Abstract
Growing in their natural environment, plants often encounter unfavorable environmental conditions that interrupt normal plant growth and productivity. Drought, high/low temperature and saline soils are the most common abiotic stresses that plants encounter in their natural environments. Molecular and genomic analyses have facilitated gene discovery and enabled genetic engineering using several functional or regulatory genes that are known to be involved in stress response and preliminary tolerance, to activate specific or broad pathways related to abiotic stress tolerance in plants. Through the use of transgenic technology, goals such as production of plants with desired traits that were unattainable with traditional selection programs are achieved. This review deals with recent advancement in understanding the role of various stress responsive genes and their critical importance for explaining the control mechanism of abiotic stress tolerance and engineering stress tolerant crops based on the expression of specific stress related genes.
Introduction
Plant productivity is severely affected by abiotic stress factors which include salinity, drought, high and low temperature. As a consequence to it, physiological and biochemical responses in plants vary and cellular aqueous and ionic equilibriums are disturbed. It is estimated that increased salinization of aerable land will have devastating global effects, resulting in 30% land loss within next 25 years, and up to 50% by the year 2050 (Wang etal. Citation2003). Plants have developed several strategies to overcome these challenges either through adoption mechanisms which allow them to survive the adverse conditions or specific growth habits to avoid stress conditions. Also, numbers of genes and their products that respond to abiotic stresses at transcriptional and translational level (Bartels and Sunkar Citation2005). Plants can sense abiotic stress and elicit appropriate response with altered metabolism, growth, and development (Cramer etal. Citation2011; Krasensky and Jonak Citation2012). At the molecular level, abiotic stress tolerance can be achieved through gene transfer in plants such as, by altering the accumulation of osmoprotectants, increase production of chaperones, enhance the superoxide radical scavenging mechanisms, exclusion, or compartmentation of ions by efficient transporter and symporter systems.
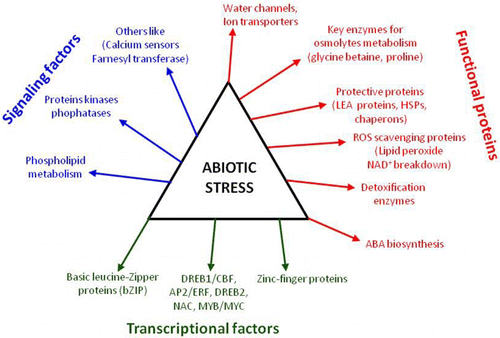
Genetic engineering has given a strong landmark over plant breeding. The recombinant transgenic technology, appreciatively, allows us to mobilize genes from virtually any organism. Different genes including those encoding enzymes involved in osmolyte biosynthesis such as proline, mannitol, glycine betaine, trehalose, etc.; genes encoding reactive oxygen species (ROS) detoxifying enzymes such as superoxide dismutase (SOD), ascorbate peroxidase (APX), and catalase; genes encoding stress-induced proteins such as late embryogenesis abundant (LEA) proteins, heat shock proteins (HSPs), etc. along with those encoding trans-acting factors such as DREB1/CBF, AP2/ERF, DREB2, NAC, MYB/MYC, basic leucine-Zipper proteins, and Zinc-finger, have been effectively utilized for developing transgenic plants tolerant against multiple stresses (Tayal etal. Citation2004) (). This review deals with the strategies that are employed for abiotic stress tolerance at molecular level, with main emphasis on genetic engineering approaches that are used to create stress resistant transgenic plants.
Figure 1. Functions of drought stress-inducible genes in stress tolerance and their response. Gene products are classified into three groups. The first group includes proteins that probably function in stress tolerance (functional proteins), the second group contains protein factors involved in further regulation of signal transduction and gene expression that probably function in stress response (regulatory proteins), and the third group encloses proteins involves in transcription, gene regulation and expression most likely function in stress tolerance (transcriptional factors).
Transgenic approaches for improved tolerance to abiotic stress
Food insecurity that has increased in recent times owing to competing claims for land, water, labor, energy, and capital, has lead more pressure to improve production per unit of land (Godfray etal. Citation2010; Varshney etal. Citation2011). Under such circumstances, increasing the yield of crop plants in normal soils and in less productive lands including salinized soils appears as absolute requirement for feeding the world. Knowing the fact that developing crops better adapted to abiotic stresses is important for crop production, transgenic technology employing genetic engineering have opened up new opportunities to improve tolerance to abiotic stresses by incorporating genes involved in stress protection from any source into agriculturally important plants (Bajaj and Mohanty Citation2005). The transgenic approach allows scientists to study mechanisms governing stress tolerance by either over expression or antisense suppression of the transgene into the model plant species and to monitor the phenotypical and biochemical changes before and after a specific abiotic stress treatment (Bartels and Phillips Citation2010; Mantri etal. Citation2012). It has met with preliminary success, and several varieties of stress tolerant lines have been produced in recent years, few of which have been utilized for further testing under field conditions (Purty etal. Citation2008; Atkinson and Urwin Citation2012). This approach seems a viable option for developing genotypes that can perform better under harsh environmental conditions particularly for complex nature of abiotic stress tolerance and reproductive barriers that limit transfer of favorable alleles from diverse genetic resources.
Genes involved in abiotic stress tolerance
To meet the growing demand for food and to contrast the detrimental effect of climatic change on crop yield, it is imperative to develop new crops with improved tolerance to abiotic stresses. Many genes and gene products have been identified which get induced in plants upon exposure to various abiotic stresses, i.e. drought, salinity, high/low temperature stress, heavy metal, anoxia, and acidity of soil etc. (). Following six categories of stress-induced genes/proteins with known function have been exploited for generating stress tolerant transgenic plant.
Table 1. Over expression of stress responsive genes in transgenic plants.
Genes involved in osmolyte biosynthesis
Natural selection is believed to be an unforgiving and relentless force in the evolution of life on earth. An organism that cannot adapt to a changing environment or an environment hostile to cell functions is at risk as a species (Singh etal. Citation2009, Citation2011). Thus, in response to harsh environmental stresses, plants accumulate low molecular weight organic compounds called osmolytes to protect their cells and macromolecular assemblies. The basic premise is that, the natural selection of protecting osmolytes is based upon selection at a particular molecular level which confers generic stabilization to all proteins without altering their functional activity (Yancey Citation2003). Compatible osmolytes are those that stabilize proteins and membranes against the denaturing effect of high concentrations of salts and other harmful solutes and can accumulate to high levels without substantively disturbing intracellular biochemistry (Yancey Citation2001). Representatives of this class include certain amino acids (e.g. proline and Ectoine), polyols (e.g. trehalose and mannitol), and other amines (e.g. Glycinebetaine) and the stresses that compatible osmolytes protect against include dehydration, high-salt environments, and extremes of temperature (Yancey etal. Citation1982). An adaptive biochemical function of osmoprotectant is scavenging of ROS that are by-products of hyperosmotic and ionic stresses, believed to cause membrane dysfunction and cell death (Bohnert etal. Citation1995, Chen and Murata Citation2002). They are synthesized in response to osmotic stress in organisms as diverse as bacteria to plants and animals (Burg and Peters Citation1998).
Osmoregulation is one of the most effective ways evolved by stress-tolerant plants to combat abiotic stress, but most crop plants lack the ability to synthesize the osmoprotectants naturally produced by stress-tolerant plants. Therefore, genes concerned with the synthesis of osmoprotectants have been transformed into transgenic plants to confer stress-tolerance. Engineering osmolyte biosynthesis is emerging as a viable approach in producing transgenics for enhanced tolerance to osmotic stresses in plants. The engineering of plants with higher concentrations of proline began with the overexpression of genes encoding the biosynthetic enzymes Δ'-Pyrroline-5-carboxylate synthase (P5CS) and Δ'-Pyrroline-5-carboxylate reductase (P5CR) catalyzing the two steps between the substrate glutamic acid and the product proline. P5CS overexpression in tobacco considerably elevated free proline in transgenic tobacco plants (Kishor etal. Citation1995). A two fold increase in free proline content achieved in tobacco plants transformed with P5CS modified by site directed mutagenesis, resulted in an improved germination and growth of seedlings under salt stress (Hong etal. Citation2000).
Ectoine (1,4,5,6-tetrahydro-2-methyl–4–pyrimidine carboxylic acid) that was first discovered in Ectothiorhodospira halochloris, is one of the most commonly found osmolytes in nature (Galinski etal. Citation1985; Lentzen and Schwarz Citation2006). Ectoine is a common compatible solute found in halophilic bacteria (e.g. Halomonas elongata). Its biosynthesis occurs from L-aspartate β-semialdehyde in three steps in presence of L-2,4-diaminobutyric acid aminotransferase, L-2,4-diaminobutyric acid acetyl transferase, and L-ectoine synthase encoded by ectB, ectA, and ectC genes (Ono etal. Citation1999). Transformed cultured tobacco cells lines with three H. elongata genes (ectA, ectB, and ectC) encoding the enzymes of ectoine synthesis under the control of the constitutive CaMV 35S promoter in a single construct, showed a small increase in resistance to osmotic stress imposed with mannitol (Nakayama etal. Citation2000). Moghaieb etal. (Citation2011) found that transgenic tomato plants expressing genes for ectoine biosynthesis shows much improved growth than that was observed in case of transgenic tobacco plants reported by Nakayama etal. (Citation2000) under salt stress condition. Besides their osmotic effect, ectoines as well as other compatible solutes have been found to improve protein folding and protect biomolecules such as enzymes, nucleic acids against heating, drying, or any chemical treatment (Knapp etal. Citation1999).
Trehalose – a nonreducing disaccharide of glucose that acts as an osmolyte compatible solute protecting membrane and proteins, is involved in conferring desiccation tolerance to cells in absence of water (Crowe etal. Citation1984). It is very rare compound for plant kingdom, but in last few years transformation for accumulation of trehalose is one of the active areas of scientific research. Engineering of trehalose metabolism by transferring trehalose-6-phosphate synthase (TPS1) gene in tobacco plants showed improved drought tolerance (Pilon-Smits etal. Citation1998). Garg etal. (Citation2002) showed rice tolerance to multiple abiotic stresses by engineering Escherichia coli trehalose biosynthesis genes (otsA and otsB) without the negative pleiotropic effects. Over-expression of trehalose synthase (TSase) gene of the edible wood fungi Grifola frondosa in tobacco was reported and the transformants were able to accumulate higher levels of products of trehalose compared to many other known transgenic plants (400-fold higher than tobacco co-transformed with E. coli TPS and TPP, twofold higher than rice transformed with a bi functional fusion gene (TPSP) of the trehalose-6-phosphate (T-6-P) synthase (TPS) and T-6-P phosphatase (TPP) of E. coli, and 12-fold higher than tobacco transformed with yeast TPS1 gene) (Zhang etal. Citation2005).
Tarczynski etal. (Citation1993) on introducing a bacterial gene (mt1D) encoding mannitol-1-phosphate dehydrogenase into tobacco plants, found enhanced mannitol accumulation in cytoplasm and as such increased tolerance to salinity. Similarly, ectopic expression of mannitol in wheat improves tolerance to water stress and salinity both at the callus and whole plant level (Abebe etal. Citation2003). In addition, accumulation of cyclic polyols such as myo-inositol and its methylated derivatives ononitol and pinitol is correlated with salinity and drought tolerance in plants. Sheveleva etal. (Citation1997) found that transgenic tobacco plants carrying imt1 gene encoding myo-inositol O-methyltransferase results in higher accumulation of D-ononitol, that in turn result in enhanced photosynthesis protection and increased recovery under drought and salt stress.
Glycine betaine (N, N, N-trimethyl glycine) – a quaternary ammonium compound, is one of the most efficient compatible solutes found in wide range of plants, animals, and bacteria (Rhodes and Hanson Citation1993; Chen and Murata Citation2002). Due to immense importance of glycine betaine as an efficient compatible solute, research has mainly focused on the cloning and isolation of individual genes of the glycine betaine biosynthetic pathway in natural accumulators. In plants, its accumulation has been widely recognized as abiotic stress response where it acts as an osmoprotectant by stabilizing both the quaternary structure of proteins and the highly ordered structure of membranes. In glycine betaine biosynthetic pathway occurring in plants, choline is converted to glycine betaine through a two-step pathway catalyzed by choline monooxygenase (CMO) and betaine-aldehyde dehydrogenase (BADH) (Rathinasabapathi etal. Citation1997), while as in E. coli and animals, the two-step pathway is catalyzed by choline dehydrogenase (CDH) and BADH, respectively (Takabe etal. Citation1998). In contrast, glycine betaine biosynthesis in some microorganisms such as Arthrobacter globiformis and Arthrobacter panescens is accomplished by a single enzyme, choline oxidase (COD), respectively, (Ikuta etal. Citation1977).
A new biosynthetic pathway of glycine betaine that begins with glycine as the precursor molecule has been discovered in two extremely halophytic microorganisms, Actinopolyspora halophila and E. halochloris (Nyyssola etal. Citation2000) and later in halotolerant cynobacterium, Aphanothece halophytica (Waditee etal. Citation2005). In these microorganisms, the glycine betaine biosynthetic pathway is accomplished by two enzymes: glycine sarcosine methyltransferase (ApGSMT) catalyzing the methylation steps from glycine to sarcosine (N-monomethylglycine) and sarcosine to dimethylglycine and sarcosine dimethylglycine methyltransferase (ApDMT) that catalyzes the steps from sarcosine to dimethylglycine and dimethylglycine to betaine.
Genes encoding reactive oxygen species scavenger proteins or antioxidants
Oxidative stress, a critical pathophysiological condition is characterized by increased production of ROS. ROS are distinguished by their high chemical reactivity and include both free radicals, such as superoxide , hydroxyl (OH•), peroxyl
, and alkoxyl (RO•) and certain nonradicals such as peroxynitrite (ONOO−), H2O2, etc. that are either oxidizing agents or get easily converted into radicals (Jan etal. Citation2011). Chemically unstable compounds such as superoxide
carry free electrons that react with macromolecules thereby results in their destabilization and by inducing a chain reaction damage DNA, essential cellular proteins and membrane lipids (lipid peroxidation) (Menvielle-Bourg Citation2005). Plants have developed several antioxidant systems comprising of enzymes such as catalase, SOD, APX, glutathione reductase (GR), and glutathione synthase to scavenge these toxic compounds. SOD is one of the most effective intracellular enzymatic antioxidants that catalyzes the dismutation of superoxide to oxygen and hydrogen peroxide, which in turn is catabolized by catalase (located in peroxisome) and peroxidase into oxygen and water.
For detailed analysis of contribution of these antioxidant enzymes to stress tolerance, a large number of experiments have been conducted with transgenic model plants overproducing these antioxidant enzymes (Wang etal. Citation2003). By constitutively expressing Mn-SOD into chloroplast and mitochondria, Bowler etal. (Citation1991) observed reduced cellular damage in response to abiotic stress. It was followed by the study conducted by Sengupta etal. (Citation1993) on transgenic tobacco expressing Cu/Zn-SOD. They found a dramatic improvement in the photosynthetic performance in transgenic tobacco by over-expression of Cu/Zn-SOD under chilling stress. Roxas etal. (Citation1997) observed additional peroxidise activity by over expressing Nt107 cDNA encoding glutathione-S-transferase in transgenic tobacco plants. Overproductions of APX3 and catalase genes have been shown to improve oxidative, cold and salt stress tolerance in transgenic tobacco plants (Wang etal. Citation1999). Similarly, transgenic rice over-expressing yeast mitochondrial Mn-SOD displayed enhanced salt tolerance (Tanaka etal. Citation1999). Arabidopsis plants over-expressing OsAPXa and OsAPXb exhibited increased tolerance to salt as compared to wild type plants (Lu, Liu, etal. Citation2007). The APX gene family has been identified and characterized by Najami etal. (Citation2008) in tomato.
Genes encoding protective proteins for cellular machinery
For survival in environmental stress, plants trigger a large set of genes that leads to the accumulation of specific stress-associated proteins (Bohnert and Sheveleva Citation1998). Among the vast majority of stress-associated proteins, HSPs, and LEA type proteins are two major types of stress-induced proteins that accumulate upon water, salinity, and extreme temperature stress. They have been shown to act as molecular chaperones, which are responsible for protein synthesis, targeting, maturation, and degradation in a broad array of normal cellular processes. Besides that molecular chaperones assist in the stabilization of proteins and membranes and in protein refolding under stress conditions (Boston etal. Citation1996).
Various studies have shown that plant HSPs involved in developmental processes such as embryo development, seed germination, somatic embryogenesis, pollen development, and fruit maturation, are not only expressed in response to heat shock but also underwater, salt, and high/low temperature along with oxidative stress. HSPs are mostly encoded by nuclear genes, but these proteins are localized in different cell compartments including cytoplasm, mitochondria, chloroplast, and endoplasmic reticulum. Genetic engineering for increased thermo-tolerance by enhancing HSP synthesis in plants has been achieved in a number of plant species. One of the low weight HSPs (HSP 17.7) has been found to be associated in imparting tolerance to thermal stress in carrot cells. Expression of HSP DnaK1 from a halotolerant cynobacterium A. halophytica has resulted in improving salt tolerance of transgenic tobacco plants (Sugino etal. Citation1999). Additionally, At-HSP17.6A expression was induced by heat and osmotic stress as well as during seed development (Sun etal. Citation2001). Hamilton and Heckathorn (Citation2001) suggested that HSPs might act as antioxidants in protecting Complex-I electron transport in mitochondria during NaCl stress.
Late embryogenesis abundant type proteins are high molecular weight proteins that are abundant during late embryogenesis and accumulate in response to water-deficit resulting from desiccation, cold, and osmotic stress in plant species. They are highly hydrophilic in nature and normally expressed in vegetative tissues or induced by osmotic stress or exogenous application of Abscisic acid (ABA). LEA-type proteins have been divided into a number of families, with diverse structures and functions (Thomashow Citation1998). Among them, proteins belonging to group 3 are predicted to play a role in sequestering ions that are concentrated during cellular dehydration while as those belonging to group 1 enhance water binding capacity. Besides that, proteins belonging to group 5 are thought to sequester ions during water loss (Bhatnagar-Mathur etal. Citation2008). The enhanced expression of transcription factors that regulates the expression of LEA type proteins was found to correlate with cold, drought, or salt stress tolerance in transgenic plants (Kasuga etal. Citation1999; Jaglo etal. Citation2001). Xu etal. (Citation1996) reported that the expression of a group 3 protein HVA1 in barley confers tolerance to water deficiency and salt stress in transgenic rice plants. Moreover, constitutive expression of the same protein in transgenic wheat plants improved biomass productivity and water-use efficiency underwater-deficit conditions (Sivamani etal. Citation2000). Constitutive expression of a wheat chloroplast LEA-like protein (WCS19) in Arabidopsis resulted in a significant increase in freezing tolerance (Ndong etal. Citation2002). Over expression of group 4 LEA protein has been shown in imparting tolerance against low temperature and salinity stresses (Imai etal. Citation1996). Transgenic rice expressing wheat LEA gene PMA80 (group 2 protein) and PMA1959 (group 1 protein) showed enhanced drought and salt tolerance in green house conditions (Cheng etal. Citation2002). The over-expression of a heterologous LEA gene Rab16A from rice enhanced salinity stress tolerance in transgenic tobacco plants (RoyChoudhury etal. Citation2007). Recently, Dalal etal. (Citation2009) reported that the constitutively as well as stress inducible expression of BnLEA4-1 gene from Brassica napus in transgenic Arabidopsis plants conferred tolerance to salt and drought stress.
Genes encoding membrane proteins or ion transporters
An important strategy for achieving tolerance to abiotic stress is to help plants to maintain and re-establish cellular ion homeostasis during stress conditions. The membrane proteins that are involved in osmotic stress tolerance include water channel proteins and transport proteins. Water channel proteins control cellular water transport in response to drought and salt stress, while as ion transporters play an important role in salt tolerance. According to Bartels and Sunkar (Citation2005), three mechanisms exist to prevent excess Na+ accumulation in the symplast of plant cells:
| • | Restricting the Na+ permeation and entry into plant cytosol by Na+ transporters. | ||||
| • | Compartmentalization of Na+ in to vacuole via, Na+/H+ antiporter. | ||||
| • | Transport of cytosolic Na+ back to the external medium or to the apoplast by plasma membrane Na+/H+ antiporter. | ||||
Ion transporters selectively transport ions in order to maintain physiologically relevant concentrations whereas Na+/H+ antiporter play a vital role in sustaining cellular ion homeostasis, thus allow plant survival and growth under saline conditions through regulation of cytoplasmic pH, sodium levels, and cell turgor (Serrano etal. Citation1999). The overexpression of the AtNHX1 gene encoding vacuolar Na+/H+ antiporter, exhibit promoted growth and development of transgenic Arabidopsis plant irrigated with 200 mM NaCl (Apse etal. Citation1999). Transgenic B. napus plants overexpressing AtNHX1 were able to grow, flower, and produce seeds even in presence of 200 mM NaCl. Moreover, their seed yields and seed oil quality were not altered by the high soil salinity (Zhang etal. Citation2001). Similarly, transgenic tomato plants overexpressing AtNHX1 gene were able to grow, flower, and produce fruit in the presence of 200 mM sodium chloride (Zhang and Blumwald Citation2001). Even though leaves accumulated high sodium concentrations, fruits displayed very low sodium content, demonstrating the potential to maintain fruit yield and quality at high salt levels. Shi etal. (Citation2003) suggested that plasma membrane Na+/H+ antiporter encoded by the SOS1 gene improves salt tolerance in transgenic Arabidopsis and increased salt tolerance was correlated with reduced Na+ accumulation. It seems that the salt overly sensitive (SOS) pathway also regulates the vacuolar Na+/H+ exchange activity and contributes to Na+ compartmentalization (Qiu etal. Citation2004). SOS pathway coordinately regulates plasma membrane and tonoplast Na+/H+ antiporter activity which leads to Na+ homeostasis and as a consequence salt tolerance.
The transport of K+ and Na+ are regulated by Saccharomyces cerevisiae cation transport systems, such as HAL1 and HAL3, respectively. The transgenic tomato lines overexpressing the HAL1 gene were showed more salt-tolerant than the wild type plants in both callus and plant growth besides exhibiting better fruit yield under salt stress (Gisbert etal. Citation2000; Rus etal. Citation2001). In plants, H+ are used as coupling ions for ion transport systems and the proton gradient generated by proton pumps found in the cell membrane acts as the driving force for nutrient uptake (Serrano etal. Citation1999). Overexpression of AVP1 gene encodes vacuolar H+-ATPase pump associated with pumping of H+ across the vacuolar membrane that acts as an additional driving force for vacuolar sodium accumulation, ultimately lead to enhanced salt and drought tolerance in transgenic plants (Gaxiola etal. Citation2001).
Genes encoding transcription factors
To make the plants more tolerant to stress, transferring a single gene encoding a single specific stress protein may not be sufficient to reach the required tolerance levels (Bohnert etal. Citation1995). To overcome such limitations, enhancing tolerance toward multiple stresses by gene-regulating protein factors that regulate gene expression, signal transduction and function of a number of genes under stress responses appears as a capable approach for improving the biotic stress tolerance in plants (Chinnusamy etal. Citation2006). These genes comprise regulatory proteins, i.e. transcription factors such as DREB, MYC, MYB, bZIP, etc., protein kinases (MAP kinase, CDP kinase, receptor protein kinase, ribosomal protein kinase, and transcription regulation protein kinase) and proteinases (phosphoesterases and phospholipase). A few important families of transcription factors are as follows.
Dehydration responsive element (DRE) or C-repeat binding factor (DREB/CBF)
Dehydration responsive element (DRE) or C-repeat (CRT), a cis-acting element, plays an important role in regulating gene expression in response to stress in an ABA independent manner (Yamaguchi-Shinozaki and Shinozaki Citation2005). DREB is a well characterized transcription factor known to play an important role in regulating gene expression in response to abiotic stresses. There are three DREB1/CBF proteins that are encoded by genes present on chromosome 4 in the order DREB1B/CBF1, DREB1A/CBF3, and DREB1C/CBF2 and two DREB2 proteins (DREB2A and DREB2B) in Arabidopsis thaliana (Liu etal. Citation1998; Gilmour etal. Citation2000; Hussain etal. Citation2011). DREB1A/CBF3, DREB1B/CBF1, and DREB1C/CBF2 mainly activate downstream genes implicated in cold response whereas, DREB2A and DREB2B regulates gene in response to drought, salt, and high temperature stresses (Yamaguchi-Shinozaki and Shinozaki Citation2005; Sakuma etal. Citation2006). The CBFs/DREB1s belong to AP2/EREBP family of transcription factors that bind to cold- and dehydration-responsive DNA regulatory element designated CRT/ DRE having a conserved CCGAC core sequence. DREB-type transcription factor genes have been reported in various plants, such as AtDREB (1A, 1B, 1C, 2A, 2B) from Arabidopsis (Liu etal. Citation1998), OsDREB (1A, 1B, 1C, 1D, 2A) from rice (Dubouzet etal. Citation2003), AhDREB1 from Atriplex hortensis (Shen, Zhang, etal. Citation2003), TaDREB1 from wheat (Shen, He, etal. Citation2003), HvDREB1 from barley (Choi etal. Citation2002), HsDREB1A from wild barley (James etal. Citation2008), ScDREB1 from rye, BnDREB1 from B. napus (Jaglo etal. Citation2001), AsDREB1 from oat (Brautigam etal. Citation2005), ZmDREB1 from maize (Qin etal. Citation2007), LpDREB1 from perennial ryegrass (Xiong and Fei Citation2006), GmDREB (a, b, c, 2A, 3) from soybean (Chen etal. Citation2007, Citation2009), and also from other plant species such as Populus sp. (Benedict etal. Citation2006), eucalyptus (El-Kayal etal. Citation2006), grape (Xiao etal. Citation2006), citrus (Champ etal. Citation2007), and birch (Welling and Palva Citation2008).
Transcription factors (TFs) activate cascade of genes that act together in enhancing tolerance toward multiple stress. Transgenic plants overexpressing DREB1/CBF3 under the control of CaMV 35S promoter also showed increased tolerance to drought, high salinity, and freezing stress (Kasuga etal. Citation1999; Gilmour etal. Citation2000; Cominelli and Tonelli Citation2010). Oh etal. (Citation2005) ectopically expressed Arabidopsis DREB1A/CBF3 in transgenic rice plants under the control of CaMV 35S promoter. Transgenic plants overexpressing DREB1/CBF3 under the control of CaMV 35S promoter showed enhanced tolerance to drought and salinity but only to a little extent to low-temperature stress without any stunted phenotype despite its constitutive expression. TINY an Arabidopsis transcription factor belonging to a subfamily closely related to DREB may function in both DRE and element binding factors (ERF) dependent signaling pathways (Sun etal. Citation2008). Transgenic Arabidopsis overexpressing TINY, strongly expressed during drought and cold, exhibited increased expression of both DRE and ERF regulatory genes.
bZIP transcription factor
bZIPs belong to a large family of transcription factor genes and possess a basic domain adjacent to leucine-zipper motif. A number of bZIP proteins are found to be involved in stress signaling (Jakoby etal. Citation2002). The first genetic evidence of importance of bZIP proteins in stress tolerance was given by Kang etal. (Citation2002) who overexpressed ABA-responsive element (ABRE) binding factor/ABA responsive element binding protein of bZIP family in transgenic A. thaliana. Xiang etal. (Citation2008) on overexpression of OsbZIP23 in transgenic rice observed that OsbZIP23 regulate expression of a wide spectrum of stress related genes (up- or down-regulation) in response to abiotic stresses (drought, salinity) through an ABA dependent pathway.
MYB and MYC transcription factors
Myb like proteins contain helix turn helix related motif and Myc like proteins have basic helix loop helix domain for DNA binding. Expression of ATMYC and/or ATMYB transcription factors involved in the ABA mediated drought stress signaling pathway and upregulate the expression of JA-mediated wounding responsive genes. ATMYC2 and/or ATMYB2 cDNAs have higher sensitive to ABA and reduced electrolyte leakage than non transformed plants (Abe etal. Citation2003). Overexpression of CpMYB10 isolate from Craterostigma plantagineum in Arabidopsis plant led to drought and salt tolerance in transgenic plants (Villalobos etal. Citation2004). Stress inducible overexpression of Arabidopsis MYB2 gene in rice conferred salt stress tolerance (Malik and Wu Citation2005). Ding etal. (Citation2009) observed that transgenic Arabidopsis overexpressing MYB15, a member of transcription factor R2R3 MYB family exhibited higher tolerance to drought and salinity stress at different developmental stages.
NAC proteins
Plant specific NAC family transcription factors contain N-terminal DNA binding domains that potentially regulates both biotic and abiotic signaling. The constitutive expression of OsNAC6 in transgenic rice enhanced tolerance to drought and high salinity stresses (Nakashima etal. Citation2007). Overexpression of ATAF2 and ATAF1 responds to both biotic and abiotic stress (Delessert etal. Citation2005; Lu, Chen, etal. Citation2007). These results suggested that NAC functions as a transcription factors in both abiotic and biotic signaling pathways.
Ethylene responsive element binding factors (ERF)
All ERFs are suggested to have a conserved 58–59 amino acid domain that can bind to C-repeat/dehydration responsive motifs (CRT/DRE). CRT or DRE motifs are involved in regulation of ABA independent gene expression under drought, salinity and cold stress. Over expression of tobacco stress induced gene (Tsi) in transgenic tobacco confirmed the role of ERF gene in conferring tolerance to osmotic stress and pathogen (Park etal. Citation2001). Over expression of ERFBP1 gene in transgenic potato further showed increased tolerance to high degree of cold and salt stress (Lee etal. Citation2004). The role of ERF transcription factor in drought tolerance was confirmed by Karaba etal. (Citation2007) through over expression of HRD gene in transgenic rice. Similarly, over expression of tomato TERF1, hot pepper JERF3, wheat ERF1, and barley ERF in transgenic plants activate the expression of GCC box containing PR genes and exhibited higher salt tolerance (Huang etal. Citation2004; Lee etal. Citation2004; Wang etal. Citation2004; Jung etal. Citation2007). These results suggested that ERFs modulate osmotic tolerance through interaction with GCC box and/ or DRE and could be potential candidate genes for engineering biotic and abiotic stress tolerance in plants.
Conclusion
World's population is increasing at an alarming rate. Around 70% of the total population lives in the rural areas of developing countries, where poverty, food insecurity and nutritional deficiency are major problems encountered in day-to-day life. Low productivity in agriculture is considered to be a major cause of poverty, food insecurity, and poor nutrition, especially in countries where agriculture is the driving force for broad economic growth and poverty alleviation. The ability of plants to tolerate stress conditions is crucial for agricultural production worldwide as they negatively influences the survival, biomass production, accumulation, and grain yield of most crops. As it is proposed that current perceptive of plant stress tolerance can be significantly polished by thorough characterization of individual genes and evaluating their contribution to stress tolerance, knowledge of basic biochemical pathways, and identification of key regulatory genes of stress response pathways appears crucial for tackling these problems. Through the use of transgenic technology, one can produce plants with desired traits such as tolerance to various abiotic stresses that includes water stress (flood and drought), temperature stress (high and low), and salt stress more precisely. Development of plant transformation technology is impacting crop improvement options in unprecedented ways.
Acknowledgements
The authors are thankful to University Grant Commission (UGC), New Delhi, India for providing funds (39–109/2010SR) to carry out this work. Authors would also like to acknowledge Council of Scientific and Industrial Research (CSIR), India, for financial assistance in terms of SRF to one of the fellow (Arif Tasleem Jan).
Additional information
Notes on contributors
Parul Singhal
Arif Tasleem Jan and Parul Singhal contributed equally to this manuscriptReferences
- Abe , H , Urao , T , Ito , T , Seki , M , Shinozaki , K and Yamaguchi-Shinozaki , K . 2003 . Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling . Plant Cell , 15 : 63 – 78 .
- Abebe , T , Guenzi , AC , Martin , B and Cushman , JC . 2003 . Tolerance of mannitol-accumulating transgenic wheat to water stress and salinity . Plant Physiol , 131 : 1748 – 1755 .
- Apse , MP , Aharon , GS , Snedden , WA and Blumwald , E . 1999 . Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis . Science , 285 : 1256 – 1258 .
- Atkinson NJ , Urwin PE . 2012 . The intereaction of plant biotic and abiotic stresses: from genes to field . J Exp Bot . doi: 10.1093/jxb/ers100 .
- Bajaj , S and Mohanty , A . 2005 . Recent advances in rice biotechnology-towards genetically superior transgenic rice . Plant Biotech J , 3 : 275 – 307 .
- Bartels , D and Phillips , J . 2010 . Genetic modification of plants. Biotechnol Agric . Forestry , 64 ( 2 ) : 139 – 157 .
- Bartels , D and Sunkar , R . 2005 . Drought and salt tolerance in plants . Crit Rev Plant Sci , 24 : 23 – 58 .
- Benedict , C , Skinner , JS , Meng , R , Chang , Y , Bhalerao , R , Huner , N , Finn , CE , Chen , T and Hurry , V . 2006 . The CBF1-dependent low temperature signalling pathway, regulon and increase in freeze tolerance are conserved in Populus spp . Plant Cell Environ , 29 : 1259 – 1272 .
- Bhatnagar-Mathur , P , Devi , MJ , Reddy , DS , Lavanya , M , Vadez , V , Serraj , R , Yamaguchi-Shinozaki , K and Sharma , KK . 2008 . Stress-inducible expression of At DREB1A in transgenic peanut (Arachis hypogaea L.) increases transpiration efficiency under water-limiting conditions . Plant Cell Rep , 26 : 2071 – 2082 .
- Bohnert , HJ , Nelson , DE and Jensen , RG . 1995 . Adaptations to environmental stresses . Plant Cell , 7 : 1099 – 1111 .
- Bohnert , HJ and Sheveleva , E . 1998 . Plant stress adaptations-making metabolism move . Curr Opin Plant Biol , 1 : 267 – 274 .
- Boston , RS , Viitanen , PV and Vierling , E . 1996 . Molecular chaperones and protein folding in plants . Plant Mol Biol , 32 : 191 – 222 .
- Bowler , C , Slooten , L , Vandenbranden , S , DeRycke , R , Botterman , J , Sybesma , C , VanMontagu , M and Inze , D . 1991 . Manganese superoxide dismutase can reduce cellular damage mediated by oxygen radicals in transgenic plants . EMBO J , 10 : 1723 – 1732 .
- Brautigam , M , Lindlo , A , Zakhrabekova , S , Gharti-Chhetri , G , Olsson , B and Olsson , O . 2005 . Generation and analysis of 9792 EST sequences from cold acclimated oat, Avena sativa . BMC Plant Biol , 5 : 18 – 25 .
- Burg , MB and Peters , EM . 1998 . Effects of glycine betaine and glycerophosphocholine on thermal stability of ribonuclease . Am J Physiol , 274 : F762 – F765 .
- Champ , KI , Febres , VJ and Moore , GA . 2007 . The role of CBF transcriptional activators in two Citrus species (poncirus and citrus) with contrasting levels of freezing tolerance . Physiol Plant , 129 : 529 – 541 .
- Chen , JR , Lu , JJ , Liu , R , Xiong , XY , Wang , TX , Chen , SY , Guo , LB and Wang , HF . 2010 . DREB1C from Medicago truncatula enhances freezing tolerance in transgenic M. truncatula and China Rose (Rosa chinensis Jacq . Plant Growth Reg , 60 : 199 – 211 .
- Chen , M , Wang , Q , Cheng , XG , Xu , ZS , Li , LC , Ye , XG , Xia , L and Ma , YZ . 2007 . GmDREB2, a soybean DRE-binding transcription factor, conferred drought and high-salt tolerance in transgenic plants . Biochem Biophys Res Commun , 353 : 299 – 305 .
- Chen , M , Xu , Z , Xia , L , Li , LC , Cheng , X , Dong , J , Wang , Q and Ma , YZ . 2009 . Cold-induced modulation and functional analyses of the DRE-binding transcription factor gene, GmDREB3, in soybean (Glycine max L.) . J Exp Bot , 60 : 121 – 135 .
- Chen , TH and Murata , N . 2002 . Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes . Curr Opin Plant Biol , 5 : 250 – 257 .
- Cheng , Z , Targolli , J , Huang , X and Wu , R . 2002 . Wheat LEA genes, PMA80 and PMA1959, enhance dehydration tolerance of transgenic rice Oryza sativa . Mol Breeding , 10 : 71 – 82 .
- Chinnusamy , V , Zhu , J and Zhu , JK . 2006 . Salt stress signaling and mechanisms of plant salt tolerance . Genet Eng , 27 : 141 – 177 .
- Choi , DW , Rodriguez , EM and Close , TJ . 2002 . Barley CBF3 gene identification, expression pattern and map . Plant Physiol , 129 : 1781 – 1787 .
- Cominelli , E and Tonelli , C . 2010 . Transgenic crops coping with water scarcity . New Biotech , 27 : 473 – 477 .
- Cramer , GR , Urano , K , Delrot , S , Pezzotti , M and Shinozaki , K . 2011 . Effect of abiotic stress on plants: a systems biology perspective . BMC Plant Biol , 11 : 163
- Crowe , JH , Crowe , LM and Chapman , D . 1984 . Preservation of membranes in anhydrobiotic organisms: the role of trehalose . Science , 223 : 701 – 703 .
- Dalal , M , Tayal , D , Chinnusamy , V and Bansal , KC . 2009 . Abiotic stress and ABA-inducible Group 4 LEA from Brassica napus plays a key role in salt and drought tolerance . J Biotechnol , 139 : 137 – 145 .
- Delessert , C , Kazan , K , Wilson , IW , VanDer Straeten , D , Manners , J , Dennis , ES and Dolferus , R . 2005 . The transcription factor ATAF2 represses the expression of pathogenesis-related genes in Arabidopsis . Plant J , 43 : 745 – 757 .
- Ding , Z , Li , S , An , X , Liu , X , Qin , H and Wang , D . 2009 . Transgenic expression of MYB15 confers enhanced sensitivity to abscisic acid and improved drought tolerance in Arabidopsis thaliana . J Genet Genom , 36 : 17 – 29 .
- Dubouzet , JG , Sakuma , Y , Ito , Y , Kasuga , M , Dubouzet , ED , Miura , S , Seki , M , Shinozaki , K and Yamaguchi-Shinozaki , K . 2003 . OsDREB genes in rice, Oryza sativa L., encoded transcription activators that function in drought, high-salt- and cold-responsive gene expression . Plant J , 33 : 751 – 763 .
- El-Kayal , W , Navarro , M , Marque , G , Keller , G , Marque , C and Teulieres , C . 2006 . Expression profile of CBF-like transcriptional factor genes from Eucalyptus in response to cold . J Exp Bot , 57 : 2455 – 2469 .
- Galinski , EA , Pfeiffer , HP and Truper , HG . 1985 . 1,4,5,6-Tetrahydro-2-methyl–4-pyrimidine carboxylic acid: a novel cyclic amino acid from halophilic phototrophic bacteria of genus Ectothiorhodospira . Eur J Biochem , 149 : 135 – 139 .
- Garg , AK , Kim , JK , Owens , TG , Ranwala , AP , Choi , YD , Kochian , LV and Wu , RJ . 2002 . Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses . Proc Natl Acad Sci USA , 99 : 15898 – 15903 .
- Gaxiola , RA , Li , J , Undurraga , S , Dang , LM , Allen , GJ , Alper , SL and Fink , GR . 2001 . Drought and salt-tolerant plants result from overexpression of the AVP1 H+-pump . Proc Natl Acad Sci USA , 98 : 11444 – 11449 .
- Gilmour , SJ , Sebolt , AM , Salazar , MP , Everard , JD and Thomashow , MF . 2000 . Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation . Plant Physiol , 124 : 1854 – 1865 .
- Gisbert , C , Rus , AM , Bolarin , MC , Lopez-Coronado , JM , Arrillaga , I , Montesinos , C , Caro , M , Serrano , R and Moreno , V . 2000 . The yeast HAL1 gene improves salt tolerance of transgenic tomato . Plant Physiol , 123 : 393 – 402 .
- Godfray , HCJ , Beddington , JR , Crute , IR , Haddad , L , Lawrence , D , Muir , JF , Pretty , J , Robinson , S , Thomas , SM and Toulmin , C . 2010 . Food security: the challenge of feeding 9 billion people . Science , 327 : 812 – 818 .
- Hamilton , EW and Heckathorn , SA . 2001 . Mitochondrial adaptations to NaCl. Complex I is protected by anti-oxidants and small heat shock proteins, whereas complex II is protected by proline and betaine . Plant Physiol , 126 : 1266 – 1274 .
- Hong , Z , Lakkineni , K , Zhang , Z and Verma , DP . 2000 . Removal of feedback inhibition of delta(1)-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress . Plant Physiol , 122 : 1129 – 1136 .
- Huang , Z , Zhang , Z , Zhang , X , Zhang , H , Huang , D and Huang , R . 2004 . Tomato TERF1 modulates ethylene response and enhances osmotic stress tolerance by activating expression of downstream genes . FEBS Lett , 573 : 110 – 116 .
- Hussain , SS , Kayani , MA and Amjad , M . 2011 . Transcription factors as tools to engineer enhanced drought stress tolerance in plants . Biotechnol Prog , 27 : 297 – 306 .
- Ikuta , S , Imamura , S , Misaki , H and Horiuti , Y . 1977 . Purification and characterization of choline oxidase from Arthrobacter globiformis . J Biochem , 82 : 1741 – 1749 .
- Imai , R , Chang , L , Ohta , A , Bray , EA and Takagi , M . 1996 . A lea-class gene of tomato confers salt and freezing tolerance when expressed in Saccharomyces cerevisiae . Gene , 170 : 243 – 248 .
- Jaglo , KR , Kleff , S , Amundsen , KL , Zhang , X , Haake , V , Zhang , JZ , Deits , T and Thomashow , MF . 2001 . Components of the Arabidopsis C-repeat/dehydration-responsive element binding factor cold-response pathway are conserved in Brassica napus and other plant species . Plant Physiol , 127 : 910 – 917 .
- Jakoby , M , Weisshaar , B , Droge-Laser , W , Vicente-Carbajosa , J , Tiedemann , J , Kroj , T and Parcy , F . 2002 . bZIP transcription factors in Arabidopsis . Trends Plant Sci , 7 : 106 – 111 .
- James , VA , Neibaur , I and Altpeter , F . 2008 . Stress inducible expression of the DREB1A transcription factor from xeric, Hordeum spontaneum L. in turf and forage grass (Paspalum notatum Flugge) enhances abiotic stress tolerance . Transgenic Res , 17 : 93 – 104 .
- Jan , AT , Ali , A and Haq , QMR . 2011 . Glutathione as an antioxidant in inorganic mercury induced nephrotoxicity . J Postgrad Med , 57 : 72 – 77 .
- Jin , T , Chang , Q , Li , W , Yin , D , Li , Z , Wang , D , Liu , B and Liu , L . 2010 . Stress-inducible expression of GmDREB1 conferred salt tolerance in transgenic alfalfa . Plant Cell Tissue Organ Cult , 100 : 219 – 227 .
- Jung , J , Won , S , Suh , S , Kim , H , Wing , R , Jeong , Y , Hwang , I and Kim , M . 2007 . The barley ERF-type transcription factor HvRAF confers enhanced pathogen resistance and salt tolerance in Arabidopsis . Planta , 225 : 575 – 588 .
- Kang , JY , Choi , HI , Im , MY and Kim , SY . 2002 . Arabidopsis basic leucine zipper proteins that mediate stress-responsive abscisic acid signaling . Plant Cell , 14 : 343 – 357 .
- Karaba , A , Dixit , S , Greco , R , Aharoni , A , Trijatmiko , KR , Marsch-Martinez , N , Krishnan , A , Nataraja , KN , Udayakumar , M and Pereira , A . 2007 . Improvement of water use efficiency in rice by expression of HARDY, an Arabidopsis drought and salt tolerance gene . Proc Natl Acad Sci USA , 104 : 15270 – 15275 .
- Kasuga , M , Liu , Q , Miura , S , Yamaguchi-Shinozaki , K and Shinozaki , K . 1999 . Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor . Nat Biotech , 17 : 287 – 291 .
- Kasuga , M , Miura , S , Shinozaki , K and Yamaguchi-Shinozaki , K . 2004 . A combination of the Arabidopsis DREB1A gene and stress-inducible rd29A promoter improved drought- and low-temperature stress tolerance in tobacco by gene transfer . Plant Cell Physiol , 45 : 346 – 530 .
- Kishor , P , Hong , Z , Miao , GH , Hu , C and Verma , D . 1995 . Overexpression of [Δ]-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants . Plant Physiol , 108 : 1387 – 1394 .
- Knapp , S , Ladenstein , R and Galinski , EA . 1999 . Extrinsic protein stabilization by naturally occurring osmolytes: hydroxyectoine and betaine . Extremophiles , 3 : 191 – 198 .
- Krasensky , J and Jonak , C . 2012 . Drought, salt and temperature stress induced metabolic rearrangements and regulatory networks . J Exp Bot , 63 : 1593 – 1608 .
- Lee , JH , Hong , JP , Oh , SK , Lee , S , Choi , D and Kim , WT . 2004 . The ethylene-responsive factor like protein 1 (CaERFLP1) of hot pepper (Capsicum annuum L.) interacts in vitro with both GCC and DRE/CRT sequences with different binding affinities: possible biological roles of CaERFLP1 in response to pathogen infection and high salinity conditions in transgenic tobacco plants . Plant Mol Biol , 55 : 61 – 81 .
- Lentzen , G and Schwarz , T . 2006 . Extremolytes: natural compounds from extremophiles for versatile applications . Appl Microbiol Biotechnol , 72 : 623 – 634 .
- Liu , Q , Kasuga , M , Sakuma , Y , Abe , H , Miura , S , Yamaguchi-Shinozaki , K and Shinozaki , K . 1998 . Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain, separate two cellular signal transduction pathways in drought and low temperature-responsive gene expression respectively in Arabidopsis . Plant Cell , 10 : 1391 – 1406 .
- Lu , PL , Chen , NZ , An , R , Su , Z , Qi , BS , Ren , F , Chen , J and Wang , XC . 2007 . A novel drought-inducible gene, ATAF1, encodes a NAC family protein that negatively regulates the expression of stress-responsive genes in Arabidopsis . Plant Mol Biol , 63 : 289 – 305 .
- Lu , Z , Liu , D and Liu , S . 2007 . Two rice cytosolic ascorbate peroxidases differentially improve salt tolerance in transgenic Arabidopsis . Plant Cell Rep , 26 : 1909 – 1917 .
- Malik , V and Wu , R . 2005 . Transcription factor AtMyb2 increased salt-stress tolerance in rice (Oryza sativa L.) . Rice Genet Newslett , 22 : 63
- Mantri , N , Patade , V , Penna , S and Pang , E . 2012 . “ Abiotic stress response in plants – present and future ” . In Abiotic stress responses in plants: metabolism to productivity , Edited by: Ahmad , P and Prasad , MNV . 1 – 20 . New York : Springer .
- Menvielle-Bourg , FJ . 2005 . Superoxide dismutase (SOD) – a powerful antioxidant is now available orally . Phytotherapie , 3 : 1 – 4 .
- Moghaieb , REA , Nakamura , A , Saneoka , H and Fujita , K . 2011 . Evaluation of salt tolerance in ectoine transgenic tomato plants (Lycopersicum esculentum) in terms of photosynthesis, osmotic adjustment and carbon partitioning . GM Crops , 2 ( 1 ) : 58 – 65 .
- Najami , N , Janda , T , Barriah , W , Kayam , G , Tal , M , Guy , M and Volokita , M . 2008 . Ascorbate peroxidase gene family in tomato: its identification and characterization . Mol Genet Genom , 279 : 171 – 182 .
- Nakashima , K , Tran , LS , VanNguyen , D , Fujita , M , Maruyama , K , Todaka , D , Ito , Y , Hayashi , N , Shinozaki , K and Yamaguchi-Shinozaki , K . 2007 . Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice . Plant J , 51 : 617 – 630 .
- Nakayama H , Yoshida K , Ono H , Murooka Y , Shinmyo A 2000 . Ectoine, the compatible solute of Halomonas elongata, confers hyperosmotic tolerance in cultured tobacco cells . Plant Physiol 122 : 1239 – 1247 .
- Ndong , C , Danyluk , J , Wilson , KE , Pocock , T , Huner , NPA and Sarhan , F . 2002 . Cold-regulated cereal chloroplast late embryogenesis abundant-like proteins. Molecular characterization and functional analyses . Plant Physiol , 129 : 1368 – 1381 .
- Nyyssola , A , Kerovuo , J , Kaukinen , P , VonWeymarn , N and Reinikainen , T . 2000 . Extreme halophiles synthesize betaine from glycine by methylation . J Biol Chem , 275 : 22196 – 22201 .
- Oh , SJ , Song , SI , Kim , YS , Jang , HJ , Kim , SY , Kim , MJ , Kim , YK , Nahm , BH and Kim , JK . 2005 . Arabidopsis CBF3/DREB1A and ABF3 in transgenic rice increased tolerance to abiotic stress without stunting growth plant . Plant Physiol , 138 : 341 – 351 .
- Ono , H , Sawada , K , Khunajakr , N , Tao , T , Yamamoto , M , Hiramoto , M , Shinmyo , A , Takano , M and Murooka , Y . 1999 . Characterization of biosynthetic enzymes for ectoine as a compatible solute in a moderately halophilic eubacterium, Halomonas elongata . J Bacteriol , 181 : 91 – 99 .
- Park , JM , Park , CJ , Lee , SB , Ham , BK , Shin , R and Paek , KH . 2001 . Overexpression of the tobacco Tsi1 gene encoding an EREBP/AP2-type transcription factor enhances resistance against pathogen attack and osmotic stress in tobacco . Plant Cell , 13 : 1035 – 1046 .
- Pilon-Smits , EAH , Terry , N , Sears , T , Kim , H , Zayed , A , Hwang , S , VanDun , K , Voogd , E , Verwoerd , TC , Krutwagen , RW and Goddijn , OJM . 1998 . Trehalose-producing transgenic tobacco plants show improved growth performance under drought stress . J Plant Physiol , 152 : 525 – 532 .
- Pino , MT , Skinner , JS , Park , EJ , Jeknic , Z , Hayes , PM , Thomashow , MF and Chen , TH . 2007 . Use of a stress inducible promoter to drive ectopic AtCBF expression improves potato freezing tolerance while minimizing negative effects on tuber yield . Plant Biotechnol J , 5 : 591 – 604 .
- Purty , RS , Kumar , G , Singla-Pareek , S and Pareek , A . 2008 . Towards salinity tolerance in Brassica: an overview . Physiol Mol Biol Plants , 14 : 39 – 49 .
- Qin , F , Kakimoto , M , Sakuma , Y , Maruyama , K , Osakabe , Y , Tran , LSP , Shinozaki , K and Yamaguchi-Shinozaki , K . 2007 . Regulation and functional analysis of ZmDREB2A in response to drought and heat stresses in Zea mays L . Plant J , 50 : 54 – 69 .
- Qiu , QS , Guo , Y , Quintero , FJ , Pardo , JM , Schumaker , KS and Zhu , JK . 2004 . Regulation of vacuolar Na+/H+exchange in Arabidopsis thaliana by the salt-overly-sensitive (SOS) pathway . J Biol Chem , 279 : 207 – 215 .
- Rathinasabapathi , B , Burnet , M , Russell , BL , Gage , DA , Liao , PC , Nye , GJ , Scott , P , Golbeck , JH and Hanson , AD . 1997 . Choline monooxygenase, an unusual iron-sulfur enzyme catalyzing the first step of glycine betaine synthesis in plants: prosthetic group characterization and cDNA cloning . Proc Natl Acad Sci USA , 94 : 3454 – 3458 .
- Rhodes , D and Hanson , AD . 1993 . Quaternary ammonium and tertiary sulfonium compounds in higher plants . Ann Rev Plant Physiol Plant Mol Biol , 44 : 357 – 384 .
- Roxas , VP , Smith , RK , Allen , ER and Allen , RD . 1997 . Overexpression of glutathione S-transferase/glutathioneperoxidase enhances the growth of transgenic tobacco seedlings during stress . Nat Biotech , 15 : 988 – 991 .
- RoyChoudhury , A , Roy , C and Sengupta , DN . 2007 . Transgenic tobacco plants overexpressing the heterologous lea gene Rab16A from rice during high salt and water deficit display enhanced tolerance to salinity stress . Plant Cell Rep , 26 : 1839 – 1859 .
- Rus , AM , Estan , MT , Gisbert , C , Garcia-Sogo , B , Serrano , R , Caro , M , Moreno , V and Bolarin , MC . 2001 . Expressing the yeast HAL1 gene in tomato increases fruit yield and enhances K+/Na+ selectivity under salt stress . Plant Cell Environ , 24 : 875 – 880 .
- Sakuma , Y , Maruyama , K , Qin , F , Osakabe , Y , Shinozaki , K and Yamaguchi-Shinozaki , K . 2006 . Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression . Proc Natl Acad Sci USA , 103 : 18822 – 18827 .
- Sengupta , A , Heinen , J , Holaday , AS , Barke , JJ and Allan , RD . 1993 . Increased resistance to oxidative stress in transgenic plants that overexpress chloroplastic Cu/Zn superoxide dismutase . Proc Natl Acad Sci USA , 90 : 1629 – 1633 .
- Serrano , R , Mulet , J , Rios , G , Marquez , J , deLarrinoa , I , Leube , M , Mendizabal , I , Pascual-Ahuir , A , Proft , M Ros , R . 1999 . A glimpse of the mechanisms of ion homeostasis during salt stress . J Exp Bot , 50 : 1023 – 1036 .
- Shen , YG , He , SJ , Zhang , WK , Zhang , JS , Liu , Q and Chen , SY . 2003 . An EREBP/AP2-type protein in Triticum aestivum was a DRE-binding transcription factor induced by cold, dehydration and ABA stress . Theor Appl Genet , 106 : 923 – 930 .
- Shen , YG , Zhang , WK , Yan , DQ , Du , BX , Zhang , JS , Liu , Q and Chen , SY . 2003 . Characterization of a DRE-binding transcription factor from a halophyte Atriplex hortensis . Theor Appl Genet , 107 : 155 – 161 .
- Sheveleva , E , Chmara , W , Bohnert , HJ and Jensen , RG . 1997 . Increased salt and drought tolerance by D-ononitol production in transgenic Nicotiana tabacum L . Plant Physiol , 115 : 1211 – 1219 .
- Shi , H , Lee , BH , Wu , SJ and Zhu , JK . 2003 . Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana . Nat Biotech , 21 : 81 – 85 .
- Singh , LR , Dar , TA and Ahmad , F . 2009 . Living with urea stress . J Biosci , 34 : 321 – 331 .
- Singh , LR , Poddar , NK , Dar , TA , Kumar , R and Ahmad , F . 2011 . Protein and DNA destabilization by osmolytes: the other side of the coin . Life Sci , 88 : 117 – 125 .
- Sivamani , E , Bahieldin , A , Wraith , JM , Al-Niemi , T , Dyer , WE , Ho , THD and Qu , R . 2000 . Improved biomass productivity and water use efficiency under water deficit conditions in transgenic wheat constitutively expressing the barley HVA1 gene . Plant Sci , 155 : 1 – 9 .
- Sugino , M , Hibino , T , Tanaka , Y , Nii , N and Takabe , T . 1999 . Overexpression of DnaK from a halotolerant cyanobacterium Aphanothece halophytica acquires resistance to salt stress in transgenic tobacco plants . Plant Sci , 146 : 81 – 88 .
- Sun , W , Bernard , C , VanDeCotte , B , VanMontagu , M and Verbruggen , N . 2001 . At-HSP17.6A, encoding a small heat-shock protein in Arabidopsis, can enhance osmotolerance upon overexpression . Plant J , 27 : 407 – 415 .
- Sun , S , Yu , JP , Chen , F , Zhao , TJ , Fang , XH , Li , YQ and Sui , SF . 2008 . TINY, a dehydration-responsive element (DRE)-binding protein-like transcription factor connecting the DRE- and ethylene-responsive element-mediated signaling pathways in Arabidopsis . J Biol Chem , 283 : 6261 – 6271 .
- Takabe T , Hayashi Y , Tanaka A , Takabe T , Kishitani S 1998 . Evaluation of glycinebetaine accumulation for stress tolerance in transgenic rice plants . Proceedings of International Workshop on Breeding and Biotechnology for Environmental Stress in Rice . Sapporo : Hokkaido National Agricultural Experiment Station and Japan International Science and Technology Exchange Center , 63 – 68 .
- Tanaka , Y , Hibino , T , Hayashi , Y , Tanaka , A , Kishitani , S , Takabe , T and Yokota , S . 1999 . Salt tolerance of transgenic rice overexpressing yeast mitochondrial Mn-SOD in chloroplasts . Plant Sci , 148 : 131 – 138 .
- Tarczynski , MC , Jensen , RG and Bohnert , HJ . 1993 . Stress protection of transgenic tobacco by production of the osmolyte mannitol . Science , 259 : 508 – 510 .
- Tayal D , Srivastava PS , Bansal KC 2004 . Transgenic crops for abiotic stress tolerance . Plant Biotechnology and Molecular Markers Kluwer Academic Publishers , Netherlands . 346 – 365 .
- Thomashow , MF . 1998 . Role of cold-responsive genes in plant freezing tolerance . Plant Physiol , 118 : 1 – 17 .
- Varshney , RK , Bansal , KC , Aggarwal , PK , Datta , SK and Craufurd , PQ . 2011 . Agricultural biotechnology for crop improvement in a variable climate: hope or hype . Trend Plant Sci , 16 : 363 – 371 .
- Villalobos , MA , Bartels , D and Iturriaga , G . 2004 . Stress tolerance and glucose insensitive phenotypes in Arabidopsis overexpressing the CpMYB10 transcription factor gene . Plant Physiol , 135 : 309 – 324 .
- Waditee , R , Bhuiyan , MN , Rai , V , Aoki , K , Tanaka , Y , Hibino , T , Suzuki , S , Takano , J , Jagendorf , AT and Takabe , T . 2005 . Genes for direct methylation of glycine provide high levels of glycinebetaine and abiotic-stress tolerance in Synechococcus and Arabidopsis . Proc Natl Acad Sci USA , 102 : 1318 – 1323 .
- Wang , H , Huang , Z , Chen , Q , Zhang , Z , Zhang , H , Wu , Y , Huang , D and Huang , R . 2004 . Ectopic overexpression of tomato JERF3 in tobacco activates downstream gene expression and enhances salt tolerance . Plant Mol Biol , 55 : 183 – 192 .
- Wang , J , Zhang , H and Allan , RD . 1999 . Overexpression of an Arabidopsis peroxisomal ascorbate peroxidise gene in tobacco increases protection against oxidative stress . Plant Cell Physiol , 40 : 725 – 732 .
- Wang , W , Vinocur , B and Altman , A . 2003 . Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance . Planta , 218 : 1 – 14 .
- Welling , A and Palva , ET . 2008 . Involvement of CBF transcription factors in winter hardiness in birch . Plant Physiol , 147 : 1199 – 1211 .
- Xiang , Y , Tang , N , Du , H , Ye , H and Xiong , L . 2008 . Charaterization of Osb-ZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice . Plant Physiol , 148 : 1938 – 1952 .
- Xiao , H , Siddiqua , M , Braybrook , S and Nassuth , A . 2006 . Three grape CBF/DREB1 genes respond to low temperature, drought and abscisic acid . Plant Cell Environ , 29 : 1410 – 1421 .
- Xiong , Y and Fei , SZ . 2006 . Functional and phylogenetic analysis of a DREB/CBF like gene in perennial ryegrass (Lolium perenne L.) . Planta , 224 : 878 – 888 .
- Xu , D , Duan , X , Wang , B , Hong , B , Ho , T and Wu , R . 1996 . Expression of a late embryogenesis abundant protein gene, HVA1, from barley confers tolerance to water deficit and salt stress in transgenic rice . Plant Physiol , 110 : 249 – 257 .
- Yamaguchi-Shinozaki , K and Shinozaki , K . 2005 . Organization of cis-acting regulatory elements in osmotic and cold stress responsive promoters . Trends Plant Sci , 10 : 88 – 94 .
- Yancey , PH . 2001 . Water stress, osmolytes and proteins . Amer Zool , 41 : 699 – 709 .
- Yancey , PH . 2003 . Proteins and counteracting osmolytes . Biologist , 50 : 126 – 131 .
- Yancey , PH , Clark , ME , Hand , SC , Bowlus , RD and Somero , GN . 1982 . Living with water stress: evolution of osmolyte systems . Science , 217 : 1214 – 1222 .
- Zhang , HX and Blumwald , E . 2001 . Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit . Nat Biotech , 19 : 765 – 768 .
- Zhang , HX , Hodson , JN , Williams , JP and Blumwald , E . 2001 . Engineering salt-tolerant Brassica plants: characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation . Proc Natl Acad Sci USA , 98 : 12832 – 12836 .
- Zhang , SZ , Yang , BP , Feng , CI and Tang , HL . 2005 . Genetic transformation of tobacco with the trehalose synthase gene from Grifola frondosa Fr. enhances the resistance to drought and salt in tobacco . J Integrat Plant Biol , 47 : 579 – 586 .