Abstract
Salt stress is a major abiotic stress limiting the productivity and the geographical distribution of many plant species. Arabidopsis thaliana is an excellent model with rich genetic resources for modern plant biology research. To comprehensively and representatively understand salt-response mechanisms in A. thaliana, we applied the first attempt to use the most data (252 of 10,469 reviewed A. thaliana protein) from public protein database for displaying the enriched protein domains, Kyoto Encyclopedia of Genes and Genomes pathways, molecular functions, and cell localizations involved in salt-response. The data were analyzed by Database for Annotation Visualization and Integrated Discovery. Our results indicated salt-response proteins cross-talked not only with drought and temperature stress as previously reported but also with further stresses such as bacterium, light, metal ion, radiation, and wounding stress. Multiple cellular localizations under salt stress indicated proteins were versatile. In addition, 27 proteins have the characteristics with response to multiple stresses and localization in multiple places. We called it the ‘space-stress’ double cross-talk effects, which indicated that A. thaliana proteins dealt with salt stress and other stresses in a reciprocal economical way. An enriched bioinformatics analysis of the large data could provide clues and basis for the development of salt-response potential biomarkers for plant growth and crop productivity.
Introduction
Soil salinity is a major environmental stress, which affects crop productivity and plants region-distribution in the world. Both agriculture irrigation and natural phenomena contribute to an increase of salt in soil (Tester and Davenport Citation2003; Horie and Schroeder Citation2004; Wiebe et al. Citation2007). High salinity content can interfere with water uptake and mineral nutrition, and finally causes ion imbalance, hyper-osmotic stress, and oxidative damage in plant (Zhu Citation2002). Under salt stress, lots of plants exhibit slow growth, wilting or death (Epstein et al. Citation1980; Munns Citation2002). To adapt to salt stress, plant regulates the expression of salt-response proteins to reestablish cellular ion and osmotic homeostasis with concomitant changes of development, morphology, physiology, and biochemistry (Taji et al. Citation2004). Therefore, many works focused on the mechanism of plant response and tolerance to salt stress (Hussain et al. Citation2008; Munns and Tester Citation2008).
Arabidopsis thaliana is an excellent model with rich genetic resources for modern plant biology research. Although A. thaliana is a true glycophyte, and not a particularly salt tolerant plant species, one consistent theme was that salt-sensitive plants do have salt tolerance genes (Zhu Citation2000; Hasegawa et al. Citation2000a, Citation2000b). It is possible to explore plant salt tolerance mechanisms using A. thaliana as the model (Møller et al. Citation2009; Katori et al. Citation2010).
Transcriptome profiling, a widely used technique to identify NaCl-responsive genes, has contributed to our understanding of salinity stress. Transcriptional profiling of NaCl-stressed A. thaliana roots was analyzed to reveal novel classes of responsive genes (Jiang and Deyholos Citation2006). The results indicated that NaCl-treated A. thaliana roots revealed dynamic changes in transcript abundance for at least 20% of the genome, including hundreds of transcription factors, kinases/phosphatases, hormone-related genes, and effectors of homeostasis, all of which highlight the complexity of this stress response.
However, transcriptome profiling has some limitations because mRNA levels are not always correlated to those of corresponding proteins, due in part to post-transcriptional regulation at both RNA and protein level. Only poor or moderate correlation between changes in the levels of specific mRNAs and their corresponding proteins has been reported in previous studies (Gygi et al. Citation1999; Mooney et al. Citation2006).
Therefore, investigation of changes in plant proteome is highly important since proteins, unlike transcripts, are direct effectors of plant stress response. These biological realities motivated us to perform an analysis of NaCl stress responses at the proteome level.
Proteomics technology has matured significantly in recent years (Brewis and Brennan Citation2010). Although knowledge about A. thaliana salt-tolerance mechanisms based on proteome technology has been improved (Ndimba et al. Citation2005; Jiang et al. Citation2007), most of these data were not publicly available and were not validated further on gene or protein levels. The big challenge of these ‘omics’ data is the extraction of the biological implications that will facilitate the discovery and characterization of important physiological mechanisms and pathways. Therefore, data analysis should be completed based on multi-disciplinary interests and integrated strategies. Now, goal of annotating all known A. thaliana proteins including over 10,000 reviewed proteins, this well-annotated resource should be possible to provide insight into a broad range of A. thaliana salt-resistance processes.
In the present work, we performed a comprehensive bioinformatics analysis of the proteins revealed from public protein database to determine its significant characteristics. Totally, 252 salt-response proteins out of 10,469 reviewed A. thaliana proteins were founded. Integrated function and pathway analyses were performed, and some significant features were clustered. Gene density on chromosomes, cell localizations, and cross-talk under other stresses of A. thaliana salt-response proteins were also analyzed and discussed. These bioinformatics classifications should serve as a useful reference for exploration of A. thaliana salt-resistance mechanisms.
Materials and methods
Selection of proteins expressed as Arabidopsis thaliana salt-response proteins from UniProt database
Uniprot (Release 2011_08, http://www.uniprot.org) was used to select A. thaliana salt-response proteins. All A. thaliana expressed proteins were extracted from Uniprot database and deposited into DAVID to analyze GOTERM_BP_FAT. Subsequently, these proteins with keyword ‘response to salt stress’ in the description of GOTERM_BP_FAT were referred as salt-response proteins. UniProt database is a high-quality database that serves as a stable, comprehensive, fully classified, richly, and accurately annotated protein sequence knowledgebase, and can provide us a comprehensive, high-quality resource of protein sequence, and functional information (Liu et al. Citation2011). The UniProt Knowledgebase (UniProtKB) gene ontology (GO) annotation program aims to provide high-quality GO annotations to proteins in the UniProtKB. The assignment of GO terms to UniProt records is an integral part of UniProt biocuration. UniProt manual and electronic GO annotations are supplemented with manual annotations supplied by external collaborating GO Consortium groups, to ensure a comprehensive GO annotation dataset is supplied to users. Its subsection of GOTERM_BP_FAT section provides information on protein response to various stresses, such as bacterium stress, light stress, radiation stress, temperature stress, metal ion stress, water stress, and wounding stress. These salient features of protein profiles are the compilation of up-to-date information, based on the available data in literature, which has been interpreted and described in the words of original researches (Schneider et al. Citation2004).
DAVID (Database for annotation, visualization and integrated discovery) V6.7 analysis
Functional analyses were performed through DAVID v6.7 which is a web-accessible program that provides a comprehensive set of functional annotation tools to understand biological meaning behind large list of genes or proteins. Uniprot IDs of A. thaliana salt-response proteins were submitted to DAVID to analyze gene ontologies, protein domains, and pathways (Huang et al. Citation2009).
Cytoscape (an open source platform for complex network analysis and visualization) V2.8.2 analysis
Arabidopsis thaliana salt-response proteins were further analyzed by Cytoscape v2.8.2. Cytoscape is an open-source software package that is widely used to integrate and visualize diverse data-sets in biology. By visualizing information about known compound–target interactions in the context of a biological network of interest, one can rapidly identify novel avenues to perturb the system with compounds and potentially identify therapeutically relevant targets. Proteins with different cellular localizations and proteins involved in different stresses were further analyzed by Cytoscape v2.8.2 (Srivas et al. Citation2011).
Results
Summary of Arabidopsis thaliana salt-response proteins
A total of 10,469 annotated proteins have been reviewed in the Uniprot database (Release 2011_08), and 252 proteins have the characteristics of salt-response (Supplementary Table 1).
Table 1. Enriched domains and pathways associated with Arabidopsis thaliana salt-response proteins.
Annotation and functional enrichment of Arabidopsis thaliana salt-response proteins
For an overall overview of the A. thaliana salt-response proteins, functional analysis including domain analysis, biological processes, molecular functions, and pathways were performed using DAVID tools.
Domain analysis using DAVID functional annotation tools and including three subset databases (Interpro, PIR-Superfamily, and SMART databases) showed 36, 8, and 3 statistically significant domains, respectively (p<0.01). Such as Serine/threonine protein kinase domain (IPR002290) can be found in 21 proteins, which were involved in protein phosphorylation process; tubulin domain (PIRSF002306) can be found in 8 proteins, which were involved in microtubule-based process; S_TKc domain (SM00220) can be found in 21 proteins, which were involved in protein amino acid phosphoryaltion ATP binding (; Supplementary Table 2).
Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis indicated that three statistically significant pathways were present in the A. thalianasalt-response proteins (p<0.01). They were arginine and proline metabolism pathway (ath00330); biosynthesis of phenylpropanoids pathway (ath01061); and citrate cycle pathway (ath00020) (; Supplementary Table 2).
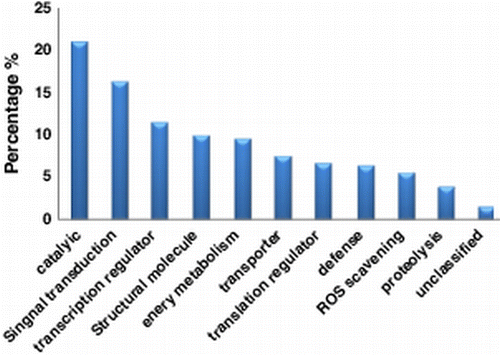
Molecular function analysis revealed that the largest number of proteins was related to metabolism (21.0%), and the next prevalent were related to signal transduction (16.3%) (; Supplementary Table 3).
Arabidopsis thaliana salt-response proteins involved in various cellular localizations
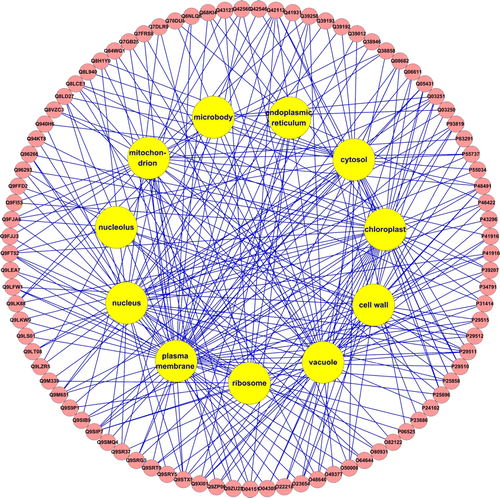
Based on the analysis of DAVID GOTERM_CC_FAT section, 183 of 252 A. thaliana salt-response proteins had cellular localizations. Eighty-nine of 183 proteins had more than one cellular localization, such as cell wall, chloroplast, cytosol, endoplasmic reticulum, microbody, mitochondrion, nucleus, nucleolus, plasma membrane, ribosome, vacuole (Supplementary Table 4). displayed the same A. thaliana salt-response proteins located in the different cellular components.
Visualization of Arabidopsis thaliana salt-response proteins involved in other stresses responses
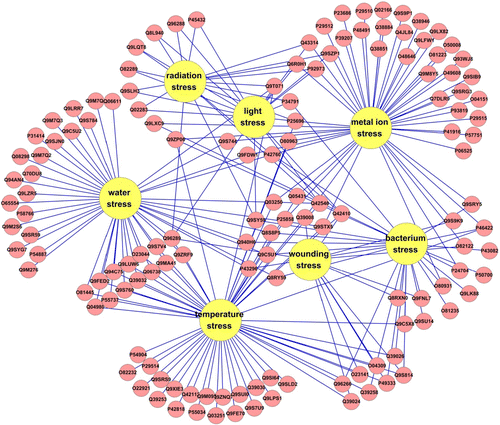
Two hundred and fifty-two A. thaliana salt-response proteins were found from Uniprot database. Based on the analysis of DAVID GOTERM_BP_FAT section, 117 of 252 A. thaliana salt-response proteins were also involved in other stresses response (). Among them, 23 proteins responded to bacterium stress; 17 proteins responded to light stress; 39 proteins responded to metal ion stress; 17 proteins responded to radiation stress; 50 proteins responded to temperature stress; 42 proteins responded to water stress; and 9 responded to wounding stress (Supplementary Table 5). Additionally, 27 proteins had the characteristics of both multi-cellular localizations and response to multi stresses (Supplementary Table 6), which was called the ‘space-stress’ double cross-talk effects.
Discussion
Although A. thaliana salt-tolerance proteins have been studied in the past years (Horie et al. Citation2009), there was no systematic attempt to organize protein pathways and natures involved in salt-tolerance exploration. Since proteins are directly involved in plant stress response, proteomics studies can significantly contribute to unravel the possible relationships between protein abundance and plant stress acclimation. Proteins involved in salt stress can be represented by the means of pathways and interactions. Up to now, many proteomes of A. thaliana under salt stress were still based on protein separation by two-dimensional electrophoresis technique (Kosová et al. Citation2011), which limited the low-abundance protein identification (Delahunty and Yates Citation2005).
In the present study, we first screened 252 of 10,469 reviewed proteins which were characterized as responsive to salt stress in A. thaliana, which exceeded the number of salt-response proteins published in any single paper. It was possible to objectively explore more mechanisms of response to salt stress using the most data. Therefore, the results of the enriched protein domains, KEGG pathways, molecular functions, and cell localizations were representative and comprehensive. This study provided us another angle to explore useful information from large amount of existing but often ignored database.
Bioinformatics analysis indicated most of the proteins response to salt stress could be clustered into different function groups and may participate in different aspects of A. thaliana physiology.
Domain analysis showed some significant enriched domains in the A. thaliana salt-response proteins, these proteins common with similar domains would regulate some aspects of A. thaliana functions coordinately. Plants use different signaling pathways to acclimate to changing environmental conditions. Fast changes in the concentration of free Ca2 + ions – so called Ca2 + signals – are among the first responses to many stress situations. These signals are decoded by different types of calcium-dependent protein kinases, which – together with mitogen-activated protein kinases (MAPK) – present two major pathways that are widely used to adapt the cellular metabolism to a changing environment. In this work, 21 proteins with Serine/threonine protein kinase domain may participate in protein phosphorylation, which was related to signal transduction (Chinnusamy et al. Citation2005). Those proteins included calcium-dependent protein kinase 32 (CPK32), mitogen-activated protein kinase 4 (MPK4), mitogen-activated protein kinase 6 (MPK6), and so on. CPK32 was involved in Ca2 +-dependent protein kinase (CDPK) pathway; and MPK4 and MPK6 were involved in MAPK pathway. They are widely used to adapt the cellular metabolism to a changing environment. Cross-talk between CDPK and MAPK signaling pathways plays an important role in the A. thaliana salt-stress response (Wurzinger et al. Citation2011).
Kyoto Encyclopedia of Genes and Genomes (KEGG) is a collection of online databases dealing with biological pathways. We found 10 proteins participating in the arginine and proline metabolism significantly. Among them, arginine decarboxylase 1 and arginine decarboxylase 2 were involved in arginine metabolism, which catalyzed the production of polyamines (Walden et al. Citation1997). Polyamines, positively charged at physiological pH, could affect physiological systems by binding to anionic sites, such as those associated with nucleic acids and membrane phospholipids. So, polyamines may be involved in the control of numerous cellular functions, including free radical scavenger and antioxidant activity, and confer protection from abiotic stresses such as salt stress (Groppa and Benavides Citation2008). Proline metabolism also played a key role in protecting A. thaliana from salt stress. Proline is an important osmolyte in stress adaptation (Verbruggen and Hermans Citation2008), accounting for up to 20% of the free amino acid pool after salt stress (Verbruggen et al. Citation1993). Therefore, arginine and proline metabolism plays a critical role in response to salt stress.
Of 11 molecular functions, the structural molecules deserved to be noted. Salt stress reduces water availability and leads to the inhibition of plant growth by increasing the threshold pressure for wall yielding in expanding cells or inducing hydraulic limitations to water uptake (Steudle Citation2000). Cell wall rigidification, the formation of a physical barrier, would protect plant roots from further dehydration underwater deficit (Neumann et al. Citation1994). Actin and tubulin dynamics have important functions in cellular homeostasis. The cytoskeleton is rapidly remodeled by the external stimuli such as NaCl (Shoji et al. Citation2006). The mechanism of structural molecules remodeling in response to salt stress needs to be further studied. By cell localization analysis, 89 salt-response proteins have been located at more than one cell component, which possibly indicated that they played different functions in various places. In case of pyrophosphate-energized vacuolar membrane proton pump 1, it can work as a H+-pump in vacuole (Martinoia et al. Citation2007), but perhaps worked as a PPi synthase to maintain the cytosolic PPi levels required for sucrose respiration on plasma membrane (Paez-Valencia et al. Citation2011). Moreover, in addition to maintaining vacuolar pH, pyrophosphate-energized vacuolar membrane proton pump 1 controls auxin transport and consequently auxin-dependent development (Li et al. Citation2005).
In general, most drought-inducible genes are also induced by high-salinity stress, and many drought-inducible genes are also induced by abscisic acid (Shinozaki et al. Citation2003). Interestingly, among 252 salt-response proteins, 117 proteins also responded to other stresses, such as bacterium stress, light stress, metal ion stress, radiation stress, temperature stress, water stress, and wounding stress, which have wider cross-talks than the above reported, and revealed more potential biomarkers participating in signaling and metabolism pathways. We assumed that the proteins responding to other stresses may also associate with salt stress, and this would provide us a wider regulatory network to discover the salt-response mechanisms.
Conclusion
The work has provided a new insight into A. thaliana salt-response mechanisms and could urge scientists in this field to integrate all the existing data to explore significant functions and pathways involved in salt-stress response. Two new understandings should be noted: one was that salt-response proteins might become potential biomarkers for other stresses and other stress-responsive proteins might also participate in salt stress; the other one was that multiple cellular localizations indicate the protein functions under stress are versatile. In addition, the ‘space-stress’ double cross-talk effects informed us that A. thaliana deals with salt stress and other stresses in a reciprocal economical way. Further studies are warranted to substantiate the enriched functions and pathways, which will contribute to a detailed protein functional characterization and surely help us to better understand the processes of plant stress acclimation and stress tolerance acquisition.
Supplementary Tables.pdf
Download PDF (365.9 KB)Acknowledgements
Supported by grants from Shandong Provincial Natural Science Foundation, China (ZR2010CQ024) and Shandong Provincial Universities Science and Technology Programme, China (J10LC72). The authors declare no competing interests.
References
- Brewis , IA and Brennan , P . 2010 . Proteomics technologies for the global identification and quantification of proteins . Adv Protein Chem Struct Biol. , 80 : 1 – 44 .
- Chinnusamy , V , Jagendorf , A and Zhu , JK . 2005 . Understanding and improving salt tolerance in plants . Crop Sci. , 45 : 437 – 448 .
- Delahunty C , Yates JR. 3rd . 2005 . Protein identification using 2D-LC-MS/MS . Methods . 35 : 248 – 255 .
- Epstein , E , Norlyn , JD , Rush , DW , Kingsbury , RW , Kelley , DB , Cunningham , GA and Wrona , AF . 1980 . Saline culture of crops, a genetic approach . Science. , 210 : 399 – 404 .
- Groppa , MD and Benavides , MP . 2008 . Polyamines and abiotic stress, recent advances . Amino Acids. , 34 : 35 – 45 .
- Gygi , SP , Rochon , Y , Franza , BR and Aebersold , R . 1999 . Correlation between protein and mRNA abundance in yeast . Mol Cell Biol. , 19 : 1720 – 1730 .
- Hasegawa , M , Bressan , R and Pardo , JM . 2000a . The dawn of plant salt tolerance genetics . Trends Plant Sci. , 8 : 317 – 319 .
- Hasegawa , PM , Bressan , RA , Zhu , JK and Bohnert , HJ . 2000b . Plant cellular and molecular responses to high salinity . Annu Rev Plant Physiol Plant Mol Biol. , 51 : 463 – 499 .
- Horie , T , Hauser , F and Schroeder , JI . 2009 . HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants . Trends Plant Sci , 14 : 660 – 668 .
- Horie , T and Schroeder , JI . 2004 . Sodium transporters in plants. Diverse genes and physiological functions . Plant Physiol. , 136 : 2457 – 2462 .
- Huang , DW , Sherman , BT and Lempicki , RA . 2009 . Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources . Nat Protoc. , 4 : 44 – 57 .
- Hussain , TM , Chandrasekhar , T , Hazara , M , Sultan , Z , Saleh , BK and Gopal , GR . 2008 . Recent advances in salt stress biology-a review . Biotech Mol Biol Rev. , 3 : 8 – 13 .
- Jiang , Y and Deyholos , MK . 2006 . Comprehensive transcriptional profiling of NaCl-stressed Arabidopsis roots reveals novel classes of responsive genes . BMC Plant Biol. , 6 : 25
- Jiang , Y , Yang , B , Harris , NS and Deyholos , MK . 2007 . Comparative proteomic analysis of NaCl stress-responsive proteins in Arabidopsis roots . J Exp Bot. , 58 : 3591 – 3607 .
- Katori , T , Ikeda , A , Iuchi , S , Kobayashi , M , Shinozaki , K , Maehashi , K , Sakata , Y , Tanaka , S and Taji , T . 2010 . Dissecting the genetic control of natural variation in salt tolerance of Arabidopsis thaliana accessions . J Exp Bot. , 61 : 1125 – 1138 .
- Kosová , K , Vítámvás , P , Prášil , IT and Renaut , J . 2011 . Plant proteome changes under abiotic stress – contribution of proteomics studies to understanding plant stress response . J Proteomics. , 74 : 1301 – 1322 .
- Li , J , Yang , H , Peer , WA , Richter , G , Blakeslee , J , Bandyopadhyay , A , Titapiwantakun , B , Undurraga , S , Khodakovskaya , M Richards , EL . 2005 . Arabidopsis H+−PPase AVP1 regulates auxin-mediated organ development . Science. , 310 : 121 – 125 .
- Liu , F , Wang , H and Li , J . 2011 . An integrated bioinformatics analysis of mouse testis protein profiles with new understanding . BMB. , 44 : 347 – 351 .
- Martinoia , E , Maeshima , M and Neuhaus , HE . 2007 . Vacuolar transporters and their essential role in plant metabolism . J Exp Bot. , 58 : 83 – 102 .
- Møller , IS , Gilliham , M , Jha , D , Mayo , GM , Roy , SJ , Coates , JC , Haseloff , J and Tester , M . 2009 . Shoot Na+ exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na+ transport in Arabidopsis . Plant Cell. , 21 : 2163 – 2178 .
- Mooney , BP , Miernyk , JA , Greenlief , CM and Thelen , JJ . 2006 . Using quantitative proteomics of Arabidopsis roots and leaves to predict metabolic activity . Physiologia Plantarum. , 128 : 237 – 250 .
- Munns , R . 2002 . Comparative physiology of salt and water stress . Plant Cell Environ. , 25 : 239 – 250 .
- Munns , R and Tester , M . 2008 . Mechanisms of salinity tolerance . Annu Rev Plant Physiol. , 59 : 651 – 681 .
- Ndimba , BK , Chivasa , S , Simon , WJ and Slabas , AR . 2005 . Identification of Arabidopsis salt and osmotic stress responsive proteins using two-dimensional difference gel electrophoresis and mass spectrometry . Proteomics. , 5 : 4185 – 4196 .
- Neumann , PM , Azaizeh , H and Leon , D . 1994 . Hardening of root cell walls, a growth inhibitory response to salinity stress . Plant Cell Environ. , 17 : 303 – 309 .
- Paez-Valencia , J , Patron-Soberano , A , Rodriguez-Leviz , A , Sanchez-Lares , J , Sanchez-Gomez , C , Valencia-Mayoral , P , Diaz-Rosas , G and Gaxiola , R . 2011 . Plasma membrane localization of the type I H+−PPase AVP1 in sieve element-companion cell complexes from Arabidopsis thaliana . Plant Sci. , 181 : 23 – 30 .
- Schneider , M , Tognolli , M and Bairoch , A . 2004 . The Swiss-Prot protein knowledgebase and ExPASy, providing the plant community with high quality proteomic data and tools . Plant Physiol Biochem. , 42 : 1013 – 1021 .
- Shinozaki , K , Yamaguchi-Shinozaki , K and Seki , M . 2003 . Regulatory network of gene expression in the drought and cold stress responses . Curr Opin Plant Biol. , 6 : 410 – 417 .
- Shoji , T , Suzuki , K , Abe , T , Kaneko , Y , Shi , H , Zhu , JK , Rus , A , Hasegawa , PM and Hashimoto , T . 2006 . Salt stress affects cortical microtubule organization and helical growth in Arabidopsis . Plant Cell Physiol. , 47 : 1158 – 1168 .
- Srivas , R , Hannum , G , Ruscheinski , J , Ono , K , Wang , PL , Smoot , M and Ideker , T . 2011 . Assembling global maps of cellular function through integrative analysis of physical and genetic networks . Nat Protoc. , 6 : 1308 – 1323 .
- Steudle , E. 2000 . Water uptake by roots, effects of water deficit . J Exp Bot. , 51 : 1531 – 1542 .
- Taji , T , Seki , M , Satou , M , Sakurai , T , Kobayashi , M , Ishiyama , K , Narusaka , Y , Narusaka , M , Zhu , JK and Shinozaki , K . 2004 . Comparative genomics in salt tolerance between Arabidopsis and Arabidopsis-related halophyte salt cress using Arabidopsis microarray . Plant Physiol. , 135 : 1697 – 1709 .
- Tester , M and Davenport , R . 2003 . Na+ tolerance and Na+ transport in higher plants . Ann Bot. , 91 : 503 – 527 .
- Verbruggen , N and Hermans , C . 2008 . Proline accumulation in plants, a review . Amino Acids. , 35 : 753 – 759 .
- Verbruggen , N , Villarroel , R and Van , MM . 1993 . Osmo regulation of a pyrroline-5-carboxylate reductase gene in Arabidopsis thaliana . Plant Physiol. , 103 : 771 – 781 .
- Walden , R , Cordeiro , A and Tiburcio , AF . 1997 . Polyamines, small molecules triggering pathways in plant growth and development . Plant Physiol. , 113 : 1009 – 1013 .
- Wiebe , BH , Eilers , RG , Eilers , WD and Brierley , JA . 2007 . Application of a risk indicator for assessing trends in dryland salinization risk on the Canadian prairies . Can J Soil Sci. , 87 : 213 – 224 .
- Wurzinger , B , Mair , A , Pfister , B and Teige , M . 2011 . Cross-talk of calcium-dependent protein kinase and MAP kinase signaling . Plant Signal Behav. , 6 : 8 – 12 .
- Zhu , JK . 2000 . Genetic analysis of plant salt tolerance using Arabidopsis . Plant Physiol. , 124 : 941 – 948 .
- Zhu , JK . 2002 . Salt and drought stress signal transduction in plants . Annu Rev Plant Biol. , 53 : 247 – 273 .