Abstract
Reintroduced environments represent stressful conditions to plants that can be observed in different ways. We evaluated the relationships between fluctuating asymmetry (FA), herbivory, and plant ontogeny of Chamaecrista semaphora (Fabaceae) under natural and restored habitat conditions. The patterns of leaf FA and herbivory by folivorous insects (chewing) on saplings and mature plants in each habitat were determined. No relationship was found between FA and herbivory on the two ontogenetic stages in both environments, suggesting that FA did not represent an indicator of stress. The frequency and amount of leaf area removed by folivores were higher in saplings compared to adult plants under the natural habitat, while the opposite trend was observed on restored habitat for adult plants. The restored habitat did not represent an environmental stress condition to C. semaphora, indicating that this endemic plant may represent a good candidate to restoration programs in harsh environments. However, we observed great differences in leaf FA and herbivory among individuals within habitats. Knowledge on plant quality, competition, physiology, and interactions with natural enemies are highly needed to support long lasting programmes on restoration of harsh environments.
Introduction
Most organisms exhibit developmental instability during ontogenetic stages as a result of environmental disturbances that can be evaluated by fluctuating asymmetry (FA). FA is defined as random morphological differences in size or shape between the two sides of bilateral characters (Palmer and Strobeck Citation1986; Whitlock Citation1996; Møller and Swadle Citation1997; Møller and Shykoff Citation1999). Under stress conditions, a deviation in the symmetry between sides suggests inability of individuals during development to minimize the influence of genetic and environmental disturbances (Alados et al. Citation1999; Hódar Citation2002; Cornelissen et al. Citation2003). Therefore, FA reflects developmental instability during ontogenetic stages as a result of environmental disturbances and hence has been considered a reliable indicator of environmentally induced stress in different taxa (Wauters et al. Citation1996; Anciles and Marini Citation2000; Leamy and Klingenberg Citation2005; Hagen et al. Citation2008; Cornelissen and Stiling Citation2011). In plants, some studies indicated higher levels of FA in disturbed habitats, pollution, urbanization, climate changes, parasitism, and herbivore pressure (Chistyakova and Kryazheva Citation2001; Cornelissen and Stiling Citation2005, Citation2011; Cuevas-Reyes et al. Citation2011a, Citation2011b; but see Telhado et al. Citation2010).
Plants respond to herbivore damage in different ways (Cuevas-Reyes et al. Citation2004), one is altering the developmental instability of leaves during ontogenetic stages (Díaz et al. Citation2004). However, a consensus on the relationship between FA and herbivory has not been reached. In some studies, leaf FA is positively related with the levels of herbivory suggesting that leaf FA serves as an indicator of plant stress as result of herbivore attack (e.g. Lempa et al. Citation2000; Cuevas-Reyes et al. Citation2011a), while in other studies no relationship has been found (Bañuelos et al. Citation2004; Díaz et al. Citation2004; Telhado et al. Citation2010). Differences in environmental conditions, enemy-free space, plant chemistry, and plant age have been suggested as possible causes for these differences (Bañuelos et al. Citation2004; Cornelissen and Stiling Citation2005; Cornelissen et al. Citation2003; Lempa et al. Citation2000). In spite of it, authors have suggested that FA serves as an indicator of plant susceptibility to damage caused by herbivores (Lempa et al. Citation2000; Cornelissen and Stiling Citation2005), in which plants with more asymmetric leaves should experience increased herbivory levels. The mechanism mediating such higher susceptibility of more asymmetrical plants is the higher nutritional quality of their leaves in comparison with symmetric leaves, suggesting that FA can also be used as an indicator of plant quality (Lempa et al. Citation2000; Cornelissen and Stiling Citation2005, Citation2011).
The quality and defense of plants are affected by a multitude of factors such as light, nutrient availability, herbivory (Waterman and Mole Citation1989; Herms and Mattson Citation1992; Lill and Marquis Citation2003), and structural and physiological differences that vary along the ontogenetic stages of the plant (Waltz and Whitham Citation1997; Karban and Thaler Citation1999). Because herbivory rates are typically higher on saplings than on mature plants (Coley and Barone Citation1996; Cuevas-Reyes et al. Citation2006), it is assumed that the foliage of mature plants must be better defended compared to saplings (Coley and Barone Citation1996; Basset Citation2001; but see Woodman and Fernandes Citation1991). In addition, variations in nutritional quality and chemical defenses along plant ontogeny may affect survival and reproduction of herbivore insects as result of ecological and physiological processes (Karban and Thaler Citation1999; Fonseca et al. Citation2006).
Restored habitats during early successional stages may represent stressful environments for plants due to various factors, including the high incidence of solar radiation and desiccation (Tripathi and Singh Citation2008). Reintroduced plant species in previously degraded environments are subject to different stress factors that potentially affect developmental instability of plants during the ontogenetic stages (Aronson et al. Citation1993). Plants subjected to some degree of stress, such as that found in habitats under environmental restoration, should become more susceptible to insect herbivore attack (see White Citation1969), particularly early in their development. The higher plant susceptibility to insect herbivores may be the result of changes in host plant physiology due to increased nitrogen availability and decreased production of secondary compounds during prolonged drought (White Citation1969). All these effects may ultimately affect the success of the environmental restoration processes (McCoy Citation1998). In general, little is known about restoration influences on the stress and herbivory experienced by plants (Suding et al. Citation2004).
One of the harsher environments in the tropics is the quartzitic outcrop fields that occur at higher altitudes in the Espinhaço mountain range in southeastern Brazil (Ribeiro and Fernandes Citation2000; Marques et al. Citation2002). In this vegetation formation, known as rupestrian fields we find the majority of the endangered species of the Brazilian Cerrado (savanna vegetation) (see Menezes and Giulietti Citation2000). In Serra do Cipó, at the southern portion of the Espinhaço mountains, extensive environmental damage caused by the construction or paving of existing highways (Viana et al. Citation2005; Moreira et al. Citation2009; see also Jacobi et al. Citation2008). As part of an endeavor to provide know-how to restore the harsh environments, a pilot study area was set in 2002 where more than 15 species were planted and their development evaluated (see Negreiros et al. Citation2008, Citation2009). One of such species was the microendemic shrub Chamaecrista semaphora (H. S. Irwin and Barneby, Fabaceae). We took advantage of the success of this species in the experimental area for, the first time, to study the relationships between FA, herbivory, and plant ontogeny in both restored and natural conditions in this harsh mountain environment. We addressed the following questions: (1) are there differences in FA patterns of C. semaphora in restored and natural environments?; (2) is leaf FA associated with the levels of herbivory under both habitat conditions?; and finally (3) how does plant age influence leaf FA?
Methods
Study area
This study was conducted in Serra do Cipó, southeastern Brazil, at the southern region of Serra do Espinhaço. The region is known for its high plant species diversity and endemism (Rizzini Citation1979; Giulietti et al. Citation1997). The climate is characterized by dry winters and rainy summers and the average annual precipitation is 1500 mm (Madeira and Fernandes Citation1999). Soil is characterized as acidic oxisols, with high aluminum levels and low cation exchange capacity (Ribeiro and Fernandes Citation2000; Medina and Fernandes Citation2007; Negreiros et al. Citation2008).
The system
Chamaecrista semaphora is an endemic shrub that occurs in ruprestrian fields of the Serra do Cipó (Irwin and Barneby Citation1982; Madeira and Fernandes Citation1999). C. semaphora is an endangered species that occur only in three natural localities as result of habitat fragmentation (Mendonça and Lins Citation2000; Silva et al. Citation2007). To aid in the conservation effort of this species, a restoration program was implemented in the Natural Reserve Vellozia in which several studies have been conducted to fill the gaps in the knowledge on the natural history and ecology of this species (Madeira and Fernandes Citation1999; Gomes et al. Citation2001; Silva et al. Citation2007; Negreiros et al. Citation2008).
Sample collection
Two independent restored and natural habitats were selected in Serra do Cipó to determine the patterns of FA and herbivory on two different plant ontogenetic stages of C. semaphora (saplings and mature individuals). Sites were distant from each other by ca. 2 km. We collected 30 leaves from each of 15 adults and 8 saplings of C. semaphora randomly sampled in each 2 habitats (e.g. 690 leaves in each habitat) at the end of the dry season (October), period were leaves are mature and completely expanded.
To determine the effects of the environment (restored vs. natural) and plant age (sapling vs. mature) on leaf FA, we obtained digital images of 30 fully developed leaves randomly collected per individual to calculate leaf FA. The sampling of leaves was conducted into 3 branches per plant in which the first 10 fully expanded leaves at the base were sampled. Leaf FA was calculated as the value of right (Ai is the right-side distance from midvein to leaf margin) minus left sides (Bi is the left-side distance from midvein to leaf margin) (∣Ai−Bi∣) divided by the average (Ai+Bi)/2, to correct for the fact that asymmetry may be size-dependent and all was yet divided by N that is the number of measurements taken (see Cornelissen and Stiling Citation2005, Citation2011). All leaves with damages caused by herbivory that could affect FA analysis were excluded from the calculations. To calculate leaf FA we use Sigma Scan Pro software.
To determine the herbivory levels we use the same 30 leaves used to calculate FA (n=690/habitat). Herbivory was caused by an unidentified species of leaf-chewing caterpillar (Geometridae: Lepidoptera) that feeds on leaf petals. The caterpillar was the only herbivore found on the plant during the study. To estimate the leaf area removed by the leaf caterpillar, we first obtained a digital image of each leaf and then estimated the total area of the leaf and the removed area using Sigma Scan Pro software. In addition, the frequency of leaves damaged by the herbivore was determined in each environment for the two different ontogenetic stages.
Statistical analyses
We conducted different statistical analysis to test each one of the hypothesis because the distribution of the response variables and the main effects were different for each hypothesis.
A linear regression analysis was performed to determine the relationship between leaf FA and herbivory levels in each environment (Hypothesis 1). We conducted a two-way ANOVA test (after arcsine transformation of the data) to determine the effect of habitat (restored vs. natural) and plant age (saplings vs. mature plants) on the leaf area removed by the herbivore (SAS Citation2000). Habitat and host plant age were considered as the independent variables while leaf area removed was used as the response variable. The proportion of leaf area affected by the herbivore was estimated for each leaf as the area removed divided by the total leaf area. We used the same analysis to evaluate the effects of environment and plant ontogeny (age) on leaf FA (Hypothesis 2). Environment and host plant ontogeny were considered as the independent variables while FA was used as the response variable. A LSMeans test was used for a posteriori comparisons. We also compared the leaf area removed by the caterpillars and leaf FA between individuals that occurred in each environment (restored and natural) using a one-way ANOVA test (after arcsine transformation of the data) (SAS Citation2000). A LSMeans test was used for a posteriori comparisons.
A logistic regression analysis using GENMOD (SAS Citation2000) was applied to test effects of habitat and plant ontogeny on frequency of caterpillar leaf damage (Hypothesis 2). The model used habitat (restored vs. natural) and plant ontogeny (saplings vs. mature plants) as the independent variables and frequency of leaf damage was used as the dependent variable.
Results
No significant relationship was found between leaf FA and leaf area damaged by the lepidopteran herbivore (F=0.42; R 2=0.001; p = 0.51). In addition, no statistical difference was found in leaf FA between habitats (F = 0.12; df = 1; p=0.72), as well as in different ontogenetic stages (F=1.51; df = 1; p=0.22).
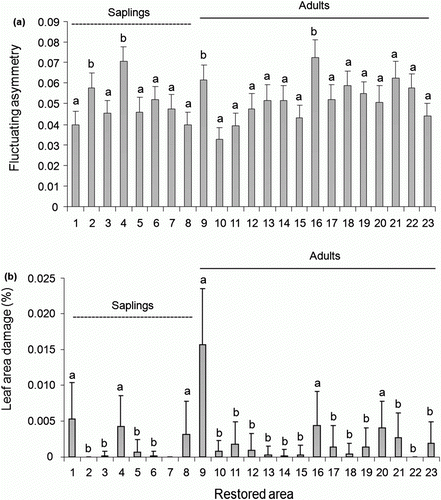
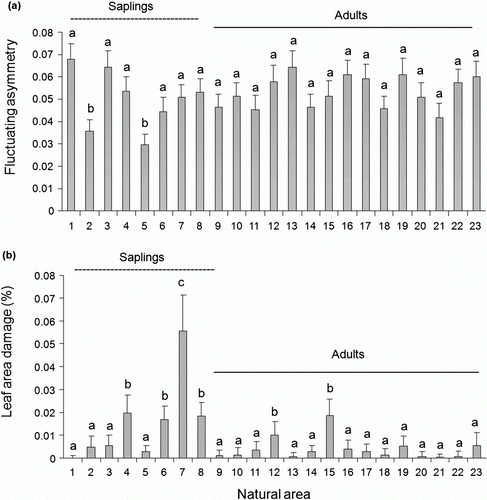
Leaf area consumed by the herbivore differed between the two habitats. In the natural habitat, the amount of leaf area removed by the caterpillar was higher in comparison with the leaf area removed in the restored habitat (F=27.33; df = 1; p=0.0001; a). The mean leaf area consumed in the natural habitat was 0.32±0.18 (mean±SE; range: 0.0–4.14 cm2), while in the restored habitat was 0.04±0.03 (mean±SE; range: 0.0–0.85 cm2). In addition, leaf area damage differed between saplings and mature plants (F=10.26, df = 1; p = 0.001, a). In the natural habitat, leaf damage was higher on saplings [0.77±0.50 (mean±SE; range: 0.0–4.13 cm2)] compared to mature plants [0.08±0.05 (mean±SE; range: 0.0–0.715 cm2)]. Additionally, variations in leaf FA (F=1.74; df = 22; p=0.02; a) and leaf area damage (F=3.21; df = 22; p=0.0001; b) were observed among individuals that occur in natural habitat.
Figure 1. Differences in leaf area damage by folivores (A) and frequency of leaf damage (B) between sites and ontogenetic stages. Non-transformed data are shown. Common letters identify means that were not statistically different according to LSMeans test (p>0.001) following ANOVA test. Error bars indicate SE.
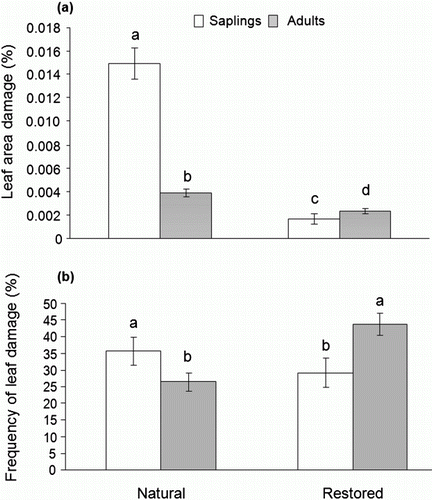
Figure 2. Fluctuating asymmetry (A) and leaf area damage (B) between individuals that occur in natural area. Common letters identify means that were not statistically different according to LSMeans test (p>0.001) following ANOVA test. Error bars indicate SE.
In contrast, in the restored habitat, leaf damage was higher on mature plants [0.06±0.05 (mean±SE; range: 0.0–0.85 cm2)] compared to saplings [0.0002±0.0001 (mean±SE; range: 0.0–0.0005 cm2)]. No significant variation in the frequency of leaf damage among ontogenetic stages was observed (F=1.25; df = 1; p = 0.26). However, the frequency of leaf damage differed between habitats (F=5.11; df = 1; p = 0.02; b). In the natural habitat, the frequency of leaf damage on saplings was higher [39.58±6.31 (mean±SE; range: 16.67–66.67 cm2)], than on mature plants [26.44±2.19 (mean±SE; range: 6.67–43.33 cm2)]. On the other hand, in the restored habitat, the frequency of leaf damage was higher on mature [43.78±3.79 (mean±SE; range: 16.67–63.33 cm2) than on saplings [29.17±2.87 (mean±SE; range: 23.33–43.33 cm2). In addition, leaf FA (F=1.94; df = 22; p = 0.006; a) and leaf area damage (F=1.86; df = 22; p=0.009; b) were significantly different among the individuals present in the restored habitat.
Discussion
Our findings demonstrate that plants of C. semaphora submitted to restoration program did not show differences in levels of FA from plants present in natural environment. The importance to develop methods to restore damaged populations has increased clearly due to the augmenting degradation of many natural ecosystems, which in turn, produces landscapes with a mosaic of sharply contrasted habitats (Paling et al. Citation2001; Tscharntke et al. Citation2002; Tscharntke and Brandl Citation2004; Bull et al. Citation2004). Reintroduced plant species in degraded environments are subject to different stress factors (e.g. extreme temperatures and low humidity) that compromise the success of the environmental restoration (McCoy Citation1998; Negreiros et al. Citation2008, Citation2009). Some authors have argued that plant stress can be evaluated by the leaf FA, as FA has been shown to increase with habitat fragmentation, pollution, soil fertility, competition, and predation (Premchand et al. Citation1998; Alados et al. Citation1999; Zvereva and Kozlov Citation2001). However, we did not detect differences in leaf FA in plants of C. semaphora in two contrasting stress conditions in a naturally harsh environment, at a mountain top in Brazil. The rupestrian fields found at the higher elevation in the Espinhaço mountains in southeastern Brazil perhaps represent the harsher natural conditions for the Cerrado (savanna) plants (Ribeiro and Fernandes Citation2000; Medina and Fernandes Citation2007), but not for which the native species are adapted to (see Barbosa et al. Citation2010).
Plant species adapted to nutrient-poor environments have developed efficient systems to minimize nutrient losses such as high rate of resorption of nutrients (Nardoto et al. Citation2006; Kozovits et al. Citation2007) and scleromorphic leaves that in turn, reduce the probability of abscission and facilitate high concentration of chemical defenses (Fernandes and Price Citation1988; Price et al. Citation1998). The endemic plants of the ruprestrian fields, such as C. semaphora, are adapted to the natural nutritional deficiencies of soil (Ribeiro and Fernandes Citation2000; Marques et al. Citation2002; Negreiros et al. Citation2008) and therefore these environmental conditions do not represent a stress factor to generate differences in developmental instability. Even when under the stress imposed by harsher conditions found in restored sites, the stress may have been not enough to influence the stability of the development of the studied species.
No relationship was found between FA and herbivore damage on C. semaphora in both natural and restored habitats. Herbivory represents a biotic stress for plants that increase the developmental instability of them (Møller Citation1996; Møller and De Lope Citation1998; Cornelissen and Stiling Citation2005, Citation2011). However, the relationship between FA and herbivory is not clear in the literature. While some studies show positive relationships (Møller Citation1995, Citation1996; Møller and De Lope Citation1998; Lempa et al. Citation2000; Cornelissen et al. Citation2003; Cornelissen and Stiling Citation2011, Citation2005), others show no relationship (Bañuelos et al. Citation2004; Díaz et al. Citation2004; Telhado et al. Citation2010). Differences in plant chemistry, plant age, environmental conditions, and enemy free-space have been suggested as possible causes for these differences (Lempa et al. Citation2000; Cornelissen et al. Citation2003; Bañuelos et al. Citation2004; Cornelissen and Stiling Citation2005; Cuevas-Reyes et al. Citation2011a, Citation2011b). Our results suggest that in both habitat conditions, symmetry of leaves did not change with the level of herbivory. Therefore, we concluded that the herbivory in harsh rupestrian fields was not a stress agent that significantly induces leaf asymmetry in C. semaphora. Future studies should focus on the potential relationship between FA and herbivory in field experiments in which herbivory would be manipulated and/or simulated, as in Bauhinia brevipes (Leguminosae) (Cornelissen and Fernandes Citation2001).
Different morphological and physiological changes are accompanied throughout the plant ontogeny that affects resistance to herbivores (Karban and Thaler Citation1999). For example, the foliage of mature plants should be better defended compared with the foliage of saplings (Basset Citation2001). However, no trend was observed in this study. Although the frequency of herbivory and leaf area removed by the lepidopteran folivore was higher on saplings than on mature plants in the natural habitat, the opposite pattern was found in plants in the restored habitat. Differences could be caused by several factors (e.g. plant chemistry, leaf palatability, local microclimate, enemy free-space) and only long-term studies could provide solid answer to the variation found (Coley and Barone Citation1996; Basset Citation2001).
In this system we did not observe any relationship between herbivory and FA, indicating that FA does not represent an indicator of stress to C. semaphora under environment restoration. On the other hand, we observed great differences in leaf FA and herbivory among individual. This variation associated with other factors such as plant quality and natural enemy pressure might be evaluated to determine the patterns of insect attack on host plants and support the recovery and conservation of endemic species in natural and reintroduced areas.
Acknowledgements
The authors thank the Programa de Pós-Graduação em Ciências Biológicas of Unimontes (PPGCB), Programa de Pós-Graduação em Ecologia, Conservação e Manejo da Vida Silvestre of UFMG (ECMVS) and the Reserva Vellozia/Planta LTDA for logistic support. This study was supported by CNPq (30 3352/2010-8, 47 4292/2010-0, 56 1883/2010-6, 15.1817/2008-1, 55.9279/2008-6) and by FAPEMIG (APQ-04105-10, RDP-00048-10, APQ 01278-08, CRA 495/07, EDT 465/07). Cuevas-Reyes P. thanks Dirección Adjunta de Desarrollo Científico y Académico del CONACYT for their generous support.
References
- Alados , CL , Navarro , TN and Cabezudo , B . 1999 . Tolerance assessment of Cistus ladanifer to serpentine soils by developmental stability analysis . Plant Ecol. , 143 : 51 – 66 .
- Anciles , M and Marini , MA . 2000 . The effects of fragmentation on fluctuating asymmetry in passerine birds of Brazilian tropical forests . J Appl Ecol. , 37 : 1013 – 1028 .
- Aronson , J , Floret , C , Lefloc'h , E , Ovalle , C and Pontanier , R . 1993 . Restoration and rehabilitation of degraded ecosystems in arid and semi-arid lands. A view from the south . Restor Ecol. , 1 : 8 – 17 .
- Bañuelos , MJ , Sierra , M and Obeso , JR . 2004 . Sex, secondary compounds and asymmetry. Effects on plant–herbivore interaction in a dioecious shrub . Acta Oecol. , 25 : 151 – 157 .
- Barbosa , NPU , Fernandes , GW , Carneiro , MAA and Carlos Júnior , LA . 2010 . Distribution of non-native invasive species and soil properties in proximity to paved roads and unpaved roads in a quartzitic mountainous grassland of southeastern Brazil (rupestrian fields) . Biol Inv. , 12 : 3745 – 3755 .
- Basset , Y . 2001 . Communities of insect herbivores foraging on saplings versus mature trees of Pouruma bicolor (Cecropiaceae) in Panama . Oecologia. , 129 : 253 – 260 .
- Bull , JS , Reed , DC and Holbrook , SJ . 2004 . An experimental evaluation of different methods of restoring Phyllospadix torreyi (Surfgrass) . Restor Ecol. , 12 : 70 – 79 .
- Chistyakova , EK and Kryazheva , NG . 2001 . Possible application of the indices of developmental stability and photosynthetic activity for studying the states of natural plat populations using the weeping birch as an example . Russ J Dev Biol. , 32 : 352 – 356 .
- Coley , PD and Barone , JA . 1996 . Herbivory and plant defenses in tropical forests . Annu Rev Ecol Syst. , 27 : 305 – 335 .
- Cornelissen , T and Stiling , P . 2005 . Perfect is best: low leaf fluctuating asymmetry reduces herbivory by leaf miners . Oecologia. , 142 : 46 – 56 .
- Cornelissen , T and Stiling , P . 2011 . Similar responses of insect herbivores to leaf fluctuating asymmetry . Arthrop-Plant Inter. , 5 : 59 – 69 .
- Cornelissen , T , Stiling , P and Drake , B . 2003 . Elevated CO2 decreases leaf fluctuating asymmetry and herbivory by leaf miners on two oak species . Global Change Biol. , 10 : 27 – 36 .
- Cornelissen , TG and Fernandes , GW . 2001 . Induced defences in the neotropical tree Bauhinia brevipes (Vog.) to herbivory: effects of damage-induced changes on leaf quality and insect attack . Trees. , 15 : 236 – 241 .
- Cuevas-Reyes , P , Mauricio , Q , Hanson , P , Dirzo , R and Oyama , K . 2004 . Diversity of gall-inducing insects in a Mexican tropical dry forest: the importance of plant species richness, life-forms, host plant age and plant density . J Ecol. , 92 : 707 – 716 .
- Cuevas-Reyes , P , Quesada , M and Oyama , K . 2006 . Abundance and leaf damage caused by gall-inducing insects in a Mexican tropical dry forest . Biotropica. , 38 : 107 – 115 .
- Cuevas-Reyes , P , Oyama , K , González-Rodríguez , A , Fernandes , GW and Mendoza-Cuenca , L . 2011a . Contrasting herbivory patterns and leaf fluctuating asymmetry in Heliocarpus pallidus between different habitat types within a Mexican tropical dry forest . J Trop Ecol. , 27 : 383 – 391 .
- Cuevas-Reyes P , Fernandes GW , González-Rodríguez A , Pimenta A . 2011b . Effects of generalist and specialist parasitic plants (Loranthaceae) on the fluctuating asymmetry patterns of ruprestrian host plants . Basic Appl Ecol . 12 : 449 – 455 .
- Díaz , M , Pulido , FJ and Møller , AP . 2004 . Herbivore effects on developmental instability and fecundity of holm oaks . Oecologia. , 139 : 224 – 234 .
- Fernandes , GW and Price , PW . 1988 . Biographical gradients in galling species richness: Test of hypotheses . Oecologia. , 76 : 161 – 167 .
- Fonseca , CR , Fleck , T and Fernandes , GW . 2006 . Processes driving ontogenetic succession of galls in a canopy tree . Biotropica , 38 : 514 – 521 .
- Giulietti AM , Pirani JR , Harley RM . 1997 . Centres of plant diversity: a guide and strategy for their conservation . World Wide Fund for Nature (WWF) & The World Conservation Union (IUCN), Cambridge. Chapter 1, Espinhaço Range Region, Eastern Brazil . 397–404 .
- Gomes , V , Madeira , JA , Fernandes , GW and Lemos-Filho , JP . 2001 . Seed dormancy and germination of sympatric species of Chamaecrista (Leguminosae) in a rupestrian field . Int J Ecol Environ Sci. , 27 : 1 – 7 .
- Hagen , SB , Ims , RA and Yoccoz , NG . 2008 . Fluctuating asymmetry as an indicator of elevation stress and distribution limits in mountain birch (Betula pubescens) . Plant Ecol. , 195 : 157 – 163 .
- Herms , DA and Mattson , WJ . 1992 . The dilemma of the plants: to grow or to defend . Q Rev Biol. , 67 : 283 – 335 .
- Hódar , JA . 2002 . Leaf fluctuating asymmetry of Holm oak in response to drought under contrasting climatic conditions . J Arid Environ. , 52 : 233 – 243 .
- Irwin , HS and Barneby , RC . 1982 . The American Cassiinae a synoptical revision of Leguminosae, Tribo Cassieae, Subtribo Cassiinae in the New World . New York Bot Gard. , 35 : 1 – 918 .
- Jacobi , CM , Carmo , FF and Vincent , RC . 2008 . Estudo fitossociológico de uma comunidade vegetal sobre canga como subsídio para a reabilitação de áreas mineradas no Quadrilátero Ferrífero, MG . Rev Árvore. , 32 : 345 – 353 .
- Karban , R and Thaler , JS . 1999 . Plant phase change and resistant to herbivory . Ecology. , 80 : 510 – 517 .
- Kozovits , AR , Bustamante , MMC , Farofalo , CR , Bucci , S , Franco , AC , Goldstein , G and Meinzer , FC . 2007 . Nutrient resorption and patterns of litter production and decomposition in a Neotropical savanna . Funct Ecol. , 21 : 1034 – 1043 .
- Leamy , LJ and Klingenberg , CP . 2005 . The genetics and evolution of fluctuating asymmetry . Annu Rev Ecol Syst. , 36 : 1 – 21 .
- Lempa , K , Martel , J , Koricheva , J , Haukioja , E , Ossipov , V , Ossipova , S and Pihlaja , K . 2000 . Covariation of fluctuating asymmetry, herbivory and chemistry during birch leaf expansion . Oecologia. , 122 : 354 – 360 .
- Lill , JT and Marquis , RJ . 2003 . Ecosystem engineering by caterpillars increases insect herbivore diversity on white oak . Ecology. , 84 : 682 – 690 .
- Madeira , JA and Fernandes , GW . 1999 . Reproductive phenology of sympatric taxa of Chamaecrista (Leguminosae) in Serra do Cipó, Brazil . J Trop Ecol. , 15 : 463 – 479 .
- Marques , AR , Fernandes , GW , Reis , IA and Assunção , RM . 2002 . Distribution of adult male and female Baccharis concinna (Asteraceae) in the rupestrian fields of Serra do Cipó, Brasil . Plant Biol. , 4 : 94 – 103 .
- McCoy S . 1998 . The dynamics of Gymnostoma maquis on ultramafic soils in New Caledonia [PhD thesis] . Canberra AU : Australian National University .
- Medina , BMO and Fernandes , GW . 2007 . Natural regeneration potential of rupestrian fields soils' in Serra do Cipó, Brazil . Rev Bras Bot. , 30 : 665 – 678 .
- Mendonça , MP and Lins , LV . 2000 . Lista Vermelha das Espécies Ameaçadas de Extinção da Flora de Minas Gerais , Minas Gerais : Biodiversitas e Fundação Zoo-Botânica de Belo Horizonte .
- Menezes , NZ and Giulietti , AM . 2000 . “ Campos rupestres ” . In Lista vermelha das espécies ameaçadas de extinção da flora de Minas Gerais , Edited by: Mendonça , MP and Lins , LV . 65 – 73 . Minas Gerais : Fundação Biodiversitas e Fundação Zoo-Botânica de Belo Horizonte .
- Møller , AP . 1995 . Patterns of fluctuating asymmetry in sexual ornaments of birds from marginal and central populations . Am Nat. , 45 : 316 – 327 .
- Møller , AP . 1996 . Parasitism and developmental instability of hosts: a review . Oikos. , 77 : 189 – 196 .
- Møller , AP and Swaddle , JP . 1997 . Asymmetry, developmental stability and evolution , Oxford : Oxford University Press .
- Møller , AP and De Lope , F . 1998 . Herbivory affects developmental instability of stone oak, Quercus rotundifolia . Oikos. , 82 : 246 – 252 .
- Møller , AP and Shykoff , JA . 1999 . Morphological developmental stability in plants: patterns and causes . Int J Plant Sci. , 160 : 135 – 146 .
- Moreira , RG , McCauley , RA , Cortés-Palomec , AC , Fernandes , GW and Oyama , K . 2009 . Spatial genetic structure of Coccoloba cereifera (Polygonaceae), a critically endangered microendemic species of Brazilian rupestrian fields . Conserv Genet. , 11 : 1247 – 1255 .
- Nardoto , GB , Bustamante , MMC and Pinto , AS . 2006 . Nutrient use efficiency at ecosytem and species level in savanna areas of Central Brazil and of fire . J Trop Ecol. , 22 : 191 – 201 .
- Negreiros , D , Moreaes , MLB and Fernandes , GW . 2008 . Caracterização da fertilidade dos solos de quatro leguminosas de campos rupestres, Serra do Cipó, MG, Brasil . J Plant Nutr Soil Sci. , 8 : 30 – 39 .
- Negreiros , D , Fernandes , GW , Silveira , FAO and Chalub , C . 2009 . Seedling growth and biomass allocation of endemic and threatened shrubs of rupestrian fields . Acta Oecol. , 35 : 301 – 310 .
- Paling , EI , Van Keulen , M , Wheeler , K , Phillips , J and Dyhrberg , R . 2001 . Mechanical seagrass transplantation in Western Australia . Ecol Eng. , 16 : 331 – 339 .
- Palmer , AR and Strobeck , C . 1986 . Fluctuating asymmetry: measurement, analysis, patterns . Annu Rev Ecol Syst. , 17 : 391 – 421 .
- Premchand , A , Mawri , F , Gladstone , S and Freeman , DC . 1998 . Is fluctuating asymmetry a reliable biomonitor of stress? A test using life history parameters in soybean . Int J Plant Sci. , 159 : 559 – 565 .
- Price , PW , Fernandes , GW , Lara , ACF , Brawn , J , Barrios , H , Wright , MG , Ribeiro , SP and Rothcliff , N . 1998 . Global patterns in local number of insect galling species . J Biogeogr. , 25 : 581 – 591 .
- Ribeiro , KT and Fernandes , GW . 2000 . Patterns of abundance of a narrow endemic species in a tropical and infetile montane habitat . Plant Ecol. , 147 : 205 – 218 .
- Rizzini , CT . 1979 . Treatise of phytogeography of Brazil: ecological aspects , 2nd ed , São Paulo : Hucited/Edusp .
- SAS . 2000 . Categorical data analysis using the SAS system . Cary (NC) : SAS Institute .
- Silva , RM , Fernandes , GW and Lovato , MB . 2007 . Genetic variation in two Chamaecrista species (Leguminosae), one endangered and narrowly distributed and another widespread in the Serra do Espinhaço, Brasil . Can J Bot. , 85 : 629 – 636 .
- Suding , KN , Gross , KL and Houseman , GR . 2004 . Alternative states and positive feedbacks in restoration ecology . Trends Ecol Evol. , 19 : 46 – 53 .
- Telhado , C , Eteves , D , Cornelissen , T , Fernandes , GW and Carneiro , MAA . 2010 . Insect herbivores of Coccoloba cereifera do not select asymmetric plants . Environ Entomol. , 39 : 849 – 855 .
- Tripathi , N and Singh , RS . 2008 . Ecological restoration of mined-out areas of dry tropical environment, Índia . Environ Monit Assess. , 146 : 325 – 337 .
- Tscharntke , T , Steffan-Dewenter , I , Kruess , A and Thies , C . 2002 . Contribution of small habitat fragments to conservation of insect communities of grassland-cropland landscapes . Ecol Appl. , 12 : 54 – 63 .
- Tscharntke , T and Brandl , R . 2004 . Plant-insect interactions in fragmented landscapes . Annu Rev Entomol. , 49 : 405 – 430 .
- Viana , LR , Fernandes , GW and Silva , CA . 2005 . Ecological road threatens endemic Brazilian plant with extinction . Plant Talk. , 41 : 15
- Waltz , AM and Whitham , TG . 1997 . Plant development affects arthropod communities: opposing impacts of species removal . Ecology. , 78 : 2133 – 2144 .
- Waterman , PG and Mole , S . 1989 . Soil nutrients and plant secondary compounds , Oxford : Blackwell Scientific Publications .
- Wauters , LA , Dhondt , AA , Knothe , H and Parkin , DT . 1996 . Fluctuating asymmetry and body size as indicators of stress in red squirrel populations in woodland fragments . J Appl Ecol. , 33 : 735 – 740 .
- White , TCR . 1969 . An in index to measure weather-induced stress of trees associated with outbreaks of psyllids in Australia . Ecology. , 50 : 905 – 909 .
- Whitlock , M . 1996 . The heritability of fluctuating asymmetry and the genetic control of developmental stability . Biol Sci. , 263 : 849 – 853 .
- Woodman , RL and Fernandes , GW . 1991 . Differential mechanical defense: herbivory, evapotranspiration, and leaf -hairs . Oikos. , 60 : 11 – 19 .
- Zvereva , EL and Kozlov , MV . 2001 . Effects of pollution-induced habitat disturbance on the response of willows to simulated herbivory . J Ecol. , 89 : 21 – 30 .