Abstract
Proof of the existence of aphid-derived molecules that induce emission of volatile organic compounds as indirect defensive responses in plants (hereafter called elicitors) has not yet been obtained. We showed here the presence and some aspects of the chemical nature of these elicitors. Exogenous application of the fed diet (liquid diet previously fed on by the Pea aphid, Acyrthosiphon pisum) on the damaged part of leaves of broad bean plants, made the plants more attractive to a parasitic wasp, Aphidius ervi, than application of the unfed diet. This result suggested that elicitors existed in the fed diet. Chemical characterization using ultrafiltration and subsequent bioassay revealed that the responsible factor was larger than 3 kDa. Heating of the fed diet did not inactivate its ability to induce attractive volatiles, which suggested thermotolerance of the elicitor. These results contribute to the mechanistic understanding of plant defenses against aphids.
Introduction
In response to herbivory, plants show various physiological changes that are related to direct and/or indirect defense against herbivores (Karban and Baldwin Citation1997; Walling Citation2000). One of the well-known changes is the emission of herbivore-induced plant volatiles (HIPVs) attractive to carnivorous natural enemies of herbivores (for reviews, Arimura et al. Citation2005; Sabelis et al. Citation2007; Arimura et al. Citation2009). Clarifying the factors of herbivore origin that are involved in the production of HIPVs (hereafter referred to as ‘elicitors’ in this article) has been an intriguing issue and has attracted many researchers’ attention. Several elicitors from Lepidopterous herbivores (folivores) have been reported (for reviews, Schmelz et al. Citation2009; Mori and Yoshinaga Citation2011). By contrast, little is known about elicitors of phloem-feeding insects and mites. Here, we focused on elicitors of pea aphid (Acyrthosiphon pisum) in a tritrophic system of broad bean plants, pea aphids, and parasitic wasps (Aphidius ervi). In previous studies, broad bean plants infested by pea aphids start emitting HIPVs which are attractive to A. ervi in a Y-tube olfactometer and in a wind tunnel (Du et al. Citation1996; Powell et al. Citation1998; Takemoto et al. Citation2009). Aphids are known to inject saliva, which can contain candidates of elicitors. Marek (1961) reported that aphids secrete gelling saliva to form sheaths. Gelling saliva will envelop the stylets for protection from apoplastic defenses by plants (Miles Citation1999). After the stylet successfully reach the sieve tube, aphids inject watery saliva into sieve elements (Martin et al. Citation1997). Watery saliva contains enzymes such as pectinase, phenoloxidase, and peroxidase (Cherqui and Tjallingii Citation2000). Although the function of watery saliva is largely unknown, it is predicted that digestive enzymes in the saliva might be involved in the induction of plant defense responses (Miles Citation1999). It is predicted that elicitors are injected by pea aphids through penetration and/or sieve-tube puncture.
Recent studies have suggested that enzymes in aphid saliva are factors causing the induction of volatiles. Volatiles from wheat plants treated with a commercially available pectinase were preferred by the aphid parasitoid, Aphidius avenae, over those from intact plants in a Y-tube olfactometer and green house experiment (Liu et al. Citation2009). The research showed that salivary enzyme(s) might induce the production of volatiles that attract parasitic wasps. However, whether aphid parasitids are attracted to a plant treated with saliva has been remained unanswered. Here, we show the presence and some aspects of the chemical nature of the elicitors of pea aphid, using an artificial liquid diet (Will et al. Citation2007) as a medium to collect the elicitors.
Materials and methods
Plants and insects
Three broad bean plants (Vicia faba var. Nintoku Issun) were grown in a vinyl cup (approximately 300 ml) in a growth chamber (20±2 °C, 16L8D, ca. 5500 lux). Seeds were embedded in vermiculite with water in the dark for 1 week, and then seedlings were transferred to the vinyl pot with a mixed soil consisting of organic soil, vermiculite, and perlite with the ratio of 6:2:1 (v:v:v) containing 1% calcium hydroxide (v/v). The pots were irrigated with water containing a fertilizer (0.05% (v/v) Hyponex; Hyponex Japan, Osaka, Japan). Seedlings of approximately 10–15 cm-height (2–3 weeks after germination) were used for experiments and rearing of aphids.
Acyrthosiphon pisum
The pea aphid, A. pisum, was obtained from Dr Yoshitaka Nakashima (Obihiro University of Agriculture and Veterinary Medicine) in April 2006. They were reared on broad bean plants in the laboratory (20±2 °C, 16L8D).
Aphidius ervi
Aphidius ervi was obtained from a culture maintained by Dr Yoshitaka Nakashima in April 2006. They were kept under laboratory conditions (20±2 °C, 16L8D, 50–70 rh) on broad bean plants with A. pisum as hosts. Since A. ervi of Hokkaido strain shows no or little olfactory responses to host-infested plant without previous experience of the odor (Takemoto et al. Citation2009), previous odor experience was conducted in the following manner. Mummies were removed from infested plants and kept in a Polyethylene terephthalate tube (6-cm diameter, 15-cm long, with the openings covered with gauze). Before emergence of naïve wasps, mummies were exposed to host-infested plant volatiles by placing the mummies in a cage next to host plants infested by A. pisum for 3–7 days. One day after emergence, female wasps were collected separately in a small vial (2-cm diameter, 12-cm long) with 50% aqueous solution of honey as food and kept for one day (20±2 °C, 16L8D) until bioassays. In this study, only female adults were used to examine their searching behavior for hosts to oviposit.
Aphid saliva collection
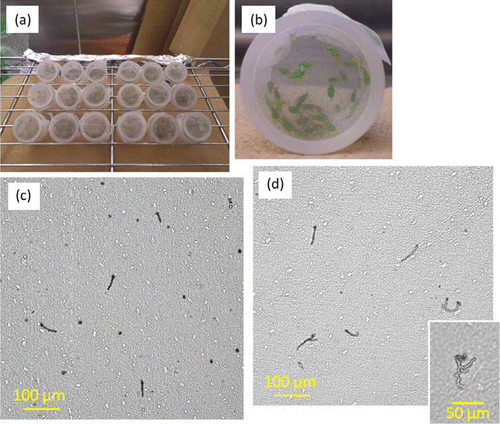
To collect aphid saliva, deionized water or 15% sucrose aq. containing 100 mM serine, 100 mM methionine and 100 mM aspartic acid was used as a liquid diet of aphids (Will et al. Citation2007). The diet solution was sterilized with a syringe filter (Whatman PES membrane, 25 mm GD/X, GE Healthcare, UK) and enclosed in two stretched Parafilm™ membranes attached to an acrylic resin tube (i.d. 25 mm, 2-mm thickness, 25-mm height with both ends open), hereafter referred to as a ‘sachet.’ Preparation of the sachet was conducted under sterile conditions. Approximately 100 mg of aphids (50–100 individuals, mixed ages) was transferred from a colony on a plant to each sachet. The open end of the sachet was closed with a stainless steel mesh to prevent the aphids from escaping. Sachets not fed upon by aphids were prepared for use as a control. Sachets were covered by a yellow film to stimulate the orientation by aphids to the membrane and placed in a climate controlled room (20±2 °C, 16L8D) for 24 hours. Then, liquid diet was collected and transferred to 5 ml vials with a sterile plastic syringe and needle, and the diet was immediately subjected to treatment on plants for odor source preparation.
Microscopic observation
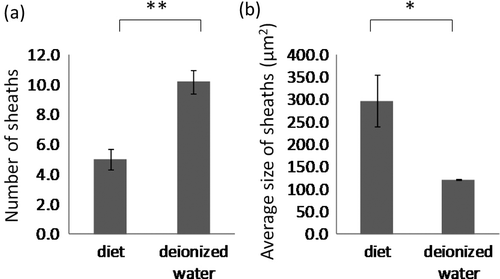
After pea aphids had fed on the sachets for 24 hours, we treated the Parafilm with Coomassie brilliant blue in order to dye the salivary sheaths left on the surface of the film. The observation procedure was based on that reported by Cherqui and Tjallingii (Citation2000). The stained sheaths were observed under a microscope (ECLIPSE TE 2000-U, Nikon, Japan). The sheaths were distributed rather uniformly on the surface of the membrane in the sachet. Thus, we randomly selected a site (660 µm×660 µm) of a sachet to take a photograph with a digital CCD camera (C4742-95-12ER, Hamamatsu, Japan). Five sachets were observed. The number of sheaths, total area of sheaths, and average size of sheaths in the photographs were measured with Image J software (Abramoff et al. Citation2004).
Y-tube olfactometer bioassay
A Y-tube olfactometer (3.5-cm inner diameter, 13-cm long for each branch with angle of 90°) was used to test the walking response of A. ervi in a climate-controlled room (20±2 °C, 40–60 rh, ca. 500 lux). The method described in Takemoto et al. (Citation2009) was applied to this study. Air was passed through activated charcoal and odor source bottles, in which either test plants or control plants were placed, to a branch of the olfactometer at the rate of 800 ml/min. A small vial with one A. ervi was placed at the base of the Y-tube with its opening facing upwind to release the wasp. When the wasp walked across a line marked in each branch, 7 cm from the Y junction, and stayed there for at least 1 min, we judged that the wasp had made a choice. A wasp that did not make choice within 5 min was recorded as a nonresponder. The control and experimental ends of the olfactometer were alternated every six bioassays. Odor sources were replaced every 12 bioassays with new ones. For each comparison, a total of 60–72 wasps were used. A binomial test was used to test the statistical significance of the difference between the distributions of the wasps that made choices of either of the two odor sources tested.
Response of A. ervi to plants treated with artificial diet subjected to different conditions
Liquid diet from 10 sachets (approximately 1000 mg of A. pisum or no aphids as a control) was applied to the lower surface of four fully expanded leaves of a potted broad bean plant. Three broad bean plants had been planted together in a pot. Before saliva application, mechanical damage with fine sandpaper was made on the lower surface of leaves (approximately 10 cm2), then the diet was applied on the damage area using paint brush. The treated plants were kept for three days in a climate-controlled room (20±2 °C, 16L8D, 50–70 rh) and used as odor sources for bioassays in a Y-tube olfactometer. The following two comparisons were conducted: (1) Vicia faba treated with deionized water previously fed on by host aphid A. pisum versus V. faba treated with deionized water not fed upon by aphids. (2) Vicia faba treated with liquid diet previously fed on by host aphid A. pisum versus V. faba treated with liquid diet not fed upon by aphids.
Chemical nature of the elicitor
The sample of liquid diet previously fed on by the aphid was prepared as shown in experiment 1. The sample was separated into two fractions (>3 kDa fraction and <3 kDa fraction) with an ultrafiltration membrane filter (Vivaspin 20 with a 3000 Da molecular weight cut-off and polyethersulfone membrane; Sartorius, Goettingen, Germany) at 4 °C. The filtration was repeated twice. Both fractions were filled up to 2 ml with liquid diet. The liquid diet without the aphid damage was also used as a control sample. The following two comparisons were made: (1) Vicia faba treated with high-molecular weight (>3 kDa) fraction of liquid diet previously fed on by host aphid A. pisum vs. V. faba treated with high-molecular weight fraction of diet not fed upon by aphids and (2) Vicia faba treated with low-MW (<3 kDa) fraction of liquid diet previously fed on by host aphid A. pisum versus V. faba treated with low-molecular weight fraction of diet not fed upon by aphids.
The sample of liquid diet previously fed on by aphids was prepared as described earlier in experiment 1. The samples were placed in air-tight 10 ml vials (20 mm-diameter, 50-mm height) and boiled at 100 °C for 3 min. The samples were then cooled down and applied to the leaves in the same manner as described earlier. The choice test, broad bean plants treated with boiled liquid diet previously fed on by host aphid A. pisum versus those treated with boiled liquid diet not fed upon by aphids, was conducted.
Results and discussion
Microscopic observation
Highly branched sheaths were only observed in the sachets containing liquid diet (c, d). The number of sheaths was significantly higher in the experiments using deionized water than in those using liquid diet (t5 = − 4.8702, df = 8, p<0.01) (a). By contrast, the average size of sheaths was significantly smaller in deionized water experiments than in liquid diet experiments (t5 = 3.096, df = 8, p<0.05) (b). While aphids insert their stylet to reach the sieve element, they secrete gelling saliva which forms the flange and the stylet sheath, and then watery saliva is secreted from the end of their stylet into the sieve tube during the next salivation phase (Tjallingii Citation2006). The difference in the features of the stylet sheaths remaining on the membrane indicated differences in the feeding behavior of aphids at the beginning of their plant penetration and salivation phases. This difference in aphid behavior might lead to quantitative and qualitative differences of the saliva secreted in plant tissue. To test this, we conducted a Y-tube olfactometer bioassay using the leaves treated with liquid diet subjected to different conditions.
Figure 1. Photographs of sachets during aphid saliva collections. (a) Pea aphids feeding on diet enclosed between two Parafilm membranes in sachets, (b) magnified view of a sachet containing ca. 100 mg of A. pisum, (c) sheaths left on the lower membrane with deionized water, and (d) sheaths left on the lower membrane with diets and highly branching structure of sheaths only observed on membrane with diet (smaller photo).
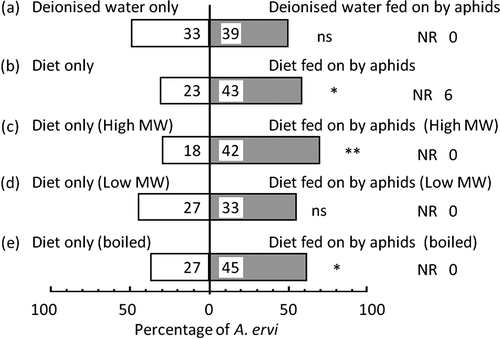
Response of A. ervi to plants treated with artificial diet subjected to different conditions
The wasps did not show a preference between broad bean plants treated with deionized water previously fed on by host aphid A. pisum and those treated with deionized water not fed upon by aphids (p=0.556, binomial test) (a). By contrast, the wasps preferred broad bean plants treated with liquid diet previously fed on by host aphid A. pisum to those treated with liquid diet not fed upon by aphids (p=0.019, binomial test) (b), indicating the presence of elicitor(s) in liquid diet which was previously infested by the pea aphid but not (or in trace amounts) in previously infested deionized water. In conjunction with the microscopic observations, these results suggested that saliva was a candidate to contain elicitors. Salivary enzymes in watery saliva have been thought to be involved in activation and inactivation of the defensive responses in plants (for review, Miles Citation1999). Recently, Liu et al. (Citation2009) reported that enzymes in the saliva of the English green aphid, Sitobion avenae, might be responsible for inducing indirect defense in wheat. In their study, volatiles from wheat plants treated with a commercially available pectinase were preferred by the aphid parasitoid, Aphidius avenae, over those from intact plants in a Y-tube olfactometer and greenhouse experiment. Their results suggest that an enzyme(s) in aphid saliva is possibly the elicitor. However, further studies on the attraction of wasps using enzymes originating from aphids are needed. We showed that wasps are attracted by plants treated with diets fed upon by aphids. This result clearly suggested that an elicitor exists in the diet fed on by aphids. We then studied the chemical nature of the elicitor.
Figure 3. Responses by Aphidius ervi to volatiles from plants in Y-tube olfactometer. (a) Plants treated with deionized water fed on by A. pisum vs. plants treated with deionized water without aphid feeding, (b) plants treated with diet fed on by A. pisum vs. plants treated with diet without aphid feeding, (c) plants treated with higher molecular weight fraction of diet fed on by A. pisum vs. plants treated with higher molecular weight fraction of diet without aphid feeding, (d) plants treated with lower molecular weight fraction of diet fed on by A. pisum vs. plants treated with lower molecular weight fraction of diet without aphid feeding, (e) plants treated with boiled deionized water fed on by A. pisum vs. plants treated with boiled diet fed on by A. pisum. *p < 0.05, **p < 0.01, ns, not significant, binomial test.
Chemical nature of the elicitor
The wasps preferred broad bean plants treated with a high-molecular weight (>3 kDa) fraction of liquid diet previously fed on by A. pisum to those treated with a high-molecular weight fraction of diet not fed upon by aphids (p=0.003, binomial test) (c). However, the wasps did not show a preference between broad bean plants treated with a low-molecular weight (<3 kDa) fraction of liquid diet previously fed on by host aphid A. pisum and those treated with a low-molecular weight fraction of diet not fed upon by aphids (p=0.519, binomial test) (d). The wasps preferred broad bean plants treated with boiled liquid diet previously fed on by host aphid A. pisum to those treated with boiled liquid diet not fed upon by aphids (p=0.044, binomial test) (e). Previous studies have suggested that an enzyme in aphid saliva is possibly one of the factors responsible for elicitor activity. Our experiments suggested that the fraction with molecular weight larger than 3 kDa possessed the activity as an elicitor. This fraction should contain proteins, including the salivary enzymes, which was consistent with the suggestion by previous researchers. Furthermore, the elicitors are thermotolerant factor(s) (e). This suggests that molecules other than enzymes may be involved in the elicitors. Oligosaccharides induce production of defensive compounds under the infection of phytopathogens in the Castor bean, Ricinus commais (Bruce and West Citation1982). A peptide, inceptin, is one of the elicitors which induce volatile production in Cowpea plants (Schmelz et al. Citation2006). The fraction larger than 3 kDa in molecular weight could contain oligosaccharides and polypeptides. These compounds are thermotolerant. Thus, oligosaccharides and polypeptides are also candidate compounds of the elicitors. Detailed chemical characterization of the elicitors will be needed.
Conclusion
Identification and characterizing elicitor molecules is important for our understanding of the mechanisms of indirect defenses in plants. We showed that elicitors from A. pisum exist in liquid diet previously fed on by the aphids. The responsible factor(s) include molecule(s) larger than 3 kDa in molecular weight and thermotolerant molecules or molecules whose breakdown products would be elicitors. Their detailed chemical characteristics need to be investigated.
Acknowledgements
We would like to thank Dr Elizabeth Nakajima and three anonymous reviewers for their comments on the article. This research was supported in part by Global Center of Excellence Program A06 ‘Formation of a Strategic Base for Biodiversity and Evolutionary Research: from Genome to Ecosystem’ of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, by a Grant-in-Aid for Scientific Research S from MEXT (No. 19101009), and by the JSPS Core-to-Core Program.
References
- Abramoff , MD , Magelhaes , PJ and Ram , SJ . 2004 . Image processing with image . J Biophoton Int. , 11 ( 7 ) : 36 – 42 .
- Arimura G , Kost C , Boland W. 2005 . Herbivore-induced, indirect plant defences . Biochim Biophys Acta, Mol Cell Biol Lipids . 1734 2 : 91 – 111 . Available from: <Go to ISI>//000229526700001
- Arimura G , Matsui K , Takabayashi J. 2009 . Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions . Plant Cell Physiol . 50 5 : 911 – 923 . Available from: <Go to ISI>//000266117000001 doi: 10.1093/pcp/pcp030
- Bruce RJ , West CA. 1982 . Elicitation of casbene synthetase activity in castor bean: the role of pectic fragments of the plant cell wall in elicitation by a fungal endopolygalacturonase . Plant Physiol. 69 5 : 1181 – 1188 . Available from: <Go to ISI> //MEDLINE:16662367 doi: 10.1104/pp.69.5.1181
- Cherqui A , Tjallingii WF. 2000 . Salivary proteins of aphids, a pilot study on identification, separation and immunolocalisation . J Insect Physiol. 46 8 : 1177 – 1186 . Available from: <Go to ISI> //000087848000004
- Du YJ , Poppy GM , Powell W. 1996 . Relative importance of semiochemicals from first and second trophic levels in host foraging behavior of Aphidius ervi . J Chem Ecol . 22 9 : 1591 – 1605 . Available from: <Go to ISI>//A1996VL10500002
- Karban , R and Baldwin , IT . 1997 . Induced responses to herbivory , Chicago : University of Chicago Press .
- Liu Y , Wang WL , Guo GX , Ji XL. 2009 . Volatile emission in wheat and parasitism by Aphidius avenae after exogenous application of salivary enzymes of Sitobion avenae . Entomol Exp Appl . 130 3 : 215 – 221 . Available from: <Go to ISI>//000263036100001 doi: 10.1111/j.1570-7458.2008.00822.x
- Marek , J . 1961 . Effects of piecing plants-cells by aphids . Entomol Exp Appl. , 4 ( 1 ) : 20 – 34 .
- Martin , B , Collar , J , Tjallingii , W and Fereres , A . 1997 . Intracellular ingestion and salivation by aphids may cause the acquisition and inoculation of nonpersistently transmitted plant viruses . J Gen Virol. , 78 : 2701 – 2705 .
- Mori N , Yoshinaga N. 2011 . Function and evolutionary diversity of fatty acid amino acid conjugates in insects . J Plant Interact . 6 2–3 : 103 – 107 . Available from: <Go to ISI>://000288257200009 doi: 10.1080/17429145.2010.544412
- Miles PW. 1999 . Aphid saliva . Biol Rev . 74 1 : 41 – 85 . Available from: <Go to ISI>://000080555700002
- Powell W , Pennacchio F , Poppy GM , Tremblay E. 1998 . Strategies involved in the location of hosts by the parasitoid Aphidius ervi Haliday (Hymenoptera: Braconidae: Aphidiinae) . Biol Control . 11 2 : 104 – 112 . Available from: <Go to ISI>://000072179400004
- Sabelis M , Takabayashi J , Janssen A , Kant M , van Wijk M , Sznajder B , Aratchige N , Lesna I , Belliure B , Schuurink R. 2007 . Ecology meets plant physiology: herbivore-induced plant responses and their indirect effects on arthropod communities . In : Ecological communities: plant mediation in indirect interaction webs . Cambridge , MA : Cambridge University Press . p. 188 – 217 .
- Schmelz EA , Carroll MJ , LeClere S , Phipps SM , Meredith J , Chourey PS , Alborn HT , Teal PEA. 2006 . Fragments of ATP synthase mediate plant perception of insect attack . Proc Natl Acad Sci USA . 103 23 : 8894 – 8899 . Available from: <Go to ISI>://000238278400056
- Schmelz EA , Engelberth J , Alborn HT , Tumlinson JH , Teal PEA . 2009 . Phytohormone-based activity mapping of insect herbivore-produced elicitors . Proc Natl Acad Sci USA . 106 2 : 653 – 657 . Available from: <Go to ISI>://000262804000054 doi: 10.1073/pnas.0811861106
- Takemoto H , Powell W , Pickett J , Kainoh Y , Takabayashi J. 2009 . Learning is involved in the response of parasitic wasps Aphidius ervi (Haliday) (Hymenoptera: Braconidae) to volatiles from a broad bean plant, Vicia faba (Fabaceae), infested by aphids Acyrthosiphon pisum (Harris) (Homoptera: Aphididae) . Appl Entomol Zool . 44 1 : 23 – 28 . Available from: <Go to ISI>://000265228100003 doi: 10.1303/aez.2009.23
- Tjallingii WF. 2006 . Salivary secretions by aphids interacting with proteins of phloem wound responses . J Exp Bot . 57 4 : 739 – 745 . Available from: <Go to ISI>://000235771700004 doi: 10.1093/jxb/erj088
- Walling LL. 2000 . The myriad plant responses to herbivores . J Plant Growth Regul . 19 2 : 195 – 216 . Available from: <Go to ISI>://000089981700007
- Will T , Tjallingii WF , Thonnessen A , van Bel AJE. 2007 . Molecular sabotage of plant defense by aphid saliva . Proc Natl Acad Sci USA . 104 25 : 10536 – 10541 . Available from: <Go to ISI>://000247500000043