Abstract
Seeds have devised sophisticated mechanisms to cope with adverse situations. Among these mechanisms the collectively referred to as redox homeostasis. We have investigated the thioredoxin (Trx)/NADPH/NADPH-dependent thioredoxin reductase (NTR) systems during the pea (Pisum sativum L.) seed germination under heavy metal stress. Cotyledon and embryonic axis were analyzed between 0 and 5 days of germination in the presence or absence of cadmium (Cd). Under Cd stress conditions, NTR activity and Trx h3 and Trx h4 expression were stimulated, while the overall activity of Trx decreased. When incubated with Cd ions in vitro, the disulfide reductase activity of Trx h3 and f isoforms was drastically inhibited, probably through the formation of protein dimers as evidenced by the electrophoresis analysis. The NADPH status was also affected, since Cd treatment provoked an increase in oxidized state of coenzyme as compared to control redox ratio. The contribution of Trx system to the redox regulation during the germination is discussed in relation with the Cd-caused oxidative stress.
Keywords:
Abbreviations
| DTNB | = |
5,5′-Dithio-bis-(2-nitrobenzoic acid) |
| DTT | = |
dithiothreitol |
| FW | = |
fresh weight |
| h | = |
hour |
| NTR | = |
NADPH-thioredoxin reductase |
| Trx | = |
Thioredoxin |
| β-MET | = |
β-Mercaptoethanol |
1. Introduction
Seeds contain several thioredoxin (Trx) systems (Berstermann et al. Citation1983). The Arabidopsis cytosolic system consists of eight different Trx h and two homodimeric NADPH-dependent thioredoxin reductases (NTR, EC 1.6.4.5) (Laloi et al. Citation2001; Meyer et al. Citation2002), while the mitochondrial and nuclei ones include a Trx o and NTR (Laloi et al. Citation2001; Martí et al. Citation2009) and endoplasmic reticulum contain a Trx s (Alkhalfioui et al. Citation2008). However, the chloroplastic Trx system is even more complex than the cytosolic one. It includes eight types of Trx (f, m, x, y, CDSP32, APR proteins, lilium proteins, and HCF164) and a heterodimeric ferredoxin-dependent Trx reductase (Cain et al. Citation2009).
Seed germination starts with the uptake of water and is completed with the emergence of the radicle (Welbaum et al. Citation1998). Subsequent events, including the mobilization of the major reserves, are associated with the growth of the seedling (Bewley Citation1997). A potential role of Trxs in redox status of storage proteins and proteolysis during germination has been already suggested (Lozano et al. Citation1996; Montrichard et al. Citation2003; Alkhalfioui et al. Citation2007). They appear to play a role in the reduction of critical disulfide bonds of proteins (Kobrehel et al. Citation1992; Besse et al. Citation1996; Lozano et al. Citation1996; Schürmann and Jacquot Citation2000). For example; Trx h acts as an early wake-up call in seed germination, facilitating the freeing of nitrogen and carbon through the activation of proteases and the inactivation of proteinaceous inhibitors (Besse et al. Citation1996; Lozano et al. Citation1996).
Another role of Trx system is the protection against oxidative injury of reactive oxygen species that are highly produced with the resumption of germinative metabolism (Aalen Citation1999; Laloi et al. Citation2004). Trx h can act as an hydrogen donor to 1Cys-peroxiredoxin, which protects the embryo macromolecules from oxidation during early imbibition (Aalen Citation1999). Moreover, the cosuppressed Arabidopsis lines lacking CDSP32 (two Trx domains) exhibit an increased sensitivity to oxidative stress (Broin and Rey Citation2003) and the expression of the cytosolic Trx h5 gene (AtTRXh5) is closely related to several oxidative stress situations (Laloi et al. Citation2004). By the functional complementation of a Saccharomyces cerevisiae double mutant, Traverso et al. (Citation2007) have also demonstrated that Trx h1 and Trx h2 are involved in the control of (1) redox-imbalance and (2) hypersensitivity to hydrogen peroxide.
In the plant life cycle, the seed germination is a key developmental stage conditioning the final yield of crops. However, redox reactions have been studied principally in adult plant and there is only little information about the onset of redox regulation during the seed germination. In this study, we have investigated the behavior of Trx-dependent system in cadmium (Cd)-poisoned germinating Pisum sativum seed. This may offer a better knowledge and a new meaning for crop protection, since several Cd-sensitive proteins can be regulated via the two major cellular redox systems (Trx and glutaredoxin) (Lemaire et al. Citation1999, Citation2004; Gillet et al. Citation2006; Hansen et al. Citation2006) which the thiol-disulfide status is important for the control of protein functions under heavy metal stress (Cobbett Citation2000).
2. Materials and methods
2.1. Germination conditions
Seeds of pea (Pisum sativum L. cv. douce province) were disinfected with 2% sodium hypochlorite for 10 min and then rinsed thoroughly and soaked in distilled water at 4°C for 30 min to obtain an initial stage (Smiri et al. Citation2009). Seeds were germinated at 25°C in the dark over two sheets of filter paper moistened with distilled water or aqueous solution of chloride salt of 5mM Cd. At harvest, the coat was removed and the embryonic axes and cotyledons were weighed and stored in liquid nitrogen until analysis.
2.2. Protein extraction
Fresh tissues were ground with a mortar and pestle in a homogenization medium (pH 8.0) consisting of 50 mM Tris-HCl, 0.4 M sucrose, 1 mM EDTA, 5 mM ascorbic acid and 1 mM MgCl2 (1:2,w:v). The homogenate was filtered through two layers of miracloth and centrifuged at 3000 g for 10 min. The resulting supernatant was centrifuged again at 20,000 g for 20 min. The pellet was washed with the homogenization medium and centrifuged in the same way. The mitochondrial pellet was then discarded for other use (Smiri et al. Citation2009). The combination of supernatants was regarded as post mitochondrial fraction which quality was routinely evaluated (Douce et al. Citation1972) using cytochrome c oxidase test (data not shown). All operations were performed at 4°C.
Protein content was determined as described by Bradford (Citation1976) using bovine serum albumin (BSA) as standard.
2.3. Electrophoresis and western-blot analysis
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed with 15% (w/v) polyacrylamide gels (Laemmli Citation1970). Proteins were then transferred to nitrocellulose sheets (Towbin et al. Citation1979) and western blotting was performed with poplar Trx h3 and Trx h4 and Arabidopsis NTR antibodies. Blocking of nonspecific binding is achieved by placing the membrane in blocking buffer [20 mM Tris-HCl (pH 7.5), 137 mM NaCl, 0.05% Tween 20 and 5% milk] for overnight. After blocking, the detection process takes place in a two-step; a dilute solution of primary antibody (dilution: 1/1000) is incubated with the membrane under gentle agitation for 2 h. After rinsing the membrane with blocking buffer for five times to remove unbound primary antibody, the membrane is exposed to a secondary antibody for 1 h. After rinsing the membrane with TBST [20 mM Tris-HCl (pH 7.5), 137 mM NaCl and 0.05% Tween 20], a horseradish peroxidase-linked secondary is used to cleave a chemiluminescent agent, and the reaction product produces luminescence in proportion to the amount of protein. A sensitive sheet of photographic film is placed against the membrane, and exposure to the light from the reaction creates an image of the antibodies bound to the blot.
2.4. Enzyme assays
The Trx assay using 5,5′-Dithio-bis-(2-nitrobenzoic acid) (DTNB) was carried out as described (Jacquot et al. Citation1994). The NTR activity (Jacquot et al. Citation1994) was measured spectrophotometrically. One unit of enzyme was defined as the amount necessary to decompose 1 µmol of substrate per min at 25°C.
2.5. Effect of Cd ions on Trx in vitro
Proteins were incubated with appropriate amounts of CdCl2 corresponding to real metal contents in cotyledons or embryonic axes of Cd-treated germinating seeds (Smiri et al. Citation2009). Trx assay (Jacquot et al. Citation1978, Citation1994) was performed after an incubation period of 30, 120, and 240 min at 4°C. The formation of Trx h3 and f dimers was analyzed by SDS-PAGE after incubation of proteins with Cd ions in the absence or in the presence of 30 mM β-mercaptoethanol (β-MET) and dithiothreitol (DTT).
2.6. NADPH/NADP+ determination
Reduced (NADPH) and oxidized (NADP+) forms were extracted from the post mitochondrial fraction according to the method of Zhao et al. (Citation1987). Enzyme cycling assay was employed as following the procedure described by Matsumura and Miyachi (Citation1980) for the coenzyme quantification.
2.7. Statistical analysis
The experiments were performed in duplicates. The data reported are the mean values (± SE) of six replicates. Data were subjected to one-way ANOVA. Means were separated with the Student Newman Keuls test (Statistica 8; StatSoft Co., USA).
3. Results
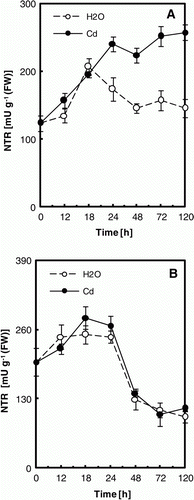
Exposure of P. sativum seeds to Cd during germination results in the increase of the NTR activity in cotyledons: about 80% from controls after 5 days of treatment (A), but no change in the embryo (B). Moreover, NTR was immunodetected with high expression level in ungerminated seed, decreased during H2O-imbibition, but induced under Cd treatment ().
Figure 2. Western blot analysis of NTR expression in cotyledons (A) and embryonic axes (B) of P. sativum seeds during germination after imbibition with H2O or 5 mM Cd. Analysis was performed after SDS-PAGE of post mitochondrial proteins obtained from several germinating seeds (20 µg/track), transferred to nitrocellulose sheet and immunodetected with AtNTR antibody. The blot is representative of two experiments.
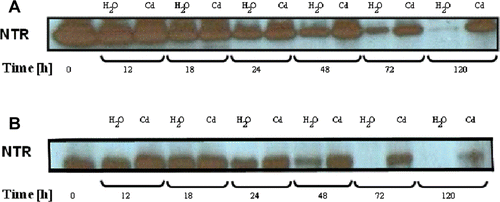
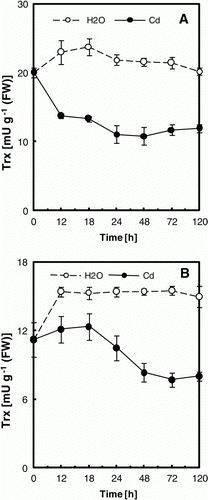
The protein levels of some isoforms, namely Trx h3 and Trx h4, decreased after H2O-imbibition, but increased following the exposure to Cd (). However, the overall Trx activity was inhibited: about 42% and 47% from control cotyledons and embryonic axes, respectively, after 5 days of treatment (). These observations led us to investigate the Trx-Cd interaction mechanisms.
Figure 3. Western blot analysis of Trx expression in cotyledons (A,C) and embryonic axes (B,D) of P. sativum seeds during germination after imbibition with H2O or 5 mM Cd. Analysis was performed after SDS-PAGE of post mitochondrial proteins obtained from several germinating seeds (20 µg/track), transferred to nitrocellulose sheet and immunodetected with poplar Trx h3 and Trx h4 antibodies. The blot is representative of two experiments.
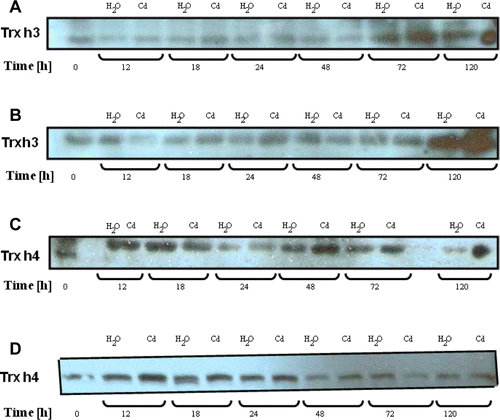
Figure 4. Trx activity in cotyledons (A) and embryonic axes (B) of P. sativum seeds during germination after imbibition with H2O or 5 mM Cd. Data are the mean of six independent measurements ±SE. Each measurement was performed in an extract obtained from several germinating seeds.
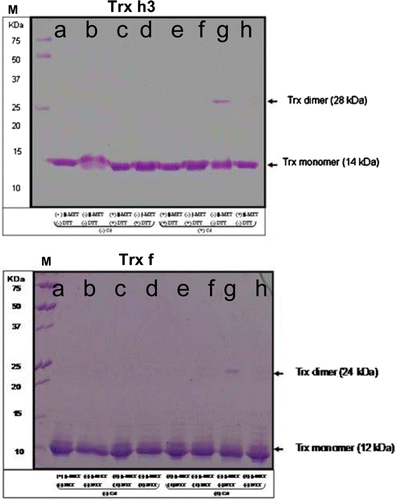
To test further the supposed mechanism of inactivation, we have analyzes the effect of pre-treatment with Cd ions on the activity of bicysteinic (active site contains two thiols) Trx h3 and f. The disulfide reductase activity of two isoforms was markedly diminished in a time-dependent manner (). This may be due, at least in part, to the formation of dimers. In fact, the electrophoretic analysis showed that the reduced form of Trx h3 (monomer; 14 KDa) and f (12 KDa) can be converted to oxidized states in the presence of Cd (dimers; 28 and 24 KDa, respectively) (). The reduced states were restored by the addition of either β-MET or DTT. These results indicate that Trxs are able to bind Cd, presumably at the level of their active site thiols. Thus, the inhibitory effect of Cd on the Trx activity can be due to the formation of dimers because the treatment of reduced forms with Cd should convert the active site to C-SS-C.
Figure 5. Effect of Cd ions on Trx activity in vitro. Poplar proteins (5 µg) were mixed with 5 µg Cd or equal volume of H2O (control) and incubated for different times before the assay which was performed using either the DTNB-NTR test (Trx h3) or the NADP-malate dehydrogenase activation test (Trx f). Data are the mean of six independent measurements ±SE.
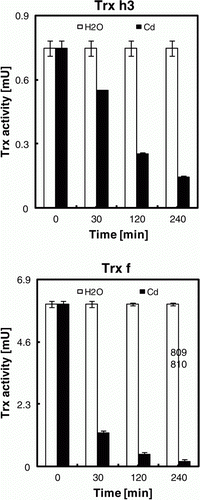
Figure 6. Effect of Cd binding on Trx. Poplar proteins (5 µg per lane) were mixed with 5 µg Cd, 30 mM β-MET and DTT (+) or equal volume of H2O (−) and incubated for 240 min before being subjected to 15% SDS-PAGE (a, Trx+ β-MET- DTT- Cd; b, Trx- β-MET- DTT- Cd; c, Trx+ β-MET+ DTT- Cd; d, Trx- β-MET+ DTT+ Cd; e, Trx + β-MET+ DTT+ Cd; f, Trx- β-MET+ DTT+ Cd; g, Trx- β-MET- DTT+ Cd; h, Trx + β-MET- DTT+ Cd). M – molecular weight markers are indicated in KDa. Trx dimer and mononer were stained with Coomassie bleue. Experiments were performed in duplicate.
The effects of Cd treatment on redox ratio of coenzyme (oxidized to reduced form) are shown in . Cd caused a significant consumption of reduced nicotinamide, as evidenced by the increase in NADP+/NADPH ratio. In fact, the redox ratios in dry seed tissues were markedly decreased during H2O-imbibition, but remained strongly high in the Cd-treated seeds ().
Table 1. Effect of Cd on redox ratio of coenzyme in germinating P. sativum seeds. Data are the means ±SE of 12 individual measurements. Each measurement was performed in an extract obtained from several germinating seeds. Values followed by an asterisk are significantly different from the respective controls at the 0.05 level of probability.
4. Discussion
The inhibitory effects of Cd on various processes in adult P. sativum and germinating seeds have been previously documented (Chaoui et al. Citation2004; Chaoui and El Ferjani Citation2005; Mihoub et al. Citation2005; Smiri et al. Citation2009, Citation2010a,Citationb,Citationc,Citationd,Citatione; Rahoui et al. Citation2010). Bioassays reflect toxicological damage at biochemical and physiological levels.
In a previous work (Smiri et al. Citation2010b), we have demonstrated that Cd causes an oxidative stress through alterations in mitochondrial redox status; we suggest that neither glutaredoxin system nor Trx one may improve the redox status of mitochondrial thiols in the embryo of germinating pea seed exposed to Cd toxicity, but in the cotyledons the contribution of Trx/NTR/NADPH can be established. Here, we studied the effects of metal on cytosolic redox Trx systems. We demonstrated that the alterations in post mitochondrial (microsomal fraction and cytosol) system can also interfere with the deleterious effects of Cd stress. The novelty of this study relative to the previous work is that Trx expression during germination in control (H2O) and treated cotyledons and embryonic axes were analyzed. However, in vitro analysis showed that Cd caused oxidation of cytosol Trx isoforms demonstrated by formation of protein dimmers. Properties of the Cd2 +-Trx interaction were examined to determine whether its binding affected Trx activity.
The pea cytosolic system consists of Trx h and homodimeric NTR (Montrichard et al. Citation2003), and Trx f and a heterodimeric ferredoxin-dependent Trx reductase for the chloroplastic Trx system (Cain et al. Citation2009).
The Trx system appears to play a fundamental role in controlling redox status in animals and plants subjected to abiotic stress as Cd (Pagano et al. Citation2000; Laloi et al. Citation2004; Aina et al. Citation2007; Traverso et al. Citation2007). The oxidized form of Trx contains a disulfide (-S-S-) bridge that is reduced to the sulfhydryl state (-SH) via either reduced ferredoxin and ferredoxin-dependent thioredoxin reductase or NADPH and NTR (Balmer et al. Citation2006; Jacquot et al. Citation2009). The stimulation of NTR expression after the exposure to Cd ( and ) led us to examine the impact of heavy metal treatment on the other components of redox system: Trx and NADPH status.
Under Cd stress conditions, the expression of some Trx isoforms (h3 and h4) was increased (), whereas the overall activity was inhibited (). In yeast, Cd induced the biosynthesis of Trx, while mutants carrying a deletion of Trx or NTR genes exhibited a decreased tolerance to Cd (Vido et al. Citation2001). In plants, the situation is more complex, because of the multiplicity of Trx. Thus different effects of Cd on synthesis or inhibition are possible according to the types of Trx and their functions in the cell. For example, regarding gene expression analysis in response to stress, Trx f and Trx m transcripts increased in P. sativum subjected to abiotic treatments (Pagano et al. Citation2000; Traverso et al. Citation2008), while an inhibition of Trx activity by Cd has been reported (Lemaire et al. Citation1999), perhaps via an oxidation phenomenon. In fact, Trx can be oxidized in response to inducers of oxidative stress; yet the functional consequences of the oxidation have not been determined (Chen et al. Citation2006).
Heavy metals are known to bind thiols with high affinity (Vallee and Ulmer Citation1972; Van Assche and Clijsters Citation1990). Consequently, the active site of reduced Trxs is a possible target of Cd fixation. To test further the mechanism of inactivation, we have analyzes the effect of pre-treatment with Cd ions on the activity of Trx h3 and f. The disulfide reductase activity of two bicysteinic isoforms was drastically inhibited (). This may be due to the formation of dimers. In fact, the electrophoretic analysis showed that the reduced form of Trx h3 (monomer; 14 KDa) and f (12 KDa) can be converted to oxidized state in the presence of Cd (dimers; 28 and 24 KDa, respectively) (). The reduced states were restored by the addition of either β-MET or DTT. These findings indicate that Trxs are able to bind Cd, presumably at the level of their active site thiols. However, it seems that heavy metal stress induces the Trx expression which should not compensate the vulnerability to dimerization and consequently to inactivation imposed by Cd ions, since the overall Trx activity was drastically inhibited.
However, the tolerance to heavy metal toxicity depends on the reducing power that can be supplied via secondary NAD(P)H recycling dehydrogenase activities (Mattioni et al. Citation1997) which are markedly inhibited in Cd-poisoned germinating P. sativum seeds (Smiri et al. Citation2009). Cd treatment caused a decrease in the reduced forms of nicotinamide, as evidenced by the increase in the redox ratio (). This suggests the comsuption of NADPH via NTR activity in Cd-treated cotyledons (A). Cd stimulated NAD(P)+/NAD(P)H ratio in embryonic axes where NTR activity was not modified by stress (B). We suggest that the increase in redox ratio of nicotinamide would be a consequence of an oxidation reaction imposed by Cd.
5. Conclusion
Under normal conditions, the intra-cellular redox state is predominately reducing, but processes like Cd-induced oxidative stress can shift the redox balance toward an oxidizing state. Our findings add new evidence for relationships between the Trx-dependent system, coenzyme status, and Cd treatment during Pisum sativum seed germination.
Acknowledgements
This research was supported by the Tunisian Ministry of Higher Education, Scientific Research, and Technology.
Additional information
Notes on contributors
Jamel El Ghoul
All authors contributed equally to this workReferences
- Aalen , RB . 1999 . Peroxiredoxin antioxidants in seed physiology . Seed Sci Res. , 9 : 285 – 295 .
- Aina , R , Labra , M , Fumagalli , P , Vannini , C , Marsoni , M , Cucchi , U , Bracale , M , Sgorbati , S and Citterio , S . 2007 . Thiol-peptide level and proteomic changes in response to cadmium toxicity in Oryza sativa L. roots . Environ Exp Bot. , 59 : 381 – 392 .
- Alkhalfioui , F , Renard , M , Frendo , P , Keichinger , C , Meyer , Y , Gelhaye , E , Hirasawa , M , Knaff , DB , Ritzenthaler , C and Montrichard , F . 2008 . A novel type of thioredoxin dedicated to symbiosis in legumes . Plant Physiol. , 148 : 424 – 435 .
- Alkhalfioui , F , Renard , M , Vensel , WH , Wong , J , Tanaka , CK , Hurkman , WJ , Buchanan , BB and Montrichard , F . 2007 . Thioredoxin-linked proteins are reduced during germination of Medicago truncatula seeds . Plant Physiol. , 144 : 1559 – 1579 .
- Balmer , Y , Vensel , WH , Cai , N , Manieri , W , Schürmann , P , Hurkman , WJ and Buchanan , BB . 2006 . A complete ferredoxin/thioredoxin system regulates fundamental processes in amyloplasts . Proc Natl Acad Sci USA. , 103 : 2988 – 2993 .
- Berstermann , A , Vogt , K and Follmann , H . 1983 . Plant seeds contain several thioredoxins of regular size . Eur J Biochem. , 131 : 139 – 344 .
- Besse , I , Wong , JH , Kobrehel , K and Buchanan , BB . 1996 . Thiocalsin: a thioredoxin- linked, substrate-specific protease dependent on calcium . Proc Natl Acad Sci USA. , 93 : 3169 – 3175 .
- Bewley DJ . 1997 . Seed germination and dormancy . The Plant Cell . 9 : 1055 – 1066 .
- Bradford , MM . 1976 . A rapid and sensitive method for the quantitation of microgram quantity of protein utilizing the principle of protein dye binding . Anal Biochem. , 72 : 248 – 254 .
- Broin , M and Rey , P . 2003 . Potato plants lacking the CDSP32 plastidic thioredoxin exhibit overoxidation of the BAS1 2-cysteine peroxiredoxin and increased lipid peroxidation in thylakoids under photooxidative stress . Plant Physiol. , 132 : 1335 – 1343 .
- Cain , P , Hall , M , Schröder , WP , Kieselbach , T and Robinson , C . 2009 . A novel extended family of stromal thioredoxins . Plant Mol Biol. , 70 : 273 – 281 .
- Chaoui , A and El Ferjani , E . 2005 . Effects of cadmium and copper on antioxidant capacities, lignification and auxin degradation in leaves of pea (Pisum sativum L.) seedling . Comptes Rendus Biol. , 328 : 23 – 31 .
- Chaoui , A , Jarrar , B and El Ferjani , E . 2004 . Effects of cadmium and copper on peroxidase, NADH oxidase and IAA oxidase activities in cell wall, soluble and microsomal membrane fractions of pea roots . J Plant Physiol. , 161 : 1225 – 1234 .
- Chen , Y , Cai , J and Jones , DP . 2006 . Mitochondrial thioredoxin in regulation of oxidant-induced cell death . FEBS Lett. , 580 : 6596 – 6602 .
- Cobbett , CS . 2000 . Phytochelatin biosynthesis and function in heavy-metal detoxification . Curr Opin Plant Biol. , 3 : 211 – 216 .
- Douce , R , Christensen , EL and Bonner , RWJR . 1972 . Preparation of intact plant mitochondria . Biochim Biophys Acta. , 275 : 148 – 160 .
- Gillet , S , Decottignies , P , Chardonnet , S and Le Maréchal , P . 2006 . Cadmium response and redoxin targets in Chlamydomonas reinhardtii: a proteomic approach . Photosyn Res. , 89 : 201 – 211 .
- Hansen , JM , Zhang , H and Jones , DP . 2006 . Differential oxidation of thioredoxin-1, thioredoxin-2, and glutathione by metal ions . Free Rad Biol Med. , 40 : 138 – 145 .
- Jacquot , JP , Eklund , H , Rouhier , N and Schürmann , P . 2009 . Structural and evolutionary aspects of thioredoxin reductases in photosynthetic organisms . Trends Plant Sci. , 14 : 336 – 343 .
- Jacquot , JP , Rivera-Madrid , R , Marinho , P , Kollarova , M , Le Maréchal , P , Miginiac-Maslow , M and Meyer , Y . 1994 . Arabidopsis thaliana NADPH thioredoxin reductase . Plant Mol Biol. , 235 : 1357 – 1363 .
- Jacquot , JP , Vidal , J , Gadal , P and Schürmann , P . 1978 . Evidence for the existence of several enzyme specific thioredoxins in plants . FEBS Lett. , 96 : 243 – 246 .
- Kobrehel , K , Wong , JH , Balogh , A , Kiss , F , Yee , BC and Buchanan , BB . 1992 . Specific reduction of wheat storage proteins by thioredoxin h . Plant Physiol. , 99 : 919 – 924 .
- Laemmli , UK . 1970 . Cleavage of structural proteins during the assembly of the head of bacteriophage T4 . Nature. , 227 : 680 – 685 .
- Laloi , C , Mestres-Ortega , D , Marco , Y , Meyer , Y and Reichheld , JP . 2004 . The Arabidopsis cytosolic thioredoxin h5 gene induction by oxidative stress and its W-box-mediated response to pathogen elicitor . Plant Physiol. , 134 : 1006 – 1016 .
- Laloi , C , Rayapuram , N , Chartier , Y , Grienenberger , JM , Bonnard , G and Meyer , Y . 2001 . Identification and characterization of a mitochondrial thioredoxin system in plants . Proc Natl Acad Sci USA. , 98 : 14144 – 14149 .
- Lemaire , SD , Guillon , B , Le Maréchal , P , Keryer , E , Miginiac- Maslow , M and Decottignies , P . 2004 . New thioredoxin targets in the unicellular photosynthetic eukaryote Chlamydomonas reinhardtii . Proc Natl Acad Sci USA. , 101 : 7475 – 7480 .
- Lemaire , SD , Keryer , E , Stein , M , Schepens , I , Issakidis-Bourguet , E , Gérard-Hirne , C , Miginiac-Maslow , M and Jacquot , JP . 1999 . Heavy-metal regulation of thioredoxin gene expression in Chlamydomonas reinhardtii . Plant Physiol. , 120 : 773 – 778 .
- Lozano , RM , Wong , JH , Yee , BC , Peters , A , Kobrehel , K and Buchanan , BB . 1996 . New evidence for a role of thioredoxin h in germination and seedling development . Planta. , 200 : 100 – 106 .
- Martí , MC , Olmos , E , Calvete , JJ , Díaz , I , Barranco-Medina , S , Whelan , J , Lázaro , JJ , Sevilla , F and Jiménez , A . 2009 . Mitochondrial and nuclear localization of a novel pea thioredoxin: identification of its mitochondrial target proteins . Plant Physiol. , 150 : 646 – 657 .
- Matsumura , H and Miyachi , S . 1980 . Cycling assay for nicotinamide adenine dinucleotides . Methods Enzymol. , 69 : 465 – 470 .
- Mattioni , C , Gabbrielli , R , Vangronsveld , J and Clijsters , H . 1997 . Nickel and cadmium toxicity and enzymatic activity in Ni-tolerant and non-tolerant populations of Silene italica Pers . J Plant Physiol. , 150 : 173 – 177 .
- Meyer , Y , Vignols , F and Reichheld , JP . 2002 . Classification of plant thioredoxins by sequence similarity and intron position . Methods Enzymol. , 347 : 394 – 402 .
- Mihoub , A , Chaoui , A and El Ferjani , E . 2005 . Biochemical changes associated with cadmium and copper stress in germinating pea seeds (Pisum sativum L.) . Comptes Rendus Biol. , 328 : 33 – 41 .
- Montrichard , F , Renard , M , Alkhalfioui , F , Duval , FD and Macherel , D . 2003 . Identification and differential expression of two thioredoxin h isoforms in germinating seeds from pea . Plant Physiol. , 132 : 1707 – 1715 .
- Pagano , EA , Chueca , A and López-Gorge , J . 2000 . Expression of thioredoxins f and m, and of their targets fructose-1,6-bisphosphatase and NADP-malate dehydrogenase, in pea plants grown under normal and light/temperature stress conditions . J Exp Bot. , 51 : 1299 – 1307 .
- Rahoui , S , Chaoui , A and El Ferjani , E . 2010 . Membrane damage and solute leakage from germinating pea seed under cadmium stress . J Hazard Mater. , 178 : 1128 – 1131 .
- Schürmann , P and Jacquot , JP . 2000 . Plant thioredoxin systems revisited . Annu Rev Plant Physiol Plant Mol Biol. , 51 : 371 – 400 .
- Smiri , M , Chaoui , A and El Ferjani , E . 2009 . Respiratory metabolism in the embryonic axis of germinating pea seed exposed to cadmium . J Plant Physiol. , 166 : 259 – 269 .
- Smiri , M , Chaoui , A and El Ferjani , E . 2010a . Interaction between heavy metals and thiol-linked redox reactions in germination . Pakistan J Biol Sci. , 13 : 877 – 883 .
- Smiri , M , Chaoui , A , Rouhier , N , Chibani , K , Gelhaye , E , Jacquot , JP and El Ferjani , E . 2010b . Cadmium induced mitochondrial redox changes in germinating pea seed . Biometals. , 23 : 973 – 984 .
- Smiri , M , Chaoui , A , Rouhier , N , Gelhaye , E , Jacquot , JP and El Ferjani , E . 2010c . Oxidative damage and redox change in pea seeds treated with cadmium . C R Biol. , 333 : 801 – 807 .
- Smiri , M , Chaoui , A , Rouhier , N , Gelhaye , E , Jacquot , JP and El Ferjani , E . 2010d . Cadmium affects the glutathione/glutaredoxin system in germinating pea seeds . Biol Trace Elem Res. , 142 : 93 – 105 .
- Smiri , M , Chaoui , A , Rouhier , N , Gelhaye , E , Jacquot , JP and El Ferjani , E . 2010e . Redox regulation of the glutathione reductase/iso-glutaredoxin system in germinating pea seed exposed to cadmium . Plant Sci. , 179 : 423 – 436 .
- Towbin , H , Staehelin , T and Gordon , J . 1979 . Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications . Proc Natl Acad Sci USA. , 76 : 4350 – 4354 .
- Traverso , JA , Vignols , F , Cazalis , R , Pulido , A , Sahrawy , M , Cejudo , FJ , Meyer , Y and Chueca , A . 2007 . PsTRXh1 and PsTRXh2 are both pea h-type thioredoxins with antagonistic behavior in redox imbalances . Plant Physiol. , 143 : 300 – 311 .
- Traverso , JA , Vignols , F , Cazalis , R , Serrato , AJ , Pulido , P , Sahrawy , M , Meyer , Y , Cejudo , FJ and Chueca , A . 2008 . Immunocytochemical localization of Pisum sativum TRXs f and m in non-photosynthetic tissues . J Exp Bot. , 59 : 1267 – 1277 .
- Vallee , BL and Ulmer , DD . 1972 . Biochemical effects of mercury, cadmium, and lead . Annu Rev Biochem. , 41 : 91 – 128 .
- Van Assche , F and Clijsters , H . 1990 . Effects of metals on enzyme activity in plants . Plant Cell Environ. , 13 : 195 – 206 .
- Vido , K , Spector , D , Lagniel , G , Lopez , S , Toledano , MB and Labarre , J . 2001 . A proteome analysis of the cadmium response in Saccharomyces cerevisiae . J Biol Chem. , 276 : 8469 – 8474 .
- Welbaum , GE , Bradford , KJ , Kyu-Ock , Y and Oluoch , MO . 1998 . Biophysical, physiological and biochemical processes regulating seed germination . Seed Sci Res. , 8 : 161 – 172 .
- Zhao , Z , Hu , X and Ross , CW . 1987 . Comparison of tissue preparation methods for assay of nicotinamide coenzymes . Plant Physiol. , 84 : 987 – 988 .