Abstract
UV-B-mediated effects in Oryza sativa L. are studied considering micronutrients’ accumulation and the photosynthetic performance. UV-B-irradiated rice leaves showed increasing membrane permeability and an inhibition of Fe accumulation linked to an antagonistic interaction with Mn. An increasing passive Cu uptake is also pointed, whereas Zn contents are associated with a higher root acidification capacity. In UV-B-irradiated leaves, photosynthetic pigments and the rates of the light reactions decreased significantly between the 15th and 28th day after germination. In the leaves grown after UV-B exposure, the electron transport rates coupled to photosystems II and II plus I increased significantly between the 21st and 28th day, whereas the Mehler reactions increased continuously from the 21st day onward. It is concluded that during vegetative growth the nutrient status is mostly linked to the inhibition of roots’ acidification capacity, but after UV-B exposure micronutrients’ accumulation favors this genotype recovery, shielding the photosynthetic light reactions.
Introduction
The depletion of the stratospheric ozone, namely caused by chlorofluorocarbons and other gaseous emissions, is triggering an increasing amount of UV-B radiation (about 10%, in particular the waveband 297–310 nm) reaching the Earth's surface (Munakata et al. Citation2006).
Although UV-B radiation constitutes only 1.5% of the solar energy, it is detrimental to terrestrial ecosystems. The majority of evidence indicates that UV-B radiation usually triggers inhibitory effects among species and cultivars, yet not all plant responses are damaging or disadvantageous (Teramura and Murali Citation1986; Mohammed and Tarpley Citation2009). It has been reported that supraoptimal UV-B doses might inhibit plant growth (Sullivan and Teramura Citation1989; Mohammed and Tarpley Citation2009), biomass production (Tosserams et al. Citation2001; Tsukaguchi and Iida Citation2008), crop yield (Teramura and Sullivan Citation1991; Corlett et al. Citation1997; Tsukaguchi and Iida Citation2008; Mohammed and Tarpley Citation2009), photosynthetic pigments (Musil et al. Citation2002; Balakrishnan et al. Citation2005) and capacity (Ilwanzik et al. Citation1983; Hidema et al. Citation1991; Teramura and Sullivan Citation1994). Yet, in contrast to the majority reports on Chl reduction following UV-B treatment, Battag and Brennan (Citation2000) showed that the concentrations of Chl a and b in cucumber (Cucumis sativus L.) were not affected by UV-B treatment, while Musil et al. (Citation2002) reported an increase in Chl concentration for three South African plant species following UV-B exposure. The carotenoid content of UV-B–exposed Colophspermum mopane and Phylica pubescens also increased (Musil et al. Citation2002), and this was proposed to be a biochemical response to alleviate UV-B stress. In this context, Sato and Kumagai (Citation1993) also examined the sensitivity to UV-B radiation of 198 rice cultivars, belonging to five Asian rice ecotypes (from the Bengal region and Indonesia) and Japanese lowland and upland rice groups, concluding that genotypes vary widely in the same ecotype and group. In addition, they concluded that rice cultivars originating from regions with higher ambient UV-B radiation do not necessarily exhibit higher levels of tolerance.
According to the United States Environmental Protection Agency (1995), the ozone layer is naturally thinner in the equatorial tropics (which is the main location area of rice production) compared with the mid- and high latitudes, and so there is less ozone to absorb the UV radiation as it passes through the atmosphere. Under the assumption that solar UV-B radiation will reach peak levels on Earth's surface in the next few years (Kakani et al. Citation2003) and considering the reports of higher UV-B fluxes in the area around the equator (United States Environmental Protection Agency 1995), a preliminary study of this type is paramount to evaluate the degree of rice necrosis and recovery mechanisms implicating the related nutrient status after supplemental UV-B irradiation. In this context, considering that the mobilization of photoassimilates is closely linked with biomass production, the UV-B–mediated effects on accumulation of micronutrients in roots, leaves and crops of Oryza sativa L. cv Safari are summarized, discussed and correlated with the plant metabolism, using as a test system the central role of the photosynthetic performance during the life cycle. In rice, the interactions among micronutrients (Lidon and Henriques Citation1993a, Citation1994, Citation1998; Lidon Citation2000, Citation2001) sharply affect biomass production (Lidon and Henriques Citation1993a; Lidon Citation2001), biosynhesis of isoprenoids (Lidon and Henriques Citation1992) and the photosynthetic performance, namely the light reactions (Lidon and Henriques Citation1991, Citation1993b, Citationc). In this context, the deleterious effects of UV-B are putatively explained, hypothesizing that during the vegetative growth the nutrient status in UV-B–treated rice is mostly linked to the inhibition of roots’ acidification capacity and photosynthetic impairment, but after UV-B exposure the patterns of micronutrients’ accumulation kinetics favors this genotype to recover, being also shielded by the photosynthetic light reactions.
Materials and methods
Rice (Oryza sativa L. cv. Safari) seeds were washed, sterilized and germinated as described by Lidon and Henriques (Citation1998). The seedlings (in 2-L pots, 10 replicates and 5 independent series from each treatment) were grown hydroponically, for 170 days, at 32–36/25–27°C day/night temperatures and under an average Photosynthetic Photon Flux Density (PPFD) of 2000 µmol m−2 s−1, during a 12-h day period.
UV-B stress was induced in an irradiation chamber with 10 narrow-band (λ=311 nm) fluorescent lamps (TL 100 W/01; Philips, Eindhoven, Holanda) and ambient PPFD intensity of about 400 µmol m−2 s−1. The intensity of UV-B radiation was measured with a RM-21 spectroradiometer (Gröbel UV-Electronics, Ettlingen, Germany). In the experiment for assessment of dose–response relationships, plants were irradiated, 8 days after germination (each day, immediately after the second hour of the daylight), with UV-B fluxes of 22 W m−2 for 1 h, for 7 days. These resulted in a total biological effective UV-B of 2.975 kJ m−2 (each day) and a total of 20,825 kJ m−2, as weighted by Caldwell's generalized plant action spectrum (Caldwell Citation1971).
The nutrient solution, developed for rice growth by Yoshida et al. (Citation1976), containing (in mg L-1) 40 N, 10 P, 40 K, 40 Ca, 40 Mg, 20 Al, 0.5 Mn, 0.2 B, 0.05 Mo, 0.01 Cu and 0.01 Zn was used. Iron was added as hexahydrated FeCl3 at 2 mg L-1. The solutions were adjusted daily to pH 4.5, the volume being restored to its original level, and renewed every 5 days.
At the end of 15, 21, 28 and 170 days (corresponding to total shoot necrosis), following germination, for the analytical measurements, plants were chosen at random, after all physically damaged or deformed plants were discarded. The analytical determinations considered the physiological alterations directly imposed by UV-B supplemental irradiation during the vegetative growth stage, thus the leaves directly UV-B irradiated (at 15 and 21 days after germination) and grown after UV-B stress (21 and 28 days old), as well as the leaves at the end of the reproductive phase (in 170-day-old plants).
Electrolytic conductance, which indicates the extent of membrane permeability, was determined with a Crison 522 conductimeter, according to Ketchie (Citation1969), with the modifications introduced by Lidon (Citation2000). Three grams of roots and leaves (youngest fully expanded) were incubated with 20 mL of deionized water for 8 h and, thereafter, the absolute conductance was measured. After a heat shock treatment, at 100°C, for 7 min, the volume of deionized water was corrected to 20 mL and the electrolytic yield was taken as 100%. The results are expressed on a percentage basis.
The measurement of the acidification capacity followed the method described by Zocchi and Cocucci (Citation1990), with the modifications introduced by Lidon (Citation2001). A preincubation was carried out for 60 min, at pH 6.2, using 8 g of excised roots in 10 mL of a 5 mL CaSO4 solution, with a shaking bath (100 oscillations/min) maintained at 26°C. Thereafter, the preincubation medium was changed for 10 mL of a fresh solution containing 0.1 mM CuSO4. After incubating for 120 min, the ATPase proton pump activity was measured with a Radiometer 64 pH-meter, which might be considered an indication of the related kinetics functioning.
Prior to Chls and carotenoids’ analysis, entire shoots were submitted to a constant flow of deionized water (10 L min−1, for 2 min). These pigments were determined spectrophotometricaly, as previously determined (Lidon and Henriques Citation1991, Citation1992), using leaf (youngest fully expanded) pigment samples suspended in an 80% acetone solution and centrifuged at 1300 g, for 4 min, in order to remove debris prior to the measurement of absorbencies at 663.2, 646.8 and 470.0 nm.
For metals’ analysis, entire roots and shoots were submitted to a constant flow of deionized water (10 L min−1, for 5 min). Concentrations of Mn, Cu, Zn and Fe were measured after mixing leaves or roots of 15 dry plants (at 100°C, for 10 days) of each UV-B treatment, from three different cultures. These samples were divided into three fractions (to obtain triplicates of three independent series), a small portion of each part being digested in HCl (20%), following Chapman and Pratt (Citation1961). A Perkin-Elmer model 3030 atomic absorption unit, equipped with a hollow cathode lamp, was used for these metals’ determinations.
The maximum photosynthetic activities were obtained, under saturating light conditions, in a Clark-type oxygen electrode (LW2, Hansatech), using subchloroplast fractions (Lidon and Henriques Citation1991) extracted from youngest fully expanded leaves. Leaves were homogenized in a medium containing 0.4 M sorbitol, 10 mM NaCl, 5 mM MgCl2, 2 mM EDTA, 1 mM MnCl2, 2 mM ascorbato, 0.4% bovine serum albumin (BSA) and 50 mM MES (pH 6.4). After filtration through four layers of nylon, chloroplasts were sedimented by centrifugation at 2000 g for 4 min. The chloroplasts were washed twice in the isolation medium and resuspended in a medium containing 0.33 M manitol, 10 mM NaCl, 1 mM MgCl2, 2 mM EDTA, 1mM MnCl2, 0.4% BSA and 50 mM HEPES (pH 7.5). The electron transport rates associated with PSII, PSI and PSII plus PSI were determined in 1 mL of reaction mixture containing 100–150 µg Chl, at 25°C, and with a photon flux density of 3000 µmol m−2 s−1 (which ensures a maximum rate for the Hill and Mehler reactions kinetics), given by a Björkman lamp. As previously described (Lidon et al. Citation2001), for measuring the PSII light reactions 2,6-dichlorophenolindophenol was used as electron acceptor from the quinine pool; to determine the electron transport associated with PSI, reduced 2,6- dichlorophenolindophenol was used as electron donor to the Cyt b6/f complex, with methylviologen as electron acceptor; sodium azide and 3(3,4-dichlorophenyl)-1,1-dimethylurea were also applied to inhibit peroxidise activities and the electron transport before the plastoquinone, respectively; to analyze the maximum activities of PSII plus PSI, methyl viologen was used as electron acceptor.
Statistic analysis was performed by two-way ANOVA (p≤0.05). For mean comparison, a Tukey test was applied, considering a 95% confidence level.
Results
The electrolytic conductance of non-UV-B–treated rice roots and leaves () did not vary significantly until 170 days after germination (even during grain production by the 80th day) but remained lower in the leaves. In UV-B–stressed rice roots, electrolytic conductance followed a similar pattern and during the entire growth cycle was lower (although nonsignificantly) relative to the control plants. Compared with the control plants, the electrolytic conductance of the mature leaves that were directly submitted to UV-B radiation was significantly higher until the 28th day after germination (all the tissues becoming necrotic 7–10 days later). In the UV-B–treated leaves, the electrolytic conductance decreased significantly between 15 and 21/28 days. In the leaves of the stressed plants grown after UV-B exposure, the electrolytic conductance increased significantly only after 170 days of germination (this value also being different relative to the control); in the 21/28-day-old plants, these leaves displayed an electrolytic conductance similar to those of non-UV-B–stressed plants.
Table 1. Root acidification capacity and root and leaves electrolytic conductance of control and UV-B-treated rice.
Until the 28th day after germination, root acidification capacity () of non-UV-B–treated rice remained stable, with a significant increase being detected only at the end of the life cycle (170 days). In UV-B–stressed rice, root acidification capacity showed a significant decrease between 15- and 21/28-day-old plants, followed by a strong increase at the end of the growth cycle. During the life cycle, root acidification capacity of the UV-B–treated rice persisted within lower (and significant) interval values relative to the non-UV-B–treated plants.
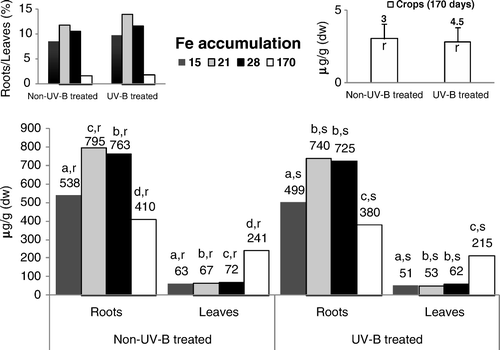
The concentration of Fe (), in the roots of nontreated UV-B rice plants, varied significantly, increasing between the 15- and 21-day-old plants, and decreasing thereafter. In UV-B–stressed rice, Fe content in the roots did not vary significantly between 21- and 28-day-old plants, but 15 days after germination was significantly lower and a higher drop occurred at the end of the growth cycle. In the leaves of control and UV-B–stressed plants, the concentration of Fe increased significantly, from the 15-day-old plants onward (except between 15 and 21 days after germination in UV-B–treated rice). By comparing the levels of Fe during the entire growth cycle, lower (and significant) values in the UV-B–stressed rice leaves were found. In addition, the level of Fe persisted systematically higher in the roots (relative to the leaves), increasing its ratio until the 21st day following germination, but decreasing thereafter.
Table 2. Concentrations of micronutrients in the roots, leaves and seeds of control and UV-B-treated rice plants.
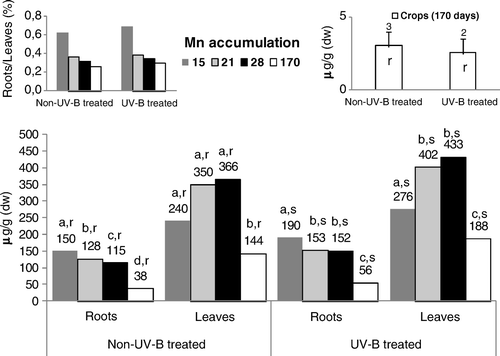
In all the experiments, the level of Mn () in the roots decreased from the 15-day-old plants onward (). Without application of UV-B radiation, the lower content of this metal in the roots was found 170 days after germination. After UV-B radiation, the concentration of Mn was significantly higher in 15-day-old roots, the lowest significant values being found in 170-day-old plants. In rice leaves, as a general pattern, the content of Mn remained systematically higher relative to that in the root tissues, yet the ratios between root and leaves’ Mn concentration decreased progressively in all the experimental periods. Without UV-B application, the content of Mn in the leaves showed only a slight (nonsignificant) increase until the 28th day after germination, followed by a significant decrease in 170-day-old plants. In UV-B–treated rice leaves, a significant increase occurred between 15 and 21/28 days following germination, but a sharp drop (also significant) was detected at the end of the life cycle. By comparing the levels of Mn concentration between UV-B–treated and non-UV-B–treated rice, it was found that during the entire growth cycle, this metal's content was consistently and significantly higher in the roots and leaves of stressed plants.
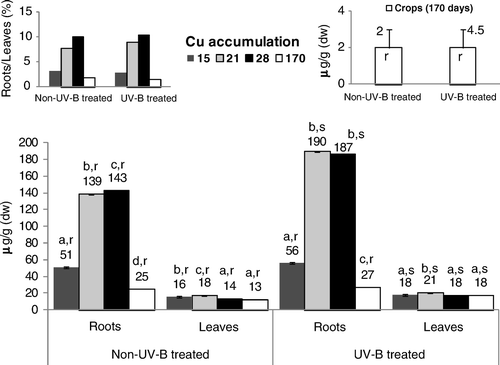
The level of Cu in rice () roots grown without UV-B application increased significantly until the 28th day after germination. In these tissues, after application of UV-B radiation, a similar pattern was found, with a significant increase in Cu level occurring between 15- and 21/28-day-old plants, followed by a significant decrease at the end of the growth cycle. In the tissues of leaves of control plants, 21 days after germination, the concentration of Cu rose significantly, decreasing thereafter. The application of UV-B triggered a similar pattern for Cu accumulation, yet in 28- and 170-day-old plants the values remained stable. As a general pattern, the concentration of this nutrient became systematically higher in the roots and leaves of UV-B–treated rice. Nevertheless, the ratio between the Cu content of roots and leaves increased progressively until the 28th day after germination, decreasing in the 170-day-old plants.
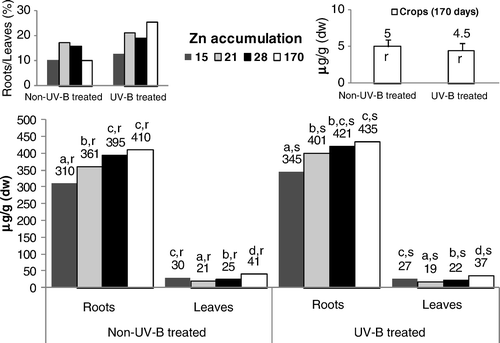
Without UV-B application, Zn content () in rice roots increased from the 15-day-old plants until the end of the growth cycle (170 days). During the entire life cycle, these tissues displayed a similar trend in UV-B–treated rice, but the values in non-UV-B–treated rice roots remained consistently lower. In the leaves of control and UV-B–stressed rice, the content of Zn decreased significantly between the 15- and 21-day-old plants, increasing thereafter until the end of the life cycle. In UV-B–treated rice, the content of Zn in the tissues of leaves also persisted significantly lower. The ratio between the Zn concentration of roots and leaves increased progressively until the 21st day after germination, decreasing in the 170-day-old plants (except in UV-B–treated plants).
In the seeds, the effects of UV-B application were not detected, the mean content values of Mn, Cu, Fe and Zn remaining similar and within interval values characteristic of this rice cultivar (Lidon Citation2001).
In non-UV-B–treated rice, the level of Chl did not vary significantly until the end of the life cycle, yet carotenoids’ content increased significantly (ca. 1.34-fold) between the 15- and 21-day-old plants, remaining thereafter without significant variations (). In rice leaves directly submitted to UV-B radiation, the content of Chl and carotenoids decreased significantly (to 61% and 55%, respectively) between 15- and 28-day-old plants. Moreover, in the leaves grown after UV-B application, the levels of these molecules did not vary significantly. In each experimental period, levels of Chl and carotenoids decreased significantly in the stressed leaves (relative to that in the non-UV-B–treated, about 58%, 64% and 76% in the 15-, 21-, and 28-day-old plants, respectively). Until the 28th day after germination, rice leaves grown after UV-B exposure showed higher Chl content relative to the control plants, whereas the level of carotenoids did not vary significantly ().
Table 3. Pigments contents of control and UV-B-treated rice plants. Each value is the mean (±SE) of three replicates of three independent series.
The electron transport rates associated with PSII, PSI and PSII plus PSI in non-UV-B–treated rice () increased significantly between 15- and 28-day-old plants (1.31-, 1.21- and 1.26-fold, respectively), decreasing thereafter (). In stressed rice leaves directly exposed to UV-B radiation, these reactions decreased consistently until the 28th day after germination. In the leaves belonging to stressed rice that grew after UV-B exposure, the electron transport rates coupled to PSII increased significantly between the 21st and 28th day after germination, but decreased at the end of the life cycle. A similar pattern was found for the acyclic photosynthetic electron transport rates (). Moreover, the electron transport rates coupled with PSI increased continuously from the 21st day after germination onward. In each experimental period, between the 15th and 28th day after germination, the rates of the photosynthetic electron transport varied significantly, but always remained higher in the control plants. At the end of the life cycle, in UV-B–treated and non-UV-B–treated rice, the rates of the PSII and PSI reactions remained consistently similar, but that of the PSII plus PSI decreased significantly.
Table 4. Photosynthetic electron transport rates of control and UV-B-treated rice plants.
Discussion
UV-B supplemental irradiation might trigger many changes in plant metabolism and also cause damage to cellular components. In this context, in the leaves directly exposed to UV-B radiation, the significant increase in membrane permeability until the 28th day after germination () impaired all the main processes of leaves, namely the nutrients’ translocation rates and the photosynthesis functioning (–), triggering, as also reported by Tevini (Citation1983) for other plant species, tissue necrosis. The increasing membrane permeability of the leaves directly submitted to UV-B radiation () inhibited Fe accumulation in rice tissues (), because although its levels remained within values metabolically controlled (Kabata-Pendias and Pendias Citation1992), the degradation of the cellular membranes in the leaves () limited citrate chelates synthesis. Accordingly, this effect might have limited its availability for Fe-chelates uptake by the roots (Chaney et al. Citation1972; Kabata-Pendias and Pendias Citation1992) and translocation () through the xylem to the shoots (Chaney et al. Citation1972; Moore Citation1972; Tiffin Citation1977). The content of Fe when correlated with the higher Mn values () found in the roots and leaves of UV-B–treated rice (relative to non-UV-B–treated rice) suggests an antagonistic interaction, since these metals are interrelated in their metabolic functions (Alvarez-Tinaut et al. Citation1980). Indeed, although the Mn uptake rates are metabolically controlled, the higher level of this metal in the leaves of both treatments (compared with the roots) resulted in its rapid translocation, as the movement of micronutrients is not bound to insoluble organic ligands in the root tissues or in the xylem fluid (Kabata-Pendias and Pendias Citation1992). Even so, although Mn content might show remarkable variations with plant species, stage of growth and different organs, this metal's availability in the control and UV-B–treated rice remained within a considered normal range for this genotype (Loneragan Citation1975; Lidon Citation2001). During the growth cycle, the higher level of Cu in the roots of both treatments () resulted in the strong capacity of these tissues to hold Cu against its transport to the leaves (Loneragan Citation1981; Kabata-Pendias and Pendias Citation1992), as previously also found in this genotype (Lidon and Henriques Citation1993a). Indeed, in Oryza sativa L. cv Safari, Cu uptake is an active process (Lidon and Henriques Citation1993a; Bravin et al. Citation2009), this metal being almost entirely retained in complexed forms (Lidon and Henriques Citation1994; Bravin et al. Citation2009), namely in cell walls (Lidon and Henriques Citation1994, Citation1998). Nevertheless, probably because of the higher electrolytic conductance, the higher content of this metal in the roots of UV-B–stressed rice () also indicated an increasing passive uptake that promoted a slight increase in this metal's concentration in the leaves. Eventually, this effect was additionally coupled with the lower acidification capacity in the UV-B–treated rice (). Although the rate of Zn absorption might differ greatly among species (Kabata-Pendias and Pendias Citation1992), this metal and Cu are absorbed by a similar mechanism (Graham Citation1981; Arnold et al. Citation2010). Until the 28th day after germination, the obtained patterns () seemed to comply with this proposal in both treatments, with the higher Zn content in the roots of UV-B–treated plants until the end of the growth cycle also suggesting a close link with the decreasing rates of roots’ acidification capacity ( ). Indeed, although disagreement exists whether Zn uptake is an active or a passive process (Kabata-Pendias and Pendias Citation1992), several authors (Moore Citation1972; Loneragan Citation1975; Arnold et al. Citation2010) support that it is mostly metabolically controlled. In addition, as it has long been known (Tiffin Citation1977), considering that Zn binds to light organic compounds in xylem fluids, the lower concentration of this metal in the leaves of UV-B–treated rice () pointed to an additional negative action, implicating the alteration of membrane permeability (). At a cellular level, Fe is a key metal in energy transformation, participating in Chl synthesis and integrating chloroplast heme proteins implicated in the photosynthetic electron transfer (Boardman Citation1975; Wild and Jones Citation1988; Lidon and Henriques Citation1992). Accordingly, in the leaves directly submitted to UV-B radiation, as Fe accumulation kinetics decreased, Chl and carotenoids’ accumulation () became inhibited and, furthermore, as this metal integrates the structures of the Cyt b6/f complex and PSI, the increasing membrane permeability further contributed to leaf tissues’ necrosis augmentation. The PSII light reactions in the UV-B–treated rice leaves also followed a pattern previously described by Trebst and Depka (Citation1990) for other plants, indicating a general failure of the photosynthetic electron transfer pathway, as well as the inhibition of oxygen evolution and of the reaction center of PSII. Nevertheless, the higher content of Mn in the leaves () points to an absence of a link between this micronutrient and the inhibition of the photosynthetic light reactions (). In addition, considering that Cu specifically integrates the structure of Pc, the higher accumulation of this metal in UV-B–treated rice () also prevented the inhibition of the PSI-associated light reactions between the cyt b6/f complex and PSI (), thus contributing to its relative insensitivity to UV-B radiation, as reported by Ilwanzik et al. (Citation1983) and Teramura and Ziska (Citation1996) for other plant species.
Figure 1. Concentrations of Fe in roots, leaves and seeds of control and UV-B treated rice plants. Each value is the mean (+SE) of three replicates of three independent series. Different letters indicate significant differences among treatments: a, b, c, d among the experimental periods; r, s among treatments within each experimental period.
In the rice leaves grown after exposure to UV-B radiation, the electrolytic release became quite similar until the 28th day after germination (), allowing the balancing of the micronutrient status as well as the structural recovery of the photosynthetic apparatus ( and ). Indeed, although revealing lower rates comparative to the non-UV-B–treated rice leaves, the photosynthetic light reactions increased until the end of the life cycle, also following a pattern previously reported for other plants (Ilwanzik et al. Citation1983; Teramura and Ziska Citation1996). In a situation of oxidative stress imposed by UV-B irradiation, chloroplast is the first organelle to show injury response (Campbell Citation1975). Yet, the higher Chl and carotenoid content of rice leaves not directly exposed to UV-B radiation, comparative to non-UV-B–treated rice, clearly pointed, as previously found (Lidon and Henriques Citation1992), to an additional synthesis of phytoene and phytofluene, implicating Mn and Fe accumulation and, thus, a higher rate of its metabolic synthesis. The higher Mn content in the leaves not directly submitted to UV-B stress also became an additional pool required for the recovery and protection of Mn structure in the oxygen evolving complex and, thus, the reaction centres of PSII shielded the maximum rates of the PSII-associated reactions. In addition, as UV-B radiation produces ROS, such as superoxide, hydrogen peroxide and singlet oxygen (Hideg et al. Citation1997), considering the recovery of the photosynthetic electron transport chain in rice leaves grown after UV-B exposure (), Zn content did not limit Cu, ZnSoD synthesis, which is essential for the control of rice leaves’ senescence (Lidon and Henriques Citation1993b, Citationc).
Figure 2. Concentrations of Mn in roots, leaves and seeds of control and UV-B treated rice plants. Each value is the mean (+SE) of three replicates of three independent series. Different letters indicate significant differences among treatments: a, b, c, d among the experimental periods; r, s among treatments within each experimental period.
In the grains of both treatments, the low Fe, Mn, Cu and Zn content () remained within the usually considered normal range (Lidon Citation2000) and resulted in the low mobility of this element within the phloem vessels, since only small amounts move to the young organs, others than roots and shoots (Lidon Citation2000). In this context, the high rates of the photosynthetic electron transport chain, until the end of the life cycle (), also become a main contributor to the mobilization of photosynthetic assimilates to the grain in UV-B–stressed rice.
Figure 3. Concentrations of Cu in roots, leaves and seeds of control and UV-B treated rice plants. Each value is the mean (+SE) of three replicates of three independent series. Different letters indicate significant differences among treatments: a, b, c, d among the experimental periods; r, s among treatments within each experimental period.
Conclusion
During the vegetative growth, the antagonistic patterns displayed by UV-B radiation on rice roots’ acidification capacity and these tissues related electrolytic conductance indicates that nutrients’ imbalances did not result in a disruption of cellular membranes selectivity. Moreover, in the leaves’ tissues, the significant disruption in membranes’ permeability is linked to the inhibition of the roots’ ATPase proton pump activity, particularly until 28 days after germination. Leaves tissues’ necrosis in the UV-B–treated rice was linked to a general impairment of the cellular metabolism, which also implicated Fe accumulation. Moreover, in the leaves grown after UV-B exposure, the patterns of micronutrients accumulation are associated with the recovery of the plant metabolism, as expressed through the photosynthetic performance.
Figure 4. Concentrations of Zn in roots, leaves and seeds of control and UV-B treated rice plants. Each value is the mean (+SE) of three replicates of three independent series. Different letters indicate significant differences among treatments: a, b, c, d among the experimental periods; r, s among treatments within each experimental period.
References
- Alvarez-Tinaut , MC , Leal , A and Recalde-Martinez , LR. 1980 . Iron-manganese interaction and its relation to boron levels in tomato plants . Plant Soil. , 55 : 377 – 388 .
- Arnold , T , Kirk , GJ , Wissuwa , M , Frei , M , Zhao , FJ , Mason , TF and Weis , DJ. 2010 . Evidence for the mechanisms of zinc uptake by rice using isotope fractionation . Plant Cell Environ. , 33 : 370 – 381 .
- Balakrishnan , V , Ravindran , KC , Venkatesan , K and Karuppusamy , S. 2005 . Effect of UV-B supplemental radiation on growth and biochemical characteristics in Crotalaria juncea L. seedlings . Electr J Environ Agric Food Chem. , 4 : 1125 – 1131 .
- Battag , PR and Brennan , TM. 2000 . Differential effects of short-term exposure to UV-B radiation upon photosynthesis in cotyledons of a resistant and a susceptible species . Int J Plant Sci. , 161 : 771 – 778 .
- Boardman , NK. 1975 . “ Trace elements in photosynthesis ” . In Trace elements in soil-plant-animal systems , Edited by: Nicholas , PJD and Egan , AR . 199 New York : Academic Press .
- Bravin , MN , Merrer , B , Denaix , L , Schneider , A and Hinsinger , P. 2009 . Copper uptake kinetics in hydroponically-grown durum wheat (Triticum turgidum durum L.) as compared with soil's ability to supply copper . Plant Soil. , 31 : 91 – 104 .
- Caldwell , MM. 1971 . “ Solar ultraviolet radiation and the growth and development of higher plants ” . In Photophysiology , Edited by: Giese , AC . 131 – 177 . New York : Academic Press. 6 .
- Campbell , WS. 1975 . “ Impacts of climatic changes on the biosphere ” . In Ultraviolet radiation effects. Monograph 5, Climatic Impact Assessment Program, U.S. Department of Transportation, Report No. DOT-TST-75-55 , Edited by: Nachtwey , NW , Caldwell , MM and Biggs , RH . 167 – 176 . Springfield , VA : National Technical Information Service .
- Chaney , RL , Brown , JC and Tiffin , LO. 1972 . Obligatory reduction of ferric chelates in iron uptake by soybeans . Plant Physiol. , 50 : 208 – 213 .
- Chapman , HD and Pratt , PF. 1961 . Methods of analysis of soils, plants and waters , Berkeley , , USA : University of California, Division of Agricultural Sciences .
- Corlett , JE , Stephen , J , Jones , HG , Woodfin , R , Mepsted , R and Paul , ND. 1997 . “ Assessing the impact of UV-B radiation on the growth and yield of field crops ” . In Plants and UV-B: responses to environmental change. Society for Experimental Biology, Seminar Series 64 , Edited by: Lumsden , PJ . Cambridge : Cambridge University Press .
- Graham , RD. 1981 . “ Absorption of copper by plant roots ” . In Copper in soil and plants , Edited by: Loneragan , JF , Robson , JF and Graham , RD . 141 – 163 . New York : Academic Press .
- Hideg , E , Mano , J , Ohno , C and Asada , K. 1997 . Increased levels of monodehydroascorbate radical in UV-B-irradiated broad bean leaves . Plant Cell Physiol. , 38 : 684 – 690 .
- Hidema , J , Makino , A , Mae , T and Ojima , T. 1991 . Photosynthetic characteristics of rice leaves under different irradiances from full expansion through senescence . Plant Physiol. , 97 : 1287 – 1293 .
- Ilwanzik , W , Tevini , M , Dohnt , G , Vos , M , Weiss , W , Gräber , P and Renger , G. 1983 . Action of UV-B radiation on photosynthetic primary reaction in spinach chloroplasts . Physiologia Plantarum. , 58 : 401 – 407 .
- Kabata-Pendias , A and Pendias , H. 1992 . Trace elements in soils and plants , 2nd ed , Boca Raton : CRC Press .
- Kakani , VG , Reddy , KR , Zhao , D and Mohammed , AR. 2003 . Effects of ultraviolet-B radiation on cotton morphology and anatomy . Annu Bot. , 91 : 817 – 826 .
- Ketchie , DO. 1969 . Methods of determining cold harness and cold injury in citrus . Proc First Int Citrus Symp. , 2 : 559 – 563 .
- Lidon , FC. 2000 . Rice adaptation to excess manganese: nutrients accumulation and implications on the quality of the crops . J Plant Physiol. , 156 : 652 – 658 .
- Lidon , FC. 2001 . Tolerance of rice to excess manganese in the early stages of vegetative growth. Characterisation of manganese accumulation . J Plant Physiol. , 158 : 1341 – 1348 .
- Lidon , FC and Henriques , FS. 1991 . Limiting step on photosynthesis of rice plants treated with varying copper levels . J Plant Physiol. , 138 : 115 – 118 .
- Lidon , FC and Henriques , FS. 1992 . Effects of excess copper on photosynthetic pigments in rice plants . Bot Bull Acad Sinica. , 33 : 141 – 149 .
- Lidon , FC and Henriques , FS. 1993a . Effects of copper toxicity on growth and metabolic uptake and translocation in rice plants . J Plant Nutr. , 16 : 1449 – 1464 .
- Lidon , FC and Henriques , FS. 1993b . Copper-mediated oxygen toxicity in rice chloroplasts . Photosynthica. , 29 : 385 – 400 .
- Lidon , FC and Henriques , FS. 1993c . Oxygen metabolism in higher plant chloroplasts . Photosynthica. , 29 : 249 – 279 .
- Lidon , FC and Henriques , FS. 1994 . Subcellular localization of copper and partial isolation of copper proteins in roots from rice exposed to excess copper . Aust J Plant Physiol. , 21 : 427 – 436 .
- Lidon , FC and Henriques , FS. 1998 . Role of rice shoot vacuoles in copper toxicity regulation . Environ Exp Bot. , 39 : 197 – 202 .
- Lidon , FC , Loureiro , AS , Vieira , DE , Bilhó , EA , Nobre , P and Costa , R. 2001 . Photoinhibition of chilling stressed wheat and maize . Photosynthetica. , 39 : 161 – 166 .
- Loneragan , JF. 1975 . “ The availability and absorption of trace elements in soil plant systems and their relation to movement and concentration of trace elements in plants ” . In Trace elements in soil-plant-animal systems , Edited by: Nicholas , DJD and Egan , AR . 109 – 134 . London : Academic Press .
- Loneragan , JF. 1981 . “ Distribution and movement of copper in plants ” . In Copper in soil and plants , Edited by: Loneragan , JF , Robson , AD and Graham , RD . 165 – 210 . New York : Academic Press .
- Mohammed , AR and Tarpley , L. 2009 . Effects of elevated ultraviolet-B radiation on productive tillers, spikelet sterility and grain characteristics of southern US rice (Oryza sativa L.) cultivars . J Agron Crop Sci. , 195 : 292 – 300 .
- Moore , DP. 1972 . “ Mechanisms of micronutrients uptake by plants ” . In Micronutrients in agriculture , Edited by: Mortvedt , JJ , Giordano , PM and Lindsay , WL . 171 – 198 . Madison , WI : Soil Science Society of America .
- Munakata , N , Cornain , S , Kanoko , M , Mulyadi , K , Lestari , S and Wirohadidjojo , W. 2006 . Biological monitoring of solar UV radiation at 17 sites in Asia, Europe and South America from 1999 to 2004 . Photochem Photobiol. , 22 : 689 – 694 .
- Musil , CF , Chimphango , SBM and Dakora , FD. 2002 . Effects of elevated ultraviolet-B radiation on native and cultivated plants of southern Africa . Annu Bot. , 90 : 127 – 137 .
- Sato , T and Kumagai , T. 1993 . Cultivar differences in resistance to the inhibitory effects of near-UV radiation among Asian ecotype and Japanese lowland and upland cultivars of rice (Oryza sativa L.) . Jpn J Breed. , 43 : 61 – 68 .
- Sullivan , JH and Teramura , AH. 1989 . The effects of ultraviolet-B radiation on loblolly pine. I. Growth, photosynthesis and pigment production in greenhouse-grown seedlings . Physiol Plantarum. , 77 : 202 – 207 .
- Teramura , AH and Murali , NS. 1986 . Intraspecific differences in growth and yield of soybean exposed to ultraviolet-B radiation under greenhouse and field conditions . Environ Exp Bot. , 26 : 89 – 95 .
- Teramura , AH and Sullivan , JH. 1991 . “ Potential effects of increased solar UV-B on global plant productivity ” . In Photobiology , Edited by: Riklis , E . 625 – 634 . New York : Plenum Press .
- Teramura , AH and Sullivan , JH. 1994 . Effects of UV-B radiation on photosynthesis and growth of terrestrial plants . Photosynthesis Res. , 39 : 463 – 473 .
- Teramura AH , Ziska LH . 1996 . Environmental stress and photosynthesis: ultraviolet-B radiation . In: Baker NR Advances in photosynthesis . London : Kluwer Press . 5 : 435 – 450 .
- Tevini , M. 1983 . UV-B radiation and ozone depletion: effects on humans, animals, plants, microorganisms and materials , Boca Raton : Lewis Publications .
- Tiffin , LO. 1977 . “ The form and distribution of metals in plants: an overview ” . In Proceedings Hanford Life Sciences Symposium , 315 – 334 . Washington , DC : U.S. Department of Energy. Symposium Series .
- Tosserams , M , Visser , A , Groen , M , Kalis , G , Magendans , E and Rozema , J. 2001 . Combined effects of CO2 concentration and enhanced UV-B radiation on faba bean . Plant Ecol. , 154 : 195 – 210 .
- Trebst , A and Depka , B. 1990 . Degradation of the D-l protein subunit of photosystem II in isolated thylakoids by UV light . Z Naturforsch. , 45c : 765 – 771 .
- Tsukaguchi , T and Iida , Y. 2008 . Effects of assimilate supply and high temperature during grain-filling period on the occurrence of various types of chalky kernels in rice plants (Oryza sativa L.) . Plant Prod Sci. , 11 : 203 – 210 .
- United States Environmental Protection Agency . 1995 . Air and radiation. A guide to the UV index and sun-safe behavior [cited 2007 June 27]. Available from: http://www.epa.gov/sunwise1/doc/sunuvu.pdf
- Wild A , Jones LHP . 1988 . Mineral nutrition in crop plants . In: Wild A, editor. Russell's soil conditions and plant growth. Harlow , Essex : Longman Scientific Technology Publications . p. 69 .
- Yoshida , SD , Forno , A , Cook , JH and Gomes , KA. 1976 . “ Routine procedure for growing rice plants in culture solution ” . In Laboratory manual for physiological studies of rice , 61 – 65 . Los Baños , Laguna, Philippines : The International Rice Research Institute .
- Zocchi , G and Cocucci , S. 1990 . Fe uptake mechanism in Fe-efficient cucumber roots . Plant Physiol. , 92 : 908 – 911 .