Abstract
Molecules released by soil microorganisms, such as arbuscular mycorrhizal fungi (AMF), trigger plant responses prior to any physical contact. Here, it is shown that disrupted spores (DS) homogenates and exudates from germinating spores (GS) of Glomus etunicatum caused marked alterations in the content of the tobacco alkaloids nicotine, anabasine, and nornicotine and the genes involved in their biosynthesis. GS and DS were applied to the base of Nicotiana tabacum seedling stems, and 3 or 10 days later, the leaves and roots were harvested for analyses. The alkaloid contents were influenced by both elicitors and varied depending on the harvest day. In general, such variations agree with the transcript levels of putrescine N-methyltransferase, oxide reductase – A622 and nicotine N-demethylase – CYP82E4. The results are discussed in light of recent insights on chemical signaling processes between plants and AMF able to trigger different elicitation responses and their possible effects on secondary metabolism in plants.
Introduction
Elicitors have been defined as signaling molecules triggering the production of secondary metabolites by stimulating plant defense systems, hypersensitive response, and/or the formation of pathogenesis-related proteins (Hahn Citation1996). These bioactive compounds have a varied chemical nature, including polysaccharides, proteins, glycoproteins, peptides, fatty acids, or cell-wall fragments derived from fungi, bacteria, and even from plants (Hahn Citation1996; Smith Citation1996). In plants, elicitation mechanisms are based on elicitor–receptor interactions that trigger a set of rapid biochemical responses. These mechanisms includes, among other events, elicitor binding to its receptor in plasmatic membranes, variations in intracellular free Ca2 + concentrations, changes in the pattern of protein phosphorylation and protein kinase activation, synthesis of jasmonates and other hormones and/or the synthesis of plant defense molecules such as phytoalexins (Zhao et al. Citation2005).
Pyridine alkaloids in Nicotiana species are inducible metabolites with ecophysiological functions under abiotic or biotic stress conditions such as tissue wounding and herbivore or pathogen attack (Baldwin and Schmelz Citation1996). For example, nicotine production (and likely other tobacco alkaloids) can be induced by endogenous signals as hormones or exogenously by jasmonates or other elicitors (Baldwin and Schmelz Citation1996; Ziegler and Facchini Citation2008). Fungal elicitors, such as cell-wall fragments, initiate a metabolic cascade that induces the biosynthesis of phytoalexins and alkaloids as part of the general plant defense response (Yoshikawa et al. Citation1993). Like other plant secondary metabolites, alkaloids biosynthesis can be stimulated by exogenous elicitors, such as fungal components (El-Sayed and Verpoorte Citation2007).
Most terrestrial plants have the capacity to establish mutualistic associations with arbuscular mycorrhizal fungi (AMF), a group of ubiquitous soil fungi that belong to the ancient phylum of Glomeromycota (Redecker et al. Citation2000). As in other plant–microbe symbiosis, AMF relationships begin with molecular and chemical dialogs between partners that trigger the events leading to an effective plant tissue colonization by microbes and to the various and complex morphophysiological alterations of the symbiotic partners (Bonfante and Requena Citation2011; Maillet et al. Citation2011). Recently, (Bonfante and Requena Citation2011) updated the knowledge regarding molecular mechanisms involved in MF perception by plants and the functions of these bioactive molecules. MFs, or at least some of them, have been identified chemically as lipochito-oligosaccharides, which are known to induce MtENOD11, a symbiosis-specific gene expressed in the roots of Medicago truncatula and involved in the formation of lateral roots (Maillet et al. Citation2011). The release of these AM fungal elicitors can activate specific signaling pathways in tobacco host plants, such as those of mitogen-activated protein kinases, which in turn are dependent on the increase of free cytosolic Ca2 + (Francia et al. Citation2011). In fact, nuclear Ca2 + spiking was induced by hyphopodium-contacted cells in legume and non-legume roots and also by exudates of germinating AMF spores. These responses were dependent on genes of the symbiosis (SYM) signaling pathway. The use of AM fungal exudates in plants to induce signs of an elicitation response, such as transient cytosolic or nuclear Ca2 + spiking and nitric oxide accumulation, has been recently and increasingly more documented (Navazio et al. Citation2007; Chabaud et al. Citation2011; Francia et al. Citation2011). In soil, AMF spores must encounter conducive host roots to continue their mandatory biotrophic lifestyle, and for that it has been recently proven that they secrete effectors proteins in planta as SP7 that entering plant cells move to the nucleus from where it plays a role in managing fungus accommodation into the roots (Kloppholz et al. Citation2011).
Considering the high complexity of AMF relationships with plants and that pyridine alkaloids can be induced in Nicotiana species by abiotic or biotic stresses as a defense response, the aim of this work was to verify, in a indirect manner, whether cellular components of AM fungi spores or exudates produced during spore germination have the capacity to act as fungal elicitors in Nicotiana tabacum plants by influencing the alkaloid concentrations and the expression of some key genes of the biosynthesis pathway. The genes coding for the enzymes putrescine N-methyltransferase (PMT), A622 (a PIP-family oxidoreductase), and CYP82E4 (nicotine N-demetilase) were chosen because their location in different branches of the pyridine alkaloids biosynthesis pathway.
Material and methods
Experimental design
To evaluate the elicitor potential of AMF components on alkaloid accumulation and biosynthesis in tobacco plants, an experiment was carried out in a completely randomized design. The experimental setup consisted of three elicitor treatments and two periods of plant collection, with eight replicates per treatment and for each collection. Two ‘elicitor solutions’ were used as treatments, one denoted ‘disrupted spores’ (DS) originated from the disruption of fungal spores and a second ‘germination solution’ (GS) composed of a filtrate from germinating AMF spore exudate media. The ‘Control’ treatment was without application of elicitors. Plant material, shoots and roots were collected 3 or 10 days after elicitor application.
Fungal material and preparation of elicitor solutions
Spores of Glomus etunicatum (Becker and Gerdemann) were obtained from the Agronomic Institute of Campinas where they were maintained in stock culture pots with Brachiaria brizantha Staf and identified as IAC-42. After the spores had been collected from pots and extracted by wet sieving, they were manually selected for uniformity in size and color under a light microscope with the aid of a micropipette. Spores were surface sterilized and stored in 15 mL Falcon tubes at 4°C (Bécard and Fortin Citation1988). To prepare the DS elicitor solution, aliquots of approximately 250 sterilized spores mL−1 were disrupted directly in the Falcon tubes using a glass micropestle and then quick-frozen in liquid nitrogen three times to improve spore cell-wall disruption. Homogenates were stored at –80°C. To prepare the GS elicitor solution, aliquots of 250 spores mL−1 of sterile distilled water were induced to germinate in horizontally placed Falcon tubes incubated at 27°C in the dark for 10 days. After 10 days, germination was checked under a light microscope. If the germination rate was estimated around 60–70% of spores, then the germination media was collected by filtration through sterilized Whatman 42 (2.5 µm) paper and stored at –80°C.
Plant material, elicitor solution application, and growth conditions
Nicotiana tabacum L. seeds were surface sterilized for 10 min with 2.5% NaClO, and five of them were sown in 50 mL plastic tubes filled with sand that had been previously sterilized by autoclaving for 1 h at 120°C. After emergence, only one seedling was left per tube. The plants were maintained in growth chambers (25±3°C, 60–70% relative humidity, 160 µmol photons m−2 s−1, and 12 h photoperiod) and irrigated with Hoagland nutrient solution (Hoagland and Arnon Citation1950) twice a week and with sterilized distilled water when necessary. After 25 days of growth, the plants received 5 mL of the elicitors solutions, applied with a micropipette directly to the base of the stems. Control plants received 5 mL of sterilized distilled water. Three or ten days later, the eight replicates were used as follows: four replicates were used for determination of alkaloids in leaves and roots; four replicates were used to analyze gene expression in the roots. Replicates for alkaloid extraction were frozen at liquid N2 and maintained at –80°C until analysis.
Plant material processing and alkaloids determination
Leaves and roots tissues collected for alkaloid extraction and stored at –80°C were freeze-dried, and approximately 0.05 g per sample was powdered with liquid nitrogen in a mortar and homogenized with acid water (deionized water + 0.01% formic acid) in a 1:1 m/v ratio. Homogenates were sonicated for 30 min and centrifuged at 10,000 rpm for 10 min, and the supernatants were collected in HPLC vials. Chromatographic separation and identification was undertaken using a UPLC-MS system (Waters) with a C-18 reverse phase column in an isocratic run (98.9% MilliQ, 1.0% acetonitrile, and 0.1% formic acid) at a flow rate of 1 mL min−1. Two microliters of authentic standard (Sigma) solutions (nicotine, anabasine, and nornicotine) and samples were injected into the system. Peak areas were extracted using the Mass Lynx software, and concentrations were calculated between 1 and 600 µg mL−1. Mass spectra were acquired between 100 and 500 m/z in the positive ion mode in a TQD (Acquity, Micromass/Waters) mass spectrometer under the following conditions: capillary 3500 (V); Cone 30 (V); source temperature 150°C; and desolvation temperature of 300°C.
RNA isolation and quantitative PCR (RT-qPCR) analysis
Total RNA was isolated from root tissue samples stored at –80°C using TRIzol reagent (Invitrogen). Three micrograms of total RNA was treated with Turbo DNase-free (Ambion) to remove DNA contamination, and total RNA was quantified by Quant-ItTM Assays (Invitrogen). First-strand cDNA was obtained with reverse transcription polymerase Superscript III RT (Invitrogen) and diluted 50 times with nuclease-free water to a final volume of 200 µL. Each PCR reaction contained a mixture of 4.5 µL diluted cDNA aliquots, 0.6 µL mixed primers (1.25 pmol mL−1), 7.5 µL QuantiFastTM SYBRR Green PCR Kit (Qiagen), and 2.4 µL of nuclease-free water. The following oligonucleotide primers were used for PCR reactions: for PMT (GenBank Accession no: AF126810.1), 5′-GCAGCATTCATTTTACCATCTTT-3′(forward) and 5′-CGTCGCATTTCACTTATTTATTCA-3′ (reverse); for A622, PIP-family oxidoreductase (D28505), 5′-TCTGCGGTGACTCTATCAAACT-3′ (forward) and 5′-CAAAAGCACTGAAGCGAAAA-3′ (reverse); for CYP82E4, nicotine N-demetilase (HM802352.1), 5′-CAAAACCCTTACCACCGAAA-3′ (forward) and 5′-AGAAAAGCTGGACGATTGGA-3′ (reverse); for NtL25, ribosomal protein (L18908), 5′-CCCCTCACCACAGAGTCTGC-3′ (forward) and 5′-AAGGGTGTTGTTGTCCTCAATCT-3′ (reverse); and for EF-1α, elongation factor 1- α (AF120093), 5′-TGAGATGCACCACGAAGCTC-3′ (forward) and 5′-CCAACATTGTCACCAGGAAGTG-3′ (reverse).
The qPCR amplification was carried out in 96-well plates on an iCycler iQTM5 Multicolor Real-Time PCR Detection System (Bio-Rad), and the cycling parameters consisted of a 95°C hold for 3 min and 40 cycles of a 95°C denaturing step for 10 sec and a 60°C annealing/extension step for 30 sec. PCR efficiency was estimated by LinReg software, and it remained between 90 and 97% for all used primers (Ramakers et al. Citation2003). Confirmation of amplicon specificity was based on the dissociation curve at the end of each run using a cDNA pool. Each primer reaction analyzed was performed in triplicate, and PCR reactions without templates were also performed as negative controls for each primer pair. The relative gene expression was quantified using the comparative CT (threshold cycle) method (Shalel-Levanon et al. Citation2005). Ribosomal protein gene NtL25 and elongation factor gene NtEF-1α were used as endogenous controls (Schmidt and Delaney Citation2010).
The obtained data were submitted to a one-way analysis of variance (ANOVA) and Tukey's test at 5% probability for comparisons of means.
Results
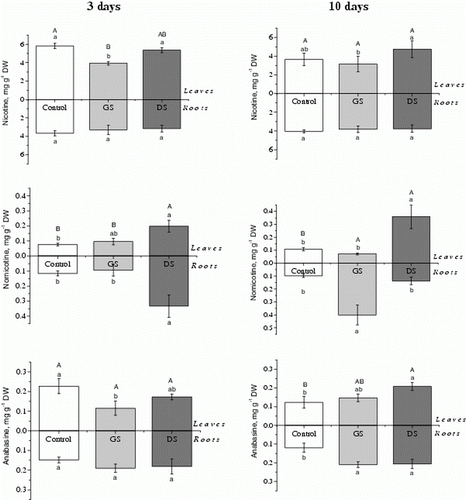
After 3 days of elicitor applications, nicotine and anabasine concentrations in tobacco leaves were significantly lower in plants that received the GS elicitor compared to control plants. In plants receiving the DS elicitor, the foliar nicotine content was similar to the control (). After 10 days of elicitor applications, the nicotine concentration was not significantly different between plants from the three treatments (). In general, after 3 days of elicitor applications, plants receiving the DS solution showed higher nornicotine contents in roots compared to control plants or plants receiving the GS solution (). However, the nornicotine content in the leaves was significantly higher than the contents observed in leaves of control plants. In plants analyzed after 10 days of either elicitor application, there was a significantly higher total nornicotine content compared to the control plants, but the below- or above-ground distribution of nornicotine was different depending on the elicitor solution. In plants receiving GS, nornicotine preferentially accumulated in the roots, while in plants receiving DS, nornicotine mainly accumulated in the leaves ().
Figure 1. Nicotine, nornicotine, and anabasine concentrations in leaves and roots of Nicotiana tabacum plants after 3 or 10 days of elicitor solution applications (Control – plants without elicitor solution application; GS – elicitor solution from germinating spore exudates; and DS – elicitor solution from disrupted AMF spores). Different lower case letters denote statistically significant differences among treatments in leaves or roots, and uppercase letters are used to compare total (leaves + roots) alkaloid contents by the Tukey's test (5%). Error bars show the standard deviation (n=4).
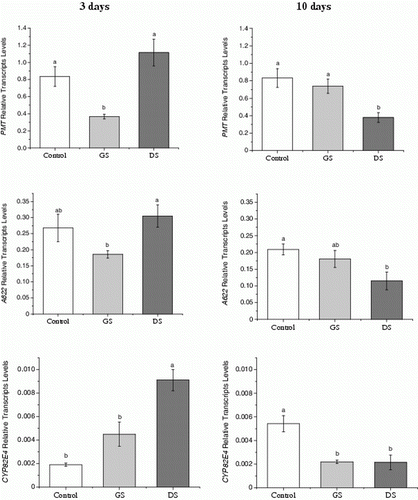
Differential gene expression analysis of the pyridine alkaloid biosynthetic key enzymes PMT, A622, and CYP82E4 was performed by RT-qPCR. In general, the transcript levels of these genes were influenced by elicitor solutions in the roots of plants 3 and 10 days after the GS or DS applications (). PMT and A622 transcript levels were lower in plants that received GS than in plants receiving DS and the control plants. CYP82E4 expression levels were significantly higher in roots treated with DS, which was correlated with the root nornicotine content. In contrast, after 10 days of the elicitor application, the plants had lower CYP82E4 expression levels than control plants and this gene's expression was not related to nornicotine content, which was lower in the control plants ().
Figure 2. Relative transcript levels of tobacco alkaloid related-enzymes in relation to elongation factor EF-1α transcript levels in roots of Nicotiana tabacum plants after 3 or 10 days of elicitor solution applications (Control – plants without elicitor solution application; GS – elicitor solution from germinating spore exudates; and DS – elicitor solution from disrupted AMF spores). Significant differences among treatments at P<0.05 were determined by one-way analysis of variance (ANOVA) followed by the Tukey's test and are indicated by different letters. Error bars show the standard deviation (n=4).
Discussion
Chemical perception is the first fulfilled event of any elicitor signal transduction pathway (Zhao et al. Citation2005), and it has been demonstrated that AMF-derived soluble signals induce the expression of specific symbiotic genes (Kosuta et al. Citation2003), are signals for transcription networks (Weidmann et al. Citation2004), increase transient cytosolic/nuclear Ca2 +spiking (Navazio et al. Citation2007), induce NO accumulation (Calcagno et al. Citation2011) and activate MAP kinases in plant cells (Francia et al. Citation2011), all without the need for previous physical contact between plant and AMF cells.
It is well known that plant secondary metabolites can accumulate in response to elicitor or signal molecules (Zhao et al. Citation2005). The biosynthesis and regulatory pathways of tobacco pyridine alkaloids form a well-studied group (Katoh and Hashimoto Citation2004; Kidd et al. Citation2006), and the alkaloids are known to accumulate through a jasmonic acid-mediated response after herbivore attacks (Keinanen et al. Citation2001; Kidd et al. Citation2006). Here, we report for the first time that AMF components either from sterilized DS or from exudates formed during the spore germination process are able to alter tobacco alkaloid biosynthesis and accumulation. However, our results do not allow us to conclude that such changes were either direct or indirect response to the AMF elicitors.
Although the compounds involved in the biosynthesis of nicotine and the majority of related enzyme activities have been documented in tobacco, the same is not true for all of the enzyme-encoding genes in each step (Shoji et al. Citation2010). In our experiments, we chose to analyze the expression of the genes coding for PMT, A622, and CYP82E4 because of the availability of a known gene sequence in the public databases and also due to their key location in different branches of the pyridine alkaloids biosynthesis pathway in tobacco. PMT leads to the biosynthesis of N-methyl putrescine (Biastoff et al. Citation2009), which in turn is condensed with nicotinic acid to form nicotine. This last step is mediated by the oxido-reductase A622. Anabasine is biosynthesized from the condensation of nicotinic acid with piperidine (formed from cadaverine) in a reaction also thought to be catalyzed by an A622 enzyme (DeBoer et al. Citation2009). Furthermore, nicotine may be converted to N-formyl-nornicotine or N-hydroxy-methylnornicotine, which may then be metabolized by CYP82E4 to nornicotine (Siminszky et al. Citation2005).
The expression of the PMT, A622, and CYP82E4 genes in tobacco roots was significantly, although differently, altered by both elicitors DS and GS. The expression level of PMT gene, corresponding to a key enzyme in nicotine biosynthesis specifically produced in root cortical cells and the pericycle (Biastoff et al. Citation2009), was lower in roots after 3 days of GS elicitor application and those plants also showed reduced nicotine contents in shoots and roots compared to control plants or plants that received the DS elicitor for the same period of time collection analyzed. Nornicotine and anabasine accumulated in much lower amounts (30–40 times less) in tobacco tissues than did nicotine. Nevertheless, their contents were increased in shoots and roots depending on the AMF elicitor solutions applied. It was also observed that the nicotine content in tobacco plants was not as altered in the extent the nornicotine or anabasine contents were, suggesting higher activity in other branches of the pyridine alkaloid biosynthesis pathway, especially after 3 days of the GS elicitor applications. The increase in CYP82E4 expression levels in plant roots may indicate an increase in further nicotine metabolism to nornicotine. CYP82E4 expression levels in roots were correlated with nornicotine contents, and both were increased in tobacco plants after 3 days of DS applications. PMT and A622 transcript levels also followed the trend observed for nicotine and anabasine contents, respectively, especially in plants analyzed after 3 days of elicitor applications. In the second period analyzed, after 10 days of elicitor applications, the total anabasine content was higher in plants that received elicitor solutions compared to control plants. The higher anabasine accumulation in plants elicited by the DS solution may indicate an oversupply of its main precursors, piperidine (derived from cadaverine) and nicotinic acid. After 10 days, plants that received the GS solution had higher nornicotine contents in roots than control plants or plants that received the DS solution.
Elicitor solutions originating from disrupted spores, named here as DS solution, are expected to contain wall components, such as chitin, other polysaccharides, and proteins, as well as high amounts of lipids, such as fatty acids, sterols, and triacylglycerides (Olsson and Johansen Citation2000). Chitin, for example, is a known fungal polysaccharide included in the microbe-associated molecular patterns (MAMPs) (Boller and Felix Citation2009) that, along with other general elicitors, can be detected by the plant defense system (Shibuya and Minami Citation2001). The perception of MAMPs by plant membrane specific receptors activates a signaling cascade related to the defense system response (Hamel and Beaudoin Citation2010).
Germinating AMF spores release different diffusible factors, such as mixtures of sulfated and non-sulfated lipochito-oligosaccharides and secreted proteins (Kloppholz et al. Citation2011; Maillet et al. Citation2011) among other spore metabolism related compounds. Consequently, it is expected to find these compounds in the GS solution. Those diffusible factors are able to rapidly induce NO accumulation, Ca2 + spiking in root cells and the expression of some plant symbiotic-related genes (Calcagno et al. Citation2011; Chabaud et al. Citation2011; Kloppholz et al. Citation2011). The induction of delayed processes, such as the formation of lateral roots and root branching, has also been observed in response to these diffusible factors released by AMF spores (Oláh et al. Citation2005).
Therefore, considering the nature of the DS and GS solutions, one may suggest that similarities exist between the alkaloid responses observed here using the DS and GS elicitors from AMF and those observed in tobacco plants subjected to different biotic and abiotic stresses (Baldwin and Schmelz Citation1996) or those after pathogen or chemical elicitation, which produce different signal molecules in the interaction process. However, it seems that we are far from understanding the complex mechanisms governing the relationship between plants and symbiotic microorganisms like AMF and rhizobia (Ercolin and Reinhardt Citation2011). For example, despite the changes caused in the nicotine content in tobacco plants exposed to AMF elicitors, it appears that tobacco displays different behaviors depending on the stimuli perceived and the location in the plant. In contrast to the increase of nicotine in leaves of Nicotiana species attacked by herbivores (Keinanen et al. Citation2001; Kidd et al. Citation2006), infection with Phytophthora nicotianae causes a reduction of the leaf alkaloid content (Ibáñez et al. Citation2010).
In conclusion, this work suggests that AMF components, such as DSs or exudates from germinating spores, are able to elicit alkaloid biosynthesis in tobacco, influencing their content and the expression of genes in the biosynthetic pathway. Independently, if these responses were a direct or indirect elicitor effect, the mechanisms leading to these responses may be diverse. Further purification studies may indicate the AMF molecules involved and which plant mechanisms were triggered, what is obviously out of the scope of this preliminary study. Under natural soil conditions both types of elicitors exist, those that arose from spores and hyphae exudates and those released after spore disruption either mechanically or by predation by other soil organisms. However, AMF interactions with plant metabolism and phytochemical biosynthesis may be more complex, and the capability of AMF exudates or cell components to elicit responses on plant secondary metabolites may have important and underestimated ecological implications that need to be further studied. Whether lipochito-oligosacharides (Maillet et al. Citation2011) were in part (GS elicitor) responsible for the alterations observed here is unknown, but under natural soil conditions where different AMF species are present and both type of elicitors (GS and DS) can exist, the alkaloid metabolic response would be even more complicated.
Acknowledgements
The authors thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for financial support through a postdoctoral and research fellowship to S.A.L. Andrade and P. Mazzafera, respectively.
References
- Baldwin , IT and Schmelz , EA . 1996 . Immunological “memory” in the induced accumulation of nicotine in wild tobacco . Ecology. , 77 : 236 – 246 .
- Bécard , G and Fortin , J . 1988 . Early events of vesicular-arbuscular mycorrhiza formation on Ri T-DNA transformed roots . New Phytol. , 108 : 211 – 218 .
- Biastoff , S , Brandt , W and Drager , B . 2009 . Putrescine N-methyltransferase-the start for alkaloids . Phytochemistry. , 70 : 1708 – 1718 .
- Boller , T and Felix , G . 2009 . A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors . Ann Rev Plant Biol. , 60 : 379 – 406 .
- Bonfante , P and Requena , N . 2011 . Dating in the dark: how roots respond to fungal signals to establish arbuscular mycorrhizal symbiosis . Curr Opin Plant Biol. , 14 : 451 – 457 .
- Calcagno , C , Novero , M , Genre , A , Bonfante , P and Lanfranco , L . 2011 . The exudate from an arbuscular mycorrhizal fungus induces nitric oxide accumulation in Medicago truncatula roots . Mycorrhiza. , 22 : 259 – 269 .
- Chabaud , M , Genre , A , Sieberer , BJ , Faccio , A , Fournier , J , Novero , M , Barker , DG and Bonfante , P . 2011 . Arbuscular mycorrhizal hyphopodia and germinated spore exudates trigger Ca2+ spiking in the legume and nonlegume root epidermis . New Phytol. , 189 : 347 – 355 .
- DeBoer , KD , Lye , JC , Aitken , CD , Su , AKK and Hamill , JD . 2009 . The A622 gene in Nicotiana glauca (tree tobacco): evidence for a functional role in pyridine alkaloid synthesis . Plant Mol Biol. , 69 : 299 – 312 .
- El-Sayed , M and Verpoorte , R . 2007 . Catharanthus terpenoid indole alkaloids: biosynthesis and regulation . Phytochem Rev. , 6 : 277 – 305 .
- Ercolin , F and Reinhardt , D . 2011 . Successful joint ventures of plants: arbuscular mycorrhiza and beyond . Trends Plant Sci. , 16 : 356 – 362 .
- Francia , D , Chiltz , A , Lo Schiavo , F , Pugin , A , Bonfante , P and Cardinale , F . 2011 . AM fungal exudates activate MAP kinases in plant cells in dependence from cytosolic Ca2+ increase . Plant Physiol Biochem. , 49 : 963 – 969 .
- Hahn , MG . 1996 . Microbial elicitors and their receptors in plants . Annu Rev Phytopathol. , 34 : 387 – 412 .
- Hamel , LP and Beaudoin , N . 2010 . Chitooligosaccharide sensing and downstream signaling: contrasted outcomes in pathogenic and beneficial planta-microbe interactions . Planta. , 232 : 787 – 806 .
- Hoagland , DR and Arnon , D . 1950 . The water culture method for growing plants without soil . Cali AES Bull. , 347 : 39
- Ibáñez , AJ , Scharte , J , Bones , P , Pirkl , A , Meldau , S , Baldwin , IT , Hillenkamp , F , Weis , E and Dreisewerd , K . 2010 . Rapid metabolic profiling of Nicotiana tabacum defence responses against Phytophthora nicotianae using direct infrared laser desorption ionization mass spectrometry and principal component analysis . Plant Methods. , 6 : 14
- Katoh , A and Hashimoto , T . 2004 . Molecular biology of pyridine nucleotide and nicotine biosynthesis . Front Biosci. , 9 : 1577 – 1586 .
- Keinanen , M , Oldham , NJ and Baldwin , IT . 2001 . Rapid HPLC screening of jasmonate-induced increases in tobacco alkaloids, phenolics, and diterpene glycosides in Nicotiana attenuata . J Agric Food Chem. , 49 : 3553 – 3558 .
- Kidd , SK , Melillo , AA , Lu , RH , Reed , DG , Kuno , N , Uchida , K , Furuya , M and Jelesko , JG . 2006 . The A and B loci in tobacco regulate a network of stress response genes, few of which are associated with nicotine biosynthesis . Plant Mol Biol. , 60 : 699 – 716 .
- Kloppholz , S , Kuhn , H and Requena , N . 2011 . A secreted fungal effector of Glomus intraradices promotes symbiotic biotrophy . Curr Biol. , 21 : 1204 – 1209 .
- Kosuta , S , Chabaud , M , Lougnon , G , Gough , C , Denarie , J , Barker , D and Becard , G . 2003 . A diffusible factor from arbuscular mycorrhizal fungi induces symbiosis-specific MtENOD11 expression in roots of Medicago truncatula . Plant Physiol. , 131 : 952 – 962 .
- Maillet , F , Poinsot , V , André , O , Puech-Pagès , V , Haouy , A , Gueunier , M , Cromer , L , Giraudet , D , Formey , D Niebel , A . 2011 . Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza . Nature. , 469 : 58 – 63 .
- Navazio , L , Moscatiello , R , Genre , A , Novero , M , Baldan , B , Bonfante , P and Mariani , P . 2007 . A diffusible signal from arbuscular mycorrhizal fungi elicits a transient cytosolic calcium elevation in host plant cells . Plant Physiol. , 144 : 673
- Oláh , B , Brière , C , Bécard , G , Dénarié , J and Gough , C . 2005 . Nod factors and a diffusible factor from arbuscular mycorrhizal fungi stimulate lateral root formation in Medicago truncatula via the DMI1/DMI2 signalling pathway . Plant J. , 44 : 195 – 207 .
- Olsson , PA and Johansen , A . 2000 . Lipid and fatty acid composition of hyphae and spores of arbuscular mycorrhizal fungi at different growth stages . Mycol Res. , 104 : 429 – 434 .
- Ramakers , C , Ruijter , JM , Deprez , RHL and Moorman , AFM . 2003 . Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data . Neurosci Lett. , 339 : 62 – 66 .
- Redecker , D , Kodner , R and Graham , LE . 2000 . Glomalean fungi from the Ordovician . Science. , 289 : 1920
- Schmidt , GW and Delaney , SK . 2010 . Stable internal reference genes for normalization of real-time RT-PCR in tobacco (Nicotiana tabacum) during development and abiotic stress . Mol Genet Genomics. , 283 : 233 – 241 .
- Shalel-Levanon , S , San , KY and Bennett , GN . 2005 . Effect of oxygen, and ArcA and FNR regulators on the expression of genes related to the electron transfer chain and the TCA cycle in Escherichia coli . Metab Eng. , 7 : 364 – 374 .
- Shibuya , N and Minami , E . 2001 . Oligosaccharide signalling for defence responses in plant . Physiol Mol Plant Pathol. , 59 : 223 – 233 .
- Shoji , T , Kajikawa , M and Hashimoto , T . 2010 . Clustered transcription factor genes regulate nicotine biosynthesis in tobacco . Plant Cell. , 22 : 3390 – 3409 .
- Siminszky , B , Gavilano , L , Bowen , SW and Dewey , RE . 2005 . Conversion of nicotine to nornicotine in Nicotiana tabacum is mediated by CYP82E4, a cytochrome P450 monooxygenase . Proc Natl Acad Sci USA. , 102 : 14919
- Smith , CJ . 1996 . Accumulation of phytoalexins: defence mechanism and stimulus response system . New Phytol. , 132 : 1 – 45 .
- Weidmann , S , Sanchez , L , Descombin , J , Chatagnier , O , Gianinazzi , S and Gianinazzi-Pearson , V . 2004 . Fungal elicitation of signal transduction-related plant genes precedes mycorrhiza establishment and requires the dmi3 gene in Medicago truncatula . Mol Plant Microbe Interact. , 17 : 1385 – 1393 .
- Yoshikawa , M , Yamaoka , N and Takeuchi , Y . 1993 . Elicitors: their significance and primary modes of action in the induction of plant defense reactions . Plant Cell Physiol. , 34 : 1163
- Zhao , J , Davis , LC and Verpoorte , R . 2005 . Elicitor signal transduction leading to production of plant secondary metabolites . Biotechnol Adv. , 23 : 283 – 333 .
- Ziegler , J and Facchini , P . 2008 . Alkaloid biosynthesis: metabolism and trafficking . Annu Rev Plant Biol. , 59 : 735