1,6-13C2]glucose on live plants of A. annua. 13C-labeled precursors have been a useful tool in studying the biosynthetic pathway of terpene compounds. A detailed biogenetic route to IPP and DMAPP through MVA and DXP pathways has been demonstrated by using 13C precursors during the course of studying the biosynthesis of andrographolide (a diterpene) in Andrographis paniculata (Srivastava and Akhila Citation2010).
Materials and methods
Reagents
CDCl3 1H δ7.24; 13C δ77.0, [1-13C]acetate, [2-13C]acetate, and [1,6-13C2]glucose (>99% isotopic abundance) were purchased from Sigma-Aldrich, USA.
Plant material and feeding methods
Artemisia annua plants grown in the farms of CIMAP were used in this study. Three different methods were tried (Srivastava and Akhila Citation2010). (1) Direct-stem injection – 60-day- old plants (40 cm high, stem: 6 mm wide) were selected, and a needle (0.30 mm) attached with 500 µl syringe (Hamilton) was inserted up to the middle of the stem (about 3 mm) about 20 cm from the flowering tips of the plant. One hundred microliters of 10% solution of 13C-labeled precursor (acetate) was injected over 12 hrs. The feeding of acetate solution was done to 20 sets of plants. All the plant material was pooled and harvested after 15 days and artemisinin (21) was extracted. (2) Plant cuttings were fed with precursor under normal environmental conditions – 30- to 40-cm-high plant twigs with intact leaves were grown under non-sterile conditions for about three days in 25 ml Hoagland's medium. The plant cuttings were then transferred to a solution (10 ml in a test tube) of 1% 13C-labeled precursors in separate set of experiments which were supplemented with 2 ml of labeled substrate for 2 weeks and then extracted for desired compound (21). (3) Precursor uptake by plant cuttings under forced transpiration – 30- to 40-cm-high plant twigs were maintained on Hoagland's medium for 3 days and then transferred to solution (10 ml in a test tube) of 1% 13C-labeled substrate. This solution was allowed to be taken up by the plant twigs under illumination (a 60 watt bulb) and forced transpiration by an air fan. The uptake of the substrate solution was supplemented by adding 13C-labeled substrate at regular intervals as soon as the level of solution dropped by 2 ml in the test tube holding the plant twigs. After the uptake of desired precursor the twigs were maintained for seven days on sterile medium to avoid contamination. The peak enrichment of desired carbons by direct stem injection method was very poor, showing that the fed-precursor could not enter the site of biosynthesis through a proper path. Similar results were obtained in an earlier experiment also (Srivastava and Akhila Citation2010). The incorporation of 13C-labeled precursors through stem cuttings under normal conditions was best followed by reasonably good incorporation under forced transpiration uptake of the substrate.
Extraction and isolation
The plant twigs (1 kg) which were fed with 13C-labeled precursors in all the above feeding experiments were air-dried, yielding about 200 g of dry plant biomass, and mixed with the dried normal plants (1.8 kg). The pooled herbage (2 kg) was extracted with cold MeOH (3×2 l for 24 hrs). The combined extracts were concentrated in vacuo to yield 225 g of dark brown residue. The residue was defatted with hexane and then separated by column chromatography (CC) on Si Gel using EtOAc–hexane (as eluent) while increasing the percentage of EtOAc [0:100; 10:90; 20:80; 30:70; 40:60; 50:50; 60:40; 70:30; 80:20] and continuously monitoring the eluted fractions for the presence of 21. The fractions obtained from EtOAc–hexane (55:50 and 60:40) afforded white crystals of 21 which were again recrystallized from hot MeOH. The structure of 21 was confirmed by 1H and 13C-NMR spectra and its comparison with the spectroscopy data available in the literature (Rimada et al. Citation2009; Schramek et al. Citation2010).
Results and discussion
Depending upon the knowledge available in the literature, the 13C-label from C-1(*)-acetate is likely to attain the positions C-1, C-3, and C-5 in MVA (5), C-3 and C-5 in IPP (6), and DMAPP (7). Further, C-1 of MVA is lost during its conversion to IPP (6) and DMAPP (7), whereas the 13C-label from C-2() will go to C-2, C-4, and C-6 of IPP (6) and DMAPP (7) as shown in . IPP (6) and DMAPP (7) are the two building blocks of terpene skeletons, and these are biosynthesized through different mechanisms and routes in cytosol and plastids via MVA and DXP pathways, respectively. Samples of 13C-enriched artemisinin (21) were isolated from cold methanol extract of flowering twigs of A. annua which were fed with [1-13C]acetate, [2-13C]acetate, and [1,6-13C2]glucose in separate set of experiments. The numbering pattern shown in IPP (6), DMAPP (7), GPP (8), FPP (9), and NPP (10) adopted in is for the convenience to understand the positions and linkages between the three C5 units (IPP + DMAPP + IPP). However, the traditional numbering pattern has been adopted in and from numbers 1 to 15 for all the 15 carbons of sesquiterpenes starting from the carbon bearing OPP at C-1. The purified crystals of (21) were subjected to 13C-NMR analysis. 13C-enriched artemisinin obtained from [1-13C]acetate showed enhancement of 13C-signals of six carbons (C-1, C-3, C-5, C-7, C-9, and C-11), whereas 13C-enriched artemisinin obtained from [2-13C]acetate showed enrichments at nine carbon atoms (C-2, C-4, C-6, C-8, C-10, C-12, C-13, C-14, and C-15) (, ). These findings could be explained through the schematic representation shown in Figures . The IPP (6) and DMAPP (7) units combine to form GPP (8) which combines with another molecule of IPP to form (E,E)-FPP (9) (Schramek et al. Citation2010). Because of the possible stereochemical requirements for cyclization to cadinane skeleton before the formation of amorphadiene (13) the (E,E)-FPP (9) may be converted to NPP (10), the (E,Z)-isomer of FPP (Picaud et al. Citation2005) (), although this step has not been claimed by many scientists but appears to be logical as mentioned above (Picaud et al. Citation2005). Later on, the enzymatic attack of amorphadiene cyclases in several steps produce amorphadiene (13). The initial attack at C-7 by nucleophile (enzyme) possibly triggers the reaction, and a nucleophilic attack by ▵6,7 on C-1 of NPP (10) releases the pyrophosphate moiety to generate a possible unstable enzyme bond intermediate (11). In the process another enzymatic attack at C-11 induces ▵10,11 to attack the electron deficient site C-1 of 11 followed by hydrogen shift from C-1 to C-6 and another hydrogen shift from C-6 to C-7. This finally eliminates the attached enzyme from C-7 and possibly produces another unstable enzyme bond species (12). 12 is biogenetically an important intermediate because at this stage the C-10 and C-11 bond becomes free for rotation, and the identity of C-12 and C-13 methyl groups, initially obtained from DMAPP, is likely to be lost. The loss of originality of C-12 and C-13 is discussed further in the experiment done by feeding [1,6-13C2]glucose. In order to produce a terminal methylene group a hydrogen is lost either from C-12 or C-13 to produce amorphadiene (13). The terminal methyl (C-12 or C-13) of isopropyl group of 13 is oxidized through a chain of reactions involving CYP (P450 enzymes), aldehyde dehydrogenase (ALDH) to form artemisinic acid (14). Further, Dbr2 and artemsinic aldehyde Δ11,13 double bond reductase convert artemisinic aldehyde to dihydroartemisinic acid (15). Artemisinic acid (14) and dihydroartemisinic acid (15) () have been considered to be the potent precursors for artemisinin (21). Further, the peroxidation of C-2, C-3 double bond and internal rearrangements may produce the unstable intermediates 16 and 17. The biosynthetic steps shown with broken arrows indicate the presence of unstable intermediates which were difficult to isolate. The cleavage of ▵2,3 in 17 is initiated by peroxidation of ▵1,2 to yield another possible intermediate (18). It is believed that the final steps of biosynthesis of artemisinin (21) take place through a series of nucleophilic additions, elimination of peroxide, cleavage of ▵2,3, and elimination of hydroxyl group () [17→18→19→20→21]. The 13C-peak enrichment of carbon atoms in artemisinin (21) support the MVA biogenetic pathway operating in the cytosol. Arteanuin B (22) and artemisitene (23) are two biogenetically important compounds found in this plant. The biogenetic pathway to these compounds probably takes an individual route from artemisinic acid (14). If the ▵12,13 of 14 is not hydrogenated a similar path [14→16→17→18→19→20→21] will result into artemisitin (23) but this needs unequivocal confirmation. Similarly the epoxidation of ▵2,3 of artemisinic acid (14) and a lactone formation in-between C1 and C12 may result in the formation of arteanuin B (22). We tried to isolate 22 and 23 from the same experiments along with artemisinin (21); however, the amounts of 13C-enriched 22 and 23 were not substantial to run a 13C-NMR spectra. The possible carbons to be enriched in 22 and 23 are shown in . This may be proved in future studies and biosynthetic correlation between 21, 22, and 23 could be established.
Figure 1. MVA and DXP pathways are shown in cytosol and plastids, respectively. Possibility of C5 and C10 units crossing over the plastidial envelope is shown. *, denote label from [1-13C]acetate and [2-13C]acetate, respectively, whereas Δ denotes label from [1,6-13C2]glucose.
![Figure 1. MVA and DXP pathways are shown in cytosol and plastids, respectively. Possibility of C5 and C10 units crossing over the plastidial envelope is shown. *, denote label from [1-13C]acetate and [2-13C]acetate, respectively, whereas Δ denotes label from [1,6-13C2]glucose.](/cms/asset/bdcf26c6-a7d0-4bf9-853c-64622b467669/tjpi_a_570869_o_f0001g.gif)
Figure 2. Proposed biosynthetic pathway from NPP (9) to allylic hydroperoxide (17) intermediate en route to artemisinin (21). Broken arrows indicate the presence of hypothetical pathway involving free or enzyme bonded intermediates which were difficult to isolate.
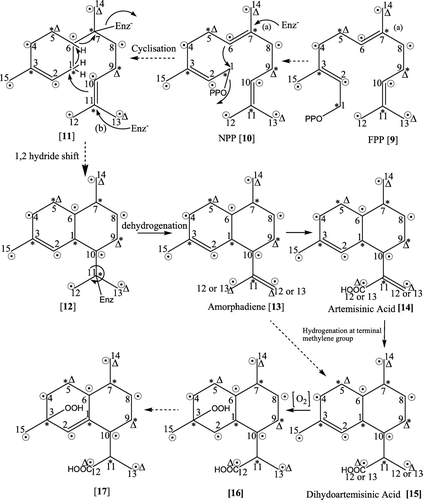
Figure 3. *, denote label from [1-13C]acetate and [2-13C]acetate when biogenetic route to artemisinin (21) is achieved from IPP and DMAPP through MVA pathway; Δ denotes label from [1,6-13C2]glucose if GPP biosynthesized from IPP and DMAPP through DXP pathway plays and important role in the biosynthesis of 21. Broken arrows indicate the presence of hypothetical pathway involving free or enzyme bonded intermediates which were difficult to isolate.
![Figure 3. *, denote label from [1-13C]acetate and [2-13C]acetate when biogenetic route to artemisinin (21) is achieved from IPP and DMAPP through MVA pathway; Δ denotes label from [1,6-13C2]glucose if GPP biosynthesized from IPP and DMAPP through DXP pathway plays and important role in the biosynthesis of 21. Broken arrows indicate the presence of hypothetical pathway involving free or enzyme bonded intermediates which were difficult to isolate.](/cms/asset/0b97d385-3360-43ae-a6b8-6bffc7aa7016/tjpi_a_570869_o_f0003g.gif)
Figure 4. (a) 13C-NMR of artemisinin isolated from A. annua. (b) 13C-NMR of artemisinin isolated from A. annua after feeding [1-13C]acetate. (c) 13C-NMR of artemisinin isolated from A. annua after feeding [2-13C]acetate. Enriched signals of 13C are seen in (b) and (c).
![Figure 4. (a) 13C-NMR of artemisinin isolated from A. annua. (b) 13C-NMR of artemisinin isolated from A. annua after feeding [1-13C]acetate. (c) 13C-NMR of artemisinin isolated from A. annua after feeding [2-13C]acetate. Enriched signals of 13C are seen in (b) and (c).](/cms/asset/ca9f8051-316e-46d7-9548-500a5db192d7/tjpi_a_570869_o_f0004g.gif)
Table 1 Incorporation of [1-13C]acetate, [2-13C]acetate, and [1,6-13C2]glucose into artemisinin (1) in Artemisia annua.
The 13C-NMR analysis of 13C-enriched artemisinin (21) obtained from A. annua twigs which were fed with [1,6-13C2]glucose gave some interesting results (). As per documentation and earlier research the glucose metabolism to IPP (6) and DMAPP (7) takes place through DXP pathway which supposedly operates in plastids of the cell () and it can also get converted to acetate by the breakdown of pyruvate (Srivastava and Akhila Citation2010) and finally to IPP (6) and DMAPP (7) through MVA pathway. The tracer from [1,6-13C2]glucose is likely to achieve different positions in IPP (6) and DMAPP (7) through MVA and DXP pathways. It has been proved in many experiments that mono- and diterpenes are formed in the plastids by the combination of IPP (6) and DMAPP (7) units through DXP pathway. Artemisinin (21) is a sesquiterpene and unlikely to obtain any incorporation from 13C-glucose through DXP pathway. However, artemisinin isolated from A. annua plants which were fed with [1,6-13C2]glucose showed enrichment at C-5, C-9, C-12, C-13, and C-14 (). Enrichment of these peaks can be explained through Figures . 13C from C-1 and C-6 (Δ)of glucose attains position C-1 and C-5 in IPP and DMAPP (), which may combine in the plastid itself in the presence of GPP synthase to make GPP (8). 13C-enriched carbons (shown as Δ) in artemisinin (21, ) are the same for GPP which are enriched with 13C as shown in . The only exception being C-12 of artemisinin which is enriched with 13C. This can be further explained that the GPP molecule travels through the plastidial membrane to cytosol where it combines with another IPP (6) which is already available there to form FPP (9) (). Later on during the cyclization process, the gem-dimethyl groups lose their identity because of the free rotation along ▵10,11 and they become identical in nature. This results in the enrichment of 13C-label as shown on C-12 and C-13. These findings are supported by earlier findings (Schramek et al. Citation2010) where GPP (10) has been shown to move from plastids to cytosol through an experiment which was conducted using universal precursor 13CO2 (Bacher and Eisenreich Citation2007).
Conclusions
The biosynthesis of artemisinin (1) has been reinvestigated using 13C-labeled precursors, and the enrichment of carbon signals in 13C-enriched artemisinin points out that MVA and DXP pathways participate in the biosynthetic process. MVA pathway is predominantly existent during the biosynthesis of 1 but it also involves geranyl (C10) moiety biosynthesized in plastids via DXP pathway and once again proof of cross talk over between the plastid and cytosol constituents is indicated.
Acknowledgements
One of the authors (NS) is thankful to CSIR for Research Internship at CIMAP. Also, we are thankful to Director, CIMAP, for encouragement during the course of work. Our thanks are also due to Mr. M.R. Khan for helping us in maintaining the plants at CIMAP farm.
References
- Akhila , A , Rani , K and Thakur , RS. 1990 . Biosynthesis of artemisinic acid in Artemisia annua . Phytochemistry. , 29 : 2129 – 2132 .
- Akhila , A , Thakur , RS and Popli , SP. 1987 . Biosynthesis of artemisinin in Artemisia annua . Phytochemistry. , 26 : 1927 – 1930 .
- Arigoni , D , Sagner , S , Latzel , C , Eisenreich , W , Bacher , A and Zenk , M. 1997 . Terpenoid biosynthesis from 1-deoxy-D-xylulose in higher plants by intramolecular skeletal rearrangement . Proc Natl Acad Sci USA. , 94 : 10600 – 10605 (and references cited therein) .
- Bacher , A and Eisenreich , W. 2007 . 13CO2 as a universal metabolic tracer in isotopologue perturbation experiments . Phytochemistry. , 68 : 2273 – 2289 .
- Bertea , CM , Voster , A , Verstappen , FWA , Maffei , M , Beekwilder , J and Bouwmeester , HJ. 2006 . Isoprenoid biosynthesis in Artemisia annua: cloning and heterologous expression of a germacrene A synthase from a glandular trichomes cDNA library . Arch Biochem Biophys. , 448 : 3 – 12 .
- Bouwmeester , HJ , Wallaart , TE , Janssen , MHA , Loo , BV , Jansen , BJM , Posthumus , MA , Schmidt , CO , Kraker , JD , Kong , WA and Franssen , MCR. 1999 . Amorpha-4,11-diene synthase catalyses the first probable step in artemisinin synthesis . Phytochemistry. , 52 : 843 – 854 .
- Brown , GD and Sy , L. 2004 . Synthesis of labeled dihydroartemisinic acid . Tetrahedron. , 60 : 1125 – 1138 .
- Brown , GD and Sy , L. 2007a . In vivo transformation of artemisininc acid in Artemisia annua plants . Tetrahedron. , 63 : 9548 – 9566 .
- Brown , GD and Sy , L. 2007b . In vivo transformation of dihydro-epi-deoxyarteannuin B in Artemisia annua plants . Tetrahedron. , 63 : 9536 – 9547 .
- Covello , PS , Teoh , KH , Polichuk , DR , Reed , DW and Nowak , G. 2007 . Functional genomics and the biosynthesis of artemisinin . Phytochemistry. , 68 : 1864 – 1871 .
- Delabays , N , Simonnet , X and Gaudin , M. 2001 . The genetics of artemisinin content in Artemisia annua L. and the breeding of yielding cultivars . Curr Med Chem. , 8 : 1795 – 1801 .
- Eisenreich , W , Rohdich , F and Bacher , A. 2001 . Deoxyxylulose phosphate pathway to terpenoids . Trends Plant Sci. , 6 : 78 – 84 .
- Eisenreich , W , Sagner , S , Zenk , MH and Bacher , A. 1997 . Monoterpenoid essential oils are not of mevalonoid origin . Tetrahedron Lett. , 38 : 3889 – 3892 .
- Eisenreich , W , Schwarz , M , Cartayrade , A , Arigoni , D , Zenk , MH and Bacher , A. 1998 . The deoxyxylulose phosphate pathway of terpenoid biosynthesis in plants and microorganisms . Chem Biol. , 5 : 221 – 233 .
- Ferreira , JFS , Laughlin , JC , Delabays , N and Magalhaes , PMD. 2005 . Cultivation and genetics of Artemisia annua L. for increased production of the antimalarial artemisinin . Plant Gen Res. , 3 ( 2 ) : 206 – 229 .
- Hemmerlin , A , Hoeffler , J , Meyer , O , Tritsch , D , Kagan , IA , Grosdemange-Billiard , C , Rohmer , M and Bach , TJ. 2003 . Crosstalk between the cytosolic mevalonate and the plastidial methylerythritol phosphate pathways in tobacco bright yellow-2 cells . J Biol Chem. , 278 : 26666 – 26676 .
- Hoeffler , J , Hammerlin , A , Grosdemange-Billiard , C , Bach , TJ and Rohmer , M. 2002 . Isoprenoid biosynthesis in higher plants and in Escherichia coli on the branching in the methylerythritol phosphate pathway and the independent biosynthesis of isopentenyl diphosphate and dimethylallyl diphosphate . Biochem J. , 366 : 573 – 583 .
- Klayman , DL. 1985 . Qinghaosu (artemisinin): an antimalarial drug from China . Science. , 228 ( 4703 ) : 1049 – 1055 .
- Klayman , DL , Lin , AJ , Acton , N , Scovill , JP , Hock , J , Milhous , WK and Theoharides , AD. 1984 . Isolation of artemisinin (qinghaosu) from Artemisia annua growing in the United States . J Nat Prod. , 47 : 715 – 717 .
- Lichtenthaler , HK. 1999 . The 1-deoxy-D-xylulose -5-phosphate pathway of isoprenoid biosynthesis in plants . Annu Rev Plant Physiol Plant Mol Biol. , 50 : 47 – 65 .
- Lichtenthaler , HK , Schwender , J , Disch , A and Rohmer , M. 1997 . Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate independent pathway . FEBS Lett. , 400 : 271 – 274 .
- Liu , JM , Ni , MY , Fan , JF , Tu , YY , Wu , ZH , Wu , YL and Chou , WS. 1979 . Structure and reaction of arteannuin . Acta Chim Sinica. , 37 : 129 – 143 .
- Mercke , P , Bengtsson , M , Bouwmeester , HJ and Posthumus , MA. 2000 . Molecular cloning, expression, and characterization of Amorpha-4,11-diene synthase, a key enzyme of artemisinin biosynthesis in Artemisia annua L . Arch Biochem Biophys. , 381 : 173 – 180 .
- Olsson , ME , Olofsson , LM , Lindahl , AL , Lundgren , A , Brodelius , M and Brodelius , PE. 2009 . Localization of enzymes of artemisinin biosynthesis to the apical cells of grandular secretory trichomes of Artemisia annua L . Phytochemistry. , 70 : 1123 – 1128 .
- Picaud , S , Mercke , P , He , X , Sterner , O , Brodelius , M , Cane , DE and Brodelius , PE. 2005 . Amorpha-4,11-diene synthase: mechanism and stereochemistry of the enzymatic cyclisation of farnesyl diphosphate . Arch Biochem Biophys. , 448 : 150 – 155 .
- Putalun , W , Luealon , W , Eknamkul , WD , Tanaka , H and Shoyama , Y. 2007 . Improvement of artemisinin production by chitosan in hairy root cultures of Artemisia annua L . Biotechnol Lett. , 29 : 1143 – 1146 .
- Rimada , RS , Gatti , WO , Jeandupeux , R and Cafferata , FR. 2009 . Isolation characterization and qualification of artemisinin by NMR from Argentinean Artemisia annua L . Boletin Latinoamericano y del Caribe de Plantas Medicinales y Aromaticas. , 8 ( 4 ) : 275 – 281 .
- Ro , D , Paradise , EM , Ouellet , M , Fisher , KJ , Newman , KL , Ndungu , JM , Ho , KA , Eachus , RA , Ham , TS Chang , MCY . 2006 . Schematic representation of engineered artemisinic acid biosynthetic pathway in S.cerevisiae strain EPY224 expressing CYP71AV1 and CPR . Nature. , 440 : 940 – 943 .
- Rohmer , M . 1999 . The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants . Nat Prod Rep. , 16 : 565 – 574 .
- Rohmer , M , Knani , M , Simonin , P , Sutter , B and Sahm , H. 1993 . Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate . Biochem J. , 295 : 517 – 524 .
- Rohmer , M , Seemann , M , Horbach , S , Bringer-Meyer , S and Sahm , H. 1996 . Glyceraldehyde 3-phosphate and pyruvate as precursors of isoprenic units in an alternative non-mevalonate pathway for terpenoid biosynthesis . J Am Chem Soc. , 118 : 2564 – 2566 .
- Schramek , N , Wang , H , Romisch-Margl , W , Keil , B , Radykewicz , T , Winzenhorlein , B , Beerhues , L , Bacher , A and Rohdich , F. 2010 . Artemisinin biosynthesis in growing plants of Artemisia annua. A 13CO2 study . Phytochemistry. , 71 : 179 – 187 .
- Schuhr , CA , Radykewicz , T , Sagner , S , Latzel , C , Zenk , MH , Arigoni , D , Bacher , A , Rohdich , F and Eisenreich , W. 2003 . Quantitative assessment of crosstalk between the two isoprenoid biosynthesis pathways in plants by NMR spectroscopy . Phytochem Rev. , 2 : 3 – 16 .
- Schwarz MK . 1994 . Terpen-biosynthese in Ginkgo biloba: Eine Oberraschende Geschichte . [Terpene biosynthesis in Ginkgo biloba: a surprising story] Thesis Nr. 10951, Schweiz: ETH Zurich .
- Srivastava , N and Akhila , A. 2010 . Biosynthesis of andrographolide in Andrographis paniculata . Phytochemistry. , 71 : 1298 – 1304 .
- Sy , L and Brown , GD. 2002 . The mechanism of the spontaneous autoxidation of dihydroartemisinic acid . Tetrahedron. , 58 : 897 – 908 .
- Towler , MJ and Weathers , PJ. 2007 . Evidence of artemisinin production from IPP streaming from both the mevalonate and the nonmevalonate pathways . Plant Cell Rep. , 26 : 2129 – 2136 .
- World Health Organization . 1981 . Report of the fourth meeting of the scientific working group on the chemotherapy of malaria. Beijing, People's Republic of China, October 6–10 . Geneva, , Switzerland : Office of Publications World Health Organization .