Abstract
Cuscuta is a stem holoparasitic plant without leaves or roots, parasitizing various types of host plants and causing major problems for certain crops. Cuscuta is known as a generalist and, thus, must have unique parasite strategies to cope with different host plants. For elucidating metabolic responses and mechanisms of parasitization, metabolomic approaches using GC/MS were applied. We compared five stages of Cuscuta japonica: early stage seedlings, with far red light (FR) cue, with contact signal, haustorium induced seedlings by both signals and adult plant parasites on host plants. Sugars, amino acids, organic acids, nucleic acids, and polyols were identified from the polar phase fraction. The apical part contained metabolite profiles different from the haustorium induced part or the basal part. Amino acid and some organic acids were up-regulated for haustorium induction but decreased after parasitization. After attachment to different host plants, metabolite profiles of Cuscuta japonica changed dramatically due to the absorption of specific host plant metabolites such as pinitol. Cuscuta seedlings attached to pinitol rich host plants contained more pinitol and showed different profiles from those attached to plants having less or lacking pinitol.
Introduction
Cuscuta is a globally widespreaded parasite plant and commonly known as dodder. It causes major damage to crops such as tomato, potato, and tobacco in the USA (Press and Phoenix Citation2005). Cuscuta develops a haustorium. This special organ, differentiated from the stem, enables the parasite that lacks leaves and roots to obtain important nutrients from various host plants. Considering the ubiquitous presence of Cuscuta and the translocation of various substances during parasitism, Cuscuta can serve as a key model plant for deciphering the mechanism of parasitism as well as for examining host plant–parasite plant interactions (Furuhashi et al. Citation2011).
Most previous studies used isotope labels and observed carbon or nitrogen flux between Cuscuta and the host plant (Jeschke et al. Citation1997; Jeschke and Hilpert Citation1997). Most of these studies, however, did not compare seedlings with a host to seedlings without a host. Some studies compared metabolites (e.g. plant hormones) in Cuscuta seedlings (haustorium-induced and/or non-induced seedlings) with Cuscuta attached to host plants (Löffler et al. Citation1999; Runyon et al. Citation2008). To date however, no comprehensive metabolite profiling has been performed.
Cuscuta haustorium induction requires both a light signal (blue and/or FR light) and a contact signal, but no chemicals (Tada et al. Citation1996). A correlation between metabolite change or signal transduction caused by light/contact and Cuscuta haustorium induction has not been investigated. Metabolic changes due to light signals in other higher plants have been reported (Bino et al. Citation2005; Lake et al. Citation2009), but no information is available on metabolic changes of thigmomorphogenesis caused by contact signals or mechanical stress.
Metabolomic approaches have been applied in plant interaction only recently (Scherling et al. Citation2010). Metabolism research on other parasitic organisms (e.g. bacterial and protozoan parasite to human) is rather advanced. However, there is some research on necrotrophic, biotrophic, and hemibiotrophic predation by herbivores (mainly insects), symbiont relationships (e.g. nitrogen-fixing fungi and legumes), biodiversity effects (Scherling et al. Citation2010) as well as parasitic relationships (e.g. parasitic nematodes) (Allwood et al. Citation2008). Most plant metabolomic research has focused on pathogenic interactions between microorganisms and host plants in view of plant pathology. Model plants, such as Arabidopsis and Medicago, have been used in modern plant Metabolomics (Weckwerth et al. Citation2004; Larrainzar et al. Citation2009). Comparisons between mutant and wild types, and stress responses under various conditions are two main research directions. Metabolomic techniques have rarely been applied to parasite plant research, an exception are studies on root parasite plants (Estabrook and Yoder Citation1998; Bouwmeester et al. Citation2003). Recently, methods were established for artificial haustorium induction (Tada et al. Citation1996). These strategies provide a unique experimental system to analyze Cuscuta seedlings under FR light and/or with a contact signal attached to different host plants.
Methods
Sample preparation
Cuscuta japonica seeds (about 200) were soaked in concentrated sulfuric acid for 10 min and washed with water (the surface of the seeds was peeled off). Seeds were then placed on cotton gauze soaked with water and incubated at 25°C in complete darkness for 2 days. Germinated seeds were transferred to flower pots and incubated at 25°C in complete darkness for 3 days, and then moved to white light (fluorescent lamp: Hitachi FL20SS EDKF2P) at the same temperature for 3 days. Seedlings were then sandwiched between two plastic plates in order to stimulate them physically. Thereafter, the seedlings were placed under far-red light (1–2 W/m2) for 15 min, followed by placement in a dark room at 25°C for 2 days. Far-red light was obtained from a lamp (Toshiba FL-20S FR-74) filtering with Deleglass A900 (Asahi kasei company). All seedling manipulations were done under green light in darkness. Negative control seedlings (no haustorium development) were not exposed to far-red light or to a contact signal.
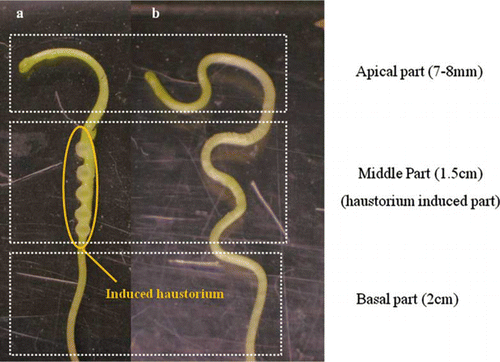
All seedlings with and without developing haustorium were excised and separated into apical (7–8 mm from tip), middle (1.5-cm length which is 1 cm from apical region, this is the same to haustorium-induced part), and basal regions (2-cm length which is 2 cm from the apical region) (). The deteriorated part was removed and samples were shock frozen and then lyophilized.
Figure 1. Photography of Cuscuta seedling with and without haustorium. (a) haustorium induced Cuscuta seedling; (b) haustorium non-induced Cuscuta seedling.
For Cuscuta seedlings with parasitization, the seedlings were prepared as follows. About 200 seeds were soaked in concentrated sulfuric acid for 10 min and then washed with water (until the surface of the seeds peeled off). Seeds were placed on cotton gauze soaked in water. Incubation was at 25°C in complete darkness for 2 days. Germinated seeds were transferred to flower pots and incubated at 25°C under white light for 3 days (fluorescent lamp: Hitachi FL20SS EDKF2P). Seedlings were then attached to host plants in the field with surgical tapes. The hosts were: Conyza sumatrensis (about 50-cm tall), Buxus microphylla (new branch of 30–40 years old plant), and Pueraria thunbergiana (10 years old). Cuscuta seedlings were attached 15–20 cm from the apex of Buxus or Conyza and 20–30 cm of Pueraria. Cuscuta was excised and collected 3 weeks after attachment. The apical part is 2 cm from the tip, the middle part bears the haustorium (~2 cm-length). After excision, seedlings were frozen and lyophilized.
Extraction of polar metabolites and derivatization
Seedlings were pooled and lyophilized. Dried samples (around 15 mg) were homogenized with a pestle. One milliliter of cold extraction buffer (methanol:chloroform:distilled water = 2.5:1:0.5) was added for 10 min with vortexing at 4°C. This was followed by centrifugation at 14,000 g for 4 min at 4°C. The supernatant was transferred to new tubes and 500 µL of water and 200 µL chloroform were added and vortexed. The mixture was centrifuged at 21,000 g for 2 min at 4°C, and the upper layer (polar phase) containing water-soluble metabolites was subsequently transferred to new tubes (modified from Weckwerth et al. Citation2004). Samples were dried completely with a micro concentrator (supply manufacturer).
As sugar and polyols are abundant metabolites in Cuscuta seedlings and mask many other metabolites, we depleted these abundant metabolites. Five hundred microliters of distilled water was added to the dried sample to dissolve it followed by incubation with anion exchange column (Dowex1X8) resin for 10 min at room temperature. The supernatant was taken as the flow-through fraction containing sugar and polyol. The resin was washed with distilled water. The amino acid and organic acid fraction was eluted with 1M HCl. Both the flow-through and eluate fraction were dried in a micro concentrator. Twenty microliters of methoximine mixture (20 mg methoxyamine hydrochloride in 500 µL pyridine) was added for 90 min at 30°C while shaking. This was followed by addition of 80 µL of MSTFA for 30 min at 37°C while shaking. The mixture was centrifugation at 21,000 g for 2 min, the supernatant was transferred into a glass micro-vial and 1 µL samples were injected into the GC/MS instrument (Thermo) in a randomized sequence.
GC/MS instrument and conditions
GC/MS measurements were carried out on a triple quad (TSQ Quantum GC: Thermo) instrument. The injector temperature was 230°C using CT split less mode. Split flow rate was 1 mL min−1. GC separation was performed on an HP-5MS capillary column (30 m×0.25 mm×0.25 µm) (Agilent Technologies, Santa Clara, CA). The temperature after a 1 min, 70°C isotherm period was programmed to 76°C at a heating rate of 1°C min−1, then to 350°C at a heating rate of 6°C min−1 and maintained for 1 min. The temperatures of the transfer line of GC/MS and the source of the mass spectrometer were 340 and 250°C, respectively. The mass spectrometer was operated in electron-impact (EI) mode at 70 eV in a scan range of m/z 40–600. Metabolites were identified based on their mass spectral characteristics and GC retention times, by comparison with retention times of reference compounds in an in-house reference library.
Identified metabolites were quantified using the following masses (m/z) for each metabolite: Adenine, 264; Ala, 116; Asp, 232; Citric acid, 273; Ile, 158; Fructose, 103; Glu, 246; Glucose, 205; Gly, 174; Glyceric acid, 189; Glycerin, 205; Leu, 158; Malic acid, 233; Myo-inositol, 305; Oxalic acid, 45; Pentonic acid, 117; Phe, 218; Pinitol, 260; Pro, 142; Pyroglutamic acid, 156; Quinic acid, 345; Ribose, 103; Ser, 204; Sucrose, 437; Thr, 117; Uracil, 241; Val, 144. Inositol 1 and 2 was quantified with a myo-inositol standard curve (Inositol 1 and 2 did not show the same retention time as the myo-inositol peaks, although they have the same MS fragmentation patter as myo-inositol. Pentonic acid was quantified with a ribonic acid standard curve, and disaccharides were quantified with a cellobiose standard curve. All metabolites were quantified and calculated in mol percentages using LC quan (Thermo), software for the analysis of mass spectrometric data. For PCA analysis, we used an in-house MATLAB tool. Three principle components were used and missing values were imputed as 1×10−14. For calculating p-value, t-test was conducted by using software called R.
Results
A typical GC/MS analysis of Cuscuta samples produced about 200 reproducible mass spectra from which 31 metabolites were identified and quantified. In the flow-through fraction of the anion-exchange column, sugars (except ribose) and polyols were observed. The HCl elution contained amino acids, organic acids, nucleic bases, and disaccharides (a, b). The flow-through fraction was the primary fraction and was more concentrated than the HCl elution fraction. In the flow-through fraction, the middle region and basal region of Cuscuta seedlings showed a similar pattern (a). A change in metabolites (e.g. sugar and polyol) was mainly seen in the apical region (a). From t-test (Appendix ), some amino acids (Val, Ile, pyro-Glutamate, Asp, Phe, Glu) and citric acid were up-regulated with any signal (b), as these metabolites showed small p-value.
Figure 2. GC/MS chromatogram of polar metabolites in haustorium induced Cuscuta seedling. (a) flow-through fraction; (b) HCl elution fraction. Flow-through fraction was 10 times diluted prior to injection, indicating most of the polar metabolites are sugars and polyols.
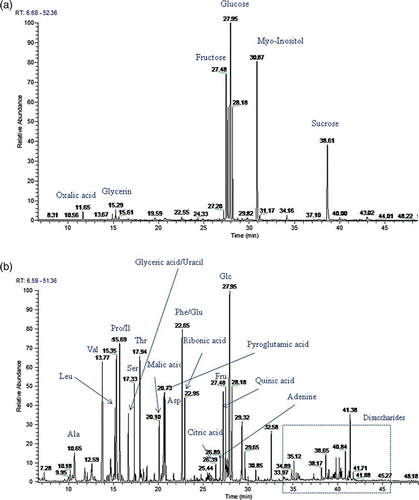
Figure 3. Molar percentage graph of metabolites. Cuscuta seedlings without any cue, seedlings with FR light signal, seedlings with contact signal and haustorium induced seedlings. (a) Graph of sugar and polyols. Apical, middle, basal indicates the regions of the seedling used for this analysis; (b) Graph of amino acids and organic acids at apical part of Cuscuta seedlings.Value is mean and error bar indicates SD (three biological replicates).
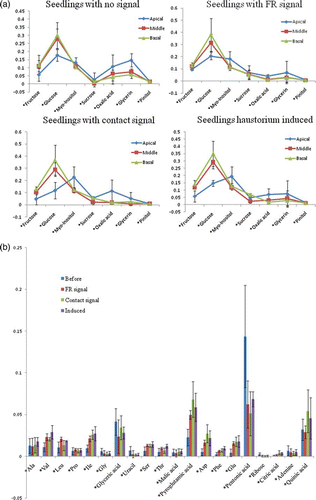
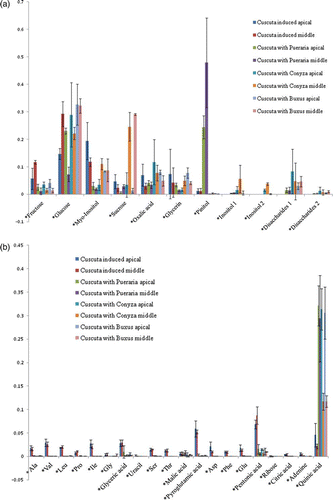
In principal components analysis (PCA), metabolites of Cuscuta parasitizing host plants differed from metabolites of Cuscuta seedlings with haustoria induced by a FR and contact signal (). In the samples the mol percentage of fructose, myo-inositol, amino acids (Ala, Val, Leu, Pro, Ile, Ser, Thr, pyro-glutamic acid, Asp, Phe, Glu), and organic acids (pentonic acid and citric acid) dropped after parasitization (except quinic acid, which increased) (a, b and Appendix ). In addition, other types of inositols and Glc–Glc disaccharides composed of two units of glucose (identified as Laminaribiose with a GMD library based on NIST software) were found. Cuscuta attached to Pueraria showed a higher (>20%) mol percentage of pinitol both in the apical and middle region (haustorium part). Cuscuta attached to Buxus and Conyza contained less pinitol and the values were even lower than in Cuscuta seedlings before parasitization. Although Cuscuta attached to Pueraria did not contain large amounts of glucose and sucrose, Cuscuta attached to Buxus and Conyza did especially in the haustorium induced parts.
Figure 4. PCA of the apical part of Cuscuta seedlings without host plants (without any cue; with FR light; with contact signal; haustorium induced), attached to pinitol poor host plants (Conyza/Buxus), and pinitol rich host plant (Pueraria). These are separated into 3 groups.
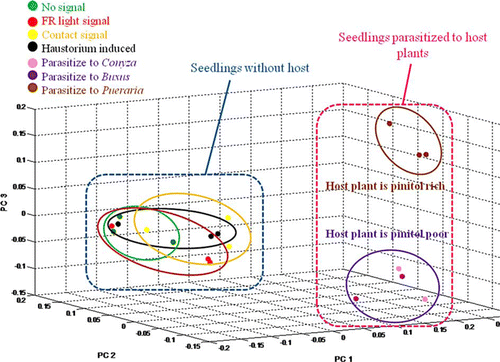
Figure 5. Molar percentage comparison between haustorium induced Cuscuta seedlings and Cuscuta seedlings attached to Pueraria/Conyza/Buxus respectively. (a) molar percentages of sugars and polyols; (b) molar percentages of amino acids and organic acids. Pinitol was only elevated in seedlings attached to Pueraria, sucrose was elevated in seedlings attached to Conyza/Buxus. Value is mean and error bar indicates SD (3 biological replicates).
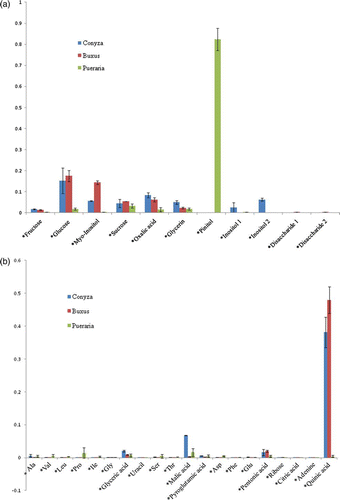
Host plants without Cuscuta parasitization clearly showed different metabolite profiling from Cuscuta seedlings. Pinitol is dominant in Pueraria, and quinic acid is dominant in Conyza and Buxus (a, b). Moreover, glucose, myo-inositol, oxalic acid were bigger in both Conyza and Buxus, but not in Pueraria.
Figure 6. Molar percentage graph of metabolites in stem part of host plants without Cuscuta parasitization (Conyza, Buxus, Pueraria). (a) molar percentages of sugars and polyols; (b) molar percentages of amino acids and organic acids. Pinitol is dominant in Pueraria and quinic acid is dominant in Conyza/Buxus.
Discussion
Significant changes in the abundance of metabolites at the apical region indicate that this region is most active during haustorium development for parasitization. In general, amino acids and organic acids appear to be up-regulated for haustorium induction, but those were decreased after parasitization except increase of quinic acid. Nevertheless, there was no prominent difference in PCA figure between Cuscuta seedlings with FR signal and with contact signal, and the effect of each signal to haustorium induction is still uncertain. Metabolite changes appear to be rather pronounced in plant–plant interaction than haustorium development. In contrast, protein profiling showed protein changes are more substantial for haustorium induction (unpublished data, manuscript in preparation).
The increase of pinitol in Cuscuta after parasitizing Pueraria (Fabaceae) is interesting because the pinitol level was low in all seedling samples before parasitization. Fabaceae are known to contain more pinitol than other plants. Pinitol is an important indicator of drought stress, and pinitol level in host plant would be increased due to drought stress caused by parasitism (Press et al. Citation1990; Ishitani et al. Citation1996). Accordingly, most of the pinitol in Cuscuta might originate from the host plant. In contrast, the sucrose increase with parasitization on Buxus and Conyza is concomitant with a lower mol percentage of pinitol. The correlation with pinitol is still poorly understood. As the connection between Cuscuta and the host plant is a direct phloem connection, all metabolites are generally moved into Cuscuta without selection. For this reason, sucrose taken up from Buxus or Conyza would already have been converted into other metabolites. The larger proportion of pinitol in Cuscuta from Pueraria might maintain low osmotic potential for unidirectional flow in the phloem connection from the host plant to Cuscuta, but Cuscuta attached to Buxus or Conyza could use sucrose and other sugars instead of pinitol for this purpose. These differences in metabolite contents suggest that different metabolites used by Cuscuta may indicate different types of drought response by the host plant, although the direct phloem connection between Cuscuta and all host plants is the same. However, these observations need further studies. Furthermore, these observations might enable in future investigation of different mechanisms of drought stress response in certain plant by analyzing the Cuscuta/host plant interaction.
The quinic acid mol percentage was strongly increased after parasitization. Quinic acid is normally present in vacuoles and not inside the phloem (Lang et al. Citation1991), and its increase after parasitization is due to an increase in Cuscuta but not a translocation from the host, because increase was seen in Cuscuta seedlings with Pueraria containing relatively poor quinic acid without Cuscuta. Consequently, there is a possibility that quinic acid of Cuscuta seedlings was up-regulated by parasitization, due to plant–plant interaction. Quinic acid is a component of cell walls, pigments, and chemicals for the defense system, but its role in Cuscuta remains uncertain. One plausible idea is that quinic acid is necessary to prevent overexposure to light. Especially after parasitization, Cuscuta can change its color from green to purple. Not only carotenoids but also quinic acid and other organic acids might be required to protect Cuscuta from too much light.
Changes (especially, reduction or loss) in metabolites after parasitization remains a complex issue and hinders simple conclusions, as there is evidence that some parasitic plants (e.g. Viscum) needs to degrade or convert some metabolites taken up from host plants (Richter and Popp Citation1992; Wanek and Richter Citation1993).
Previous pathogenic interaction research is hampered by technical difficulties. For example, it is almost impossible to distinguish whether metabolites in the parasite were absorbed from the host or produced by the parasite, especially in the case of intracellular parasites (Kafsack and Llinas Citation2010). The holostemparasitic plant Cuscuta can serve as an important system for studies on plant–plant interactions. Different responses from host plants to Cuscuta might be able to partially clarify some potential tendencies of plant stress response between different plant taxa, and may also suggest unknown stress response mechanisms in host plants.
Acknowledgements
Andreas Richter provided useful comments at an early stage of the manuscript. Wolfgang Hoehenwarter and Michael Stachowitsch helped with comments and suggestions.
References
- Allwood , JW , Ellis , DI and Goodacre , R . 2008 . Metabolomic technologies and their application to the study of plants and plant–host interactions . Physiol Plant. , 132 : 117 – 135 .
- Bino , RJ , Ric de Vos , CH , Lieberman , M , Hall , RD , Bovy , A , Jonker , HH , Tikunov , Y , Lommen , A , Moco , S and Levin , I . 2005 . The light-hyperresponsive high pigment-2dg mutation of tomato: alterations in the fruit metabolome . New Phytol. , 166 : 427 – 438 .
- Bouwmeester , HJ , Matusova , R , Zhongkui , S and Beale , MH . 2003 . Secondary metabolite signalling in host–parasitic plant interactions . Curr Opinion Plant Biol. , 6 : 358 – 364 .
- Estabrook , EM and Yoder , JI . 1998 . Plant-plant communications: rhizosphere signaling between parasitic angiosperms and their hosts . Plant Physiol. , 116 : 1 – 7 .
- Furuhashi T , Furuhashi K , Weckwerth W . 2011 . The parasitic mechanism of the holostemparasitic plant Cuscuta . J Plant Inter .
- Ishitani , M , Majumder , AL , Bornhouser , A , Michalowski , CB , Jensen , RG and Bohnert , HJ . 1996 . Coordinate transcriptional induction of myo-inositol metabolism during environmental stress . Plant J. , 9 ( 4 ) : 537 – 548 .
- Jeschke , WD , Baig , A and Hilpert , A . 1997 . Sink-stimulated photosynthesis, increased transpiration and increased demand-dependent stimulation of nitrate uptake: nitrogen and carbon relations in the parasitic association Cuscuta reflexa-Coleus blumei . JExp Bot. , 48 ( 309 ) : 915 – 925 .
- Jeschke , WD and Hilpert , A . 1997 . Sink-Stimulated photosynthesis and sink-dependent increase in nitrate uptake: nitrogen and carbon relations of the parasitic association Cuscuta reflexa-Ricinus communis . Plant Cell Environ. , 20 : 47 – 56 .
- Kafsack , BFC and Llinas , M . 2010 . Eating at the table of another: metabolomics of host-parasite interactions . Cell Host Microbe. , 7 : 90 – 99 .
- Lake , JA , Field , KJ , Davey , MP , Beerling , DJ and Lomax , BH . 2009 . Metabolomic and physiological responses reveal multi-phasic acclimation of Arabidopsis thaliana to chronic UV radiation . Plant Cell Environ. , 32 : 1377 – 1389 .
- Lang , M , Stober , F and Lichtenthaler , HK . 1991 . Fluorescence emission spectra of plant leaves and plant constituents . Radiat Environ Biophys. , 30 : 333 – 347 .
- Larrainzar , E , Wienkoop , S , Scherling , C , Kempa , S , Ladrera , R , Arrese-Igor , C , Weckwerth , W and Gonzalez , EM . 2009 . Carbon metabolism and bacteroid functioning are involved in the regulation of nitrogen fixation in Medicago truncatula under drought and recovery . Mol Plant Microbe Interact. , 22 : 1565 – 1576 .
- Löffler , C , Czygan , FC and Proksch , P . 1999 . Role of indole-3-acetic acid in the interaction of the phanerogamic parasite cuscuta and host plants . Plant Biol. , 1 : 613 – 617 .
- Press , MC , Graves , JD and Stewart , GR . 1990 . Physiology of the interaction of angiosperm parasites and their higher plant hosts . Plant Cell Environ. , 13 : 91 – 104 .
- Press , MC and Phoenix , GK . 2005 . Impacts of parasitic plants on natural communities . New Phytol. , 166 : 737 – 751 .
- Richter , A and Popp , M . 1992 . The physiological importance of accumulation of cyclitols in Visum album L . New Phytol. , 121 : 431 – 438 .
- Runyon , JB , Mescher , MC and Moraes , CMD . 2008 . Parasitism by Cuscuta pentagona attenuates host plant defenses against insect herbivores . Plant Physiol. , 146 : 987 – 995 .
- Scherling C , Roscher C , Giavalisco P , Schulze ED , Weckwerth W . 2010 . Metabolomics unravel contrasting effects of biodiversity on the performance of individual plant species . Plos One . 5 : e12569
- Tada , Y , Sugai , M and Furuhashi , K . 1996 . Haustoria of Cuscuta japonica, a holoparasitic flowering plant, are induced by cooperative effect of far-red light and tactile stimuli . Plant Cell Physiol. , 37 ( 8 ) : 1049 – 1053 .
- Wanek , W and Richter , A . 1993 . L-Idiol: NAD + 5-oxidoreductase in Viscum album: utilization of host-derived sorbitol . Plant Physiol Biochem. , 31 ( 2 ) : 205 – 211 .
- Weckwerth , W , Wenzel , K and Fiehn , O . 2004 . Process for the integrated extraction, identification and quantification of metabolites, proteins and RNA to reveal their co-regulation in biochemical networks . Proteomics. , 4 : 78 – 83 .