Abstract
Nitrogen availability is closely related to crop senescence and productivity, but its associated effect on reserve remobilization is not yet fully understood. In this study, we observed that nitrogen deficiency (N−) led to significant decreases in the activities of superoxide dismutase (SOD) (P<0.05), guaiacol peroxidase (P<0.05), and catalase (P<0.05) as well as a higher concentration of reactive oxygen species (ROS) (P<0.05) in wheat (Triticum aestivum L.) peduncles during the middle grain-filling compared with the application of 225 kg N ha−1 (N+). Callose concentration showed the same trend of temporal changes as ROS. Histochemical staining revealed that both ROS and callose predominantly occurred in vascular bundles of peduncles. Ultimately, grain filling rates and grain weight in N− wheat were reduced compared with N+ plant. These data suggest that the grain yield decline in N− wheat may be at least partially attributed to the higher callose deposition in peduncle vascular bundles and ROS level is closely associated with the increase in the callose deposition in wheat peduncle vascular bundles.
Introduction
Grain filling in cereal crops, with senescence as a typical trait, is a highly complicated process that is under genetic control as well as depends on environmental conditions and can be modified by fertilizer application (Bahrani et al. Citation2011). Specific changes in reactive oxygen species (ROS) may be an important signal for causing senescence and regulating grain-filling rate (Agüera et al. Citation2010). In Arabidopsis, increase of ROS concentration might play a role in modifying gene expression in response to the nitrogen (N) deprivation (Shin et al. Citation2005). In wheat, less availability of N during the post-anthesis period resulted in earlier plant senescence and a decrease in grain yield (Bogard et al. Citation2011). The proper availability of soil N and higher amount of N accumulated pre-anthesis in vegetable organs is highly associated with the higher translocation rates of N to grains (Barbottin et al. Citation2003; Bahrani et al. Citation2011).
Peduncle, the first internode directly below the ear, has a diversity of important roles in wheat growth and productivity. In a recent study, we found that the exposed peduncle has higher stomatal density and higher activity of heat-tolerant PEPCase over the flag leaf, possesses anatomical, ultrastructural, and physiological advantages for performing photosynthesis, and has a superior ability to adapt to the ecological environment at the later stages of grain filling. Thus, the peduncle makes a crucial contribution to grain growth (Kong et al. Citation2010). Because vascular system is highly developed in peduncle and essential for transporting assimilates to the filling grain (Wardlaw Citation1990), peduncle has, therefore, been thought of as a key transporting organ controlling the assimilate remobilization.
Callose, a β-1,3-linked homopolymer of glucose that contains some β-1,6-branches, is known to be involved in various biological processes in plants. For example, callose is temporarily deposited at cell plates during cytokinesis and the reversible accumulation of callose at the neck region of plasmodesmata (Pd) is involved in regulating Pd permeability to macromolecules (Northcote et al. Citation1989; Thiele et al. Citation2009). Besides these developmental processes, callose deposition is induced by various biotic and abiotic stresses, including metal exposure, pathogen attack, and wounding (Iwano et al. Citation2002; Jacobs et al. Citation2003; Vellosillo et al. Citation2010; Luna et al. Citation2011; Zavaliev et al. Citation2011).
Despite the widespread deposition of callose in plants, little is known about the regulations of callose occurrence in stem tissues and callose's functions in modulating the reserve remobilization. The objective of this study was to investigate whether ROS is involved in the assimilate remobilization via regulation of the callose deposition in peduncle vascular bundles in wheat under N deficient stress.
Materials and methods
Plant material
Winter wheat (Triticum aestivum L.) of cultivar Jimai 22, that is currently used in local production, was sown on 7 October 2010 at an experimental station (36°42′ N, 117°4′ E; altitude 48 m) at the Shandong Academy of Agricultural Sciences, China.
The soil type of experimental site was classified as sandy loam (pH 6.6). The top 40 cm of soil contains 0.98% organic matter, 75.6 kg ha−1 of water-hydrolysable N, 38.0 mg kg−1 of rapidly available phosphorous, and 87.3 mg kg−1 of rapidly available potassium. Prior to tillage, 120 kg P2O5 ha−1 and 112.5 kg K2O ha−1 were applied to all plots with the maize (the previous crop) residue retention. The top soil of 18 cm was tilled twice with a rotary tiller. The plots were 4 m wide and 50 m long. Wheat was sown at 330 grains m−2 with 20-cm spacing and supplied with 0 kg N ha−1 (N−) and total of 225 kg N ha−1 (N+). For N+ treatment, N was applied as urea at planting, Zadoks 31, and Zadoks 41 of growth stage (75 kg N ha −1 each stage) (Zadoks et al. Citation1974). The topdressing of N fertilizers was surface supplied on 20-cm spacing and immediately incorporated with irrigations. These treatments were arranged in a randomized complete block design with four replications.
Enzyme extraction and activity assay
Exposed peduncles (2−3 cm beneath the ear) were harvested from more than 20 plants per replicate, cut into segments, and mixed. About 0.5 g FW of these segments was ground into a fine power with a mortar and pestle containing liquid nitrogen. The powder was transferred into 5 ml of extraction buffer [100 mM potassium phosphate, pH 7.5, containing 5 mM dithiothreitol, 0.5 mM ethylenediaminetetraacetic acid (EDTA), and 2% (w/v) soluble polyvinylpyrrolidone (PVP-40)]. After centrifugation at 12,000 g for 20 min at 4°C, the supernatant was used to determine enzyme activities.
Superoxide dismutase (SOD) (EC 1.15.1.1) activity was assayed as described by Giannopolitis and Ries (Citation1977) with slight modifications. A 3-ml reaction mixture consisted of 50 µM nitroblue tetrazolium (NBT), 13 mM methionine, 75 µM NBT chloride, 0.1 mM EDTA, 50 mM potassium phosphate buffered solution (PBS; pH 7.8), 100 µl enzyme extract, and 1.3 µM riboflavin was irradiated under a light bank (15-W fluorescent lamps) for 15 min. The absorbances of the irradiated and nonirradiated solutions were determined at 560 nm using an UV/Vis spectrophotometer (Beckman DU 800, USA). One unit of SOD activity was defined as the amount of enzyme required to cause 50% inhibition of NBT photoreduction.
Guaiacol peroxidase (POD) (EC 1.11.1.7) was evaluated by the method described by Chance and Maehly (Citation1955). The reaction was triggered by the addition of 2.75 mM H2O2 to the solution containing 50 mM PBS (pH 6.4), 8 mM guaiacol, and 100–200 µl enzyme extract. Absorbance of the reaction mixture was recorded at 470 nm 180 s after H2O2 were added. One unit of POD activity was defined as the amount of enzyme that could produce 1 µmol tetraguaiacol per min per mg of total protein (extinction coefficient is 26.6 mM−1 cm−1 at 470 nm).
The enzymatic activity of catalase (CAT; EC 1.11.1.6) was assayed by following the initial rate of H2O2 degradation (Aebi Citation1984). A 3-ml reaction mixture contained 100 mM PBS (pH 7.0), 10 mM H2O2, and 100 µl of enzyme extract. Enzyme activity was assayed by monitoring the decrease in absorbance at 240 nm as a consequence of H2O2 consumption. The enzyme activity was calculated using the extinction coefficient (0.036 mM−1 cm−1) for H2O2. One unit of CAT specific activity was defined as the amount to decompose 1 µmol of H2O2 per min per mg of total protein.
Soluble protein concentration assay
The water soluble protein concentration was determined by Bradford (Citation1976) assay using bovine serum albumin (BSA) as a standard.
Determination of ROS and histochemical staining
Reactive oxygen species (ROS) levels were determined by using 2′,7′-Dichlorofluorescein diacetate (DCFH-DA) (Sigma-Aldrich, Germany). A 25 mM solution was prepared in dimethyl sulphoxide and was kept at −20°C for pending use. Cross sections cut from the peduncles were washed in 50 mM methyl ethanesulfonate buffer (pH 6.2), and transferred to 200 µl of fresh buffer in small wells of ELISA plates containing 10 µM of DCFH-DA. Following incubation for 20 min at 25°C, the fluorescence was measured with an excitation wavelength at 485 nm and emission wavelength at 535 nm, using an ELISA plate reader (GENios Pro, Tecan, Switzerland). Changes in absorbance were measured every 10 min until 60 min after the incubation.
The production of ROS in the wheat peduncle tissue was detected using the ROS-sensitive fluorochrome DCFH-DA as described by Tyburski et al. (Citation2009). Segments of peduncles (3 mm in length) were immersed in 20 mM PBS (pH 6.8) containing 25 µM DCFH-DA and vacuum-infiltrated. After staining, the samples were rinsed with fresh buffer to remove excess fluorophore and were then mounted on microscopic slides. Green fluorescence was observed under a microscope (excitation 495 nm and emission 518 nm) (Leica DM 2500; Microsystems, Wetzlar, Germany) and the images were captured by a Leica DFC 420 CCD camera. Experiments were performed with 4–5 peduncles from different plants per replicate in each experiment.
Callose extraction and assay
Exposed peduncles (2–3 cm beneath the ear) were harvested from more than 20 plants per replicate, cut into segments, and mixed. About 0.5 g FW of these segments was washed with deionized water and then fixed in 96% (v/v) ethanol for 60 min. Fixed samples were ground with a mortar and pestle containing liquid nitrogen. After centrifugation at 12,000 g for 20 min, the supernatant was discarded and the pellet was dissolved in 400 µl of 1 M NaOH and incubated at 80°C for 30 min to solubilize the callose. After centrifugation at room temperature at 12,000 g for 20 min, the supernatant was used to callose determination.
Callose was determined by addition of 200 µl of the callose extraction and 200 µl of 0.1% (w/v) aniline blue (pH 8.2) as described by Voigt et al. (Citation2006). After incubation at 50°C for 30 min and subsequently at room temperature for 30 min under shaking conditions, fluorescence was quantified with a fluorescence spectrophotometer (F96S; Shanghai, China) at an excitation wavelength of 400 nm and an emission wavelength of 485 nm. Standard curves were generated with β-1, 3-glucan from Euglena gracilis (Sigma-Aldrich, Germany). All measurements were repeated twice with four replicates each. Amounts of callose were expressed as E. gracilis glucan-equivalents.
Histochemical staining to detect callose deposition
Freshly cut tissue of peduncles was stained with 0.1% (w/v) decolorized aniline blue prepared with 100 mM PBS (pH 8.2) for approximately 2 min. The cross sections were then immediately mounted and viewed in a fluorescent microscope using a UV filter (Leica DM 2500; Microsystems, Wetzlar, Germany). Digital images were captured using a Leica DFC 420 CDD camera.
H2O2 treatment
For H2O2 treatment, N+ plants were sprayed with 20 mM H2O2 at 16 days after anthesis (DAA), with those sprayed with distilled water as controls. The callose concentration in wheat peduncles was determined at 1, 2, 3, 4, 5, and 6 days after H2O2 treatment (DAHT). Anthesis was scored when yellow anthers were visible on 50% of the ears, corresponding to 1100 degree days after sowing.
Number of fertile tillers, grain number, thousand kernel, and grain weight
Three samples of 1-m length row were randomly selected from each plot for measuring the number of fertile tillers. For grain weight, ears were harvested from the N+ and N− wheat plants at eight-day intervals after anthesis (in our experiment wheat flowered at the same time under high and low N conditions) and were then oven dried at 60°C for 48 h to a constant mass and manually threshed. Grains were weighed and the data were averaged from five replicates, each with 30 ears. The ears sampled at 32 DAA were also used to calculate the grain number per ear and 1000-grain weight for both N level treatments.
Statistical analysis
All of the data were subjected to an analysis of variance (ANOVA) using DPS statistical software (v.7.55; Refine Information Tech. Co., Ltd., Hangzhou, Zhejiang, China). The data are presented as the mean±SD. The significance of differences between mean values was determined with a t-test. Differences at P<0.05 were considered significant. The relationship between ROS and callose concentration was evaluated using correlation analyses.
Results
Antioxidant enzymes
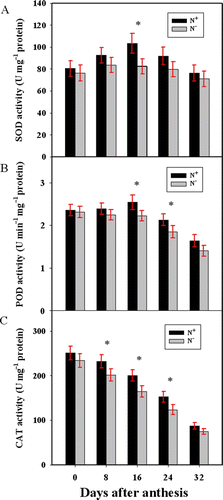
Under both N+ and N− conditions, the activity of SOD isolated from peduncle gradually increased after anthesis, reached a peak at 16 DAA, and then declined until 32 DAA. Although the SOD activity is consistently higher in N+ than in N− plants throughout grain-filling, a significant difference in SOD activity was only observed at 16 DAA (P<0.05) (A). Different from that in N− plants, where the activity of POD isolated from peduncle gradually decreased after anthesis, POD activity in N+ peduncle increased after anthesis and reached a peak at 16 DAA. As a consequence, significant differences in the activity were observed between 16 and 24 DAA (P<0.05) (B). The activity of CAT isolated from peduncle declined with grain filling over both N treatments. However, CAT activity was constantly higher during grain filling and significantly higher at 8, 16, and 24 DAA in N+ than in N− plants (P <0.05) (C).
ROS relative concentration
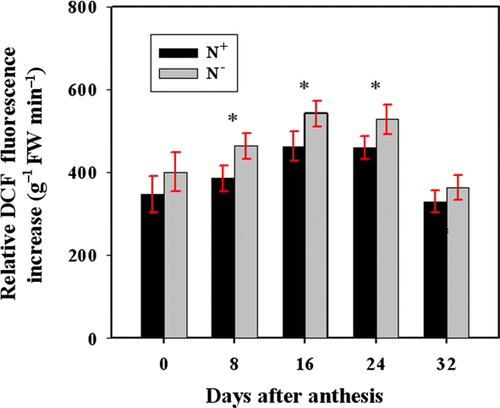
Reactive oxygen species (ROS) relative concentration was evaluated by measuring the fluorescence arising from oxidation of DCFH-DA occurred in both N− and N+ plants. It was found that the ROS relative concentration was constantly higher in N− peduncles than in N+ peduncles (). However, significant difference was only observed at 8–24 DAA.
Figure 2. Comparison of the relative DCFH-DA fluorescence intensity in the N− and N+ peduncles. ROS concentration was measured using DCFH-DA that is oxidized by ROS to DCF. Fluorescence was determined every 10 min during 20−60 min after the incubation of peduncle cross sections with DCFH-DA. Bars represent mean±SD of four replicates. For the ANOVA, *denotes significant effects at P<0.05.
Callose concentration
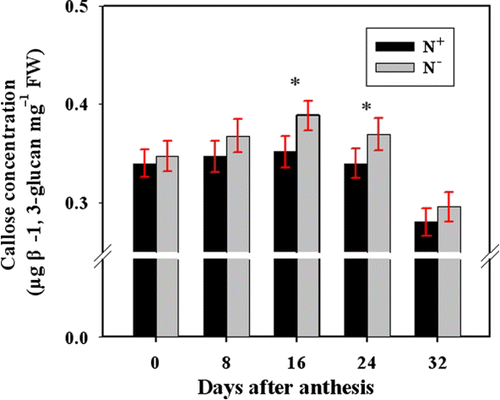
In general, callose concentration in N− peduncles was higher than in N+ peduncles throughout grain filling (). A significant difference was observed at 16 and 24 DAA. These data suggest that N deficiency increased callose deposition in peduncles.
Relationship between ROS and callose
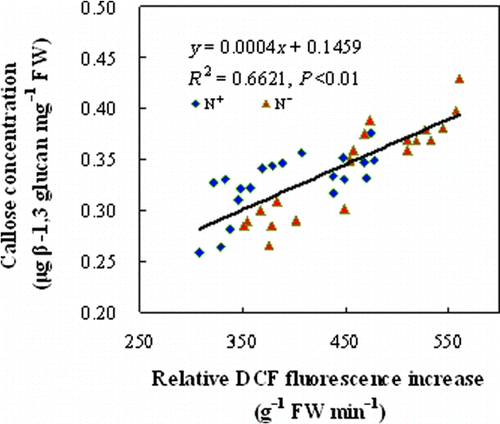
Regression analysis based on the above data showed that the callose concentration was positively related to ROS concentration in peduncle (). More evidence was obtained by using histochemical staining and exogenous H2O2 treatment. Staining with DCFH-DA for ROS in segments of wheat peduncles revealed that high amounts of ROS were mainly localized in vascular bundles (A). Interestingly, callose deposition, visualized by an intense yellow-green fluorescence under UV light after staining with aniline blue, were also preferentially found in vascular bundles in the cross sections of wheat peduncles (B). The treatment of N+ wheat with 20 mM H2O2 led to an increase in callose concentration in N+ peduncle throughout 1–6 DAHT, with a most significant increase observed 4 DAHT (0.349 vs. 0.377 µg β-1, 3-glucan mg−1 FW) (P<0.05), indicating that the higher callose deposition was induced upon H2O2 treatment.
Grain yield components
Fertile tillers and 1000-grain weight for N+ wheat were significantly higher than N− treatment. However, no difference in grain number per ear was observed between N+ and N− treatments (). As can be seen from these data, the difference in grain yield per unit area between two N treatments was less significant than expected. This may be due to that fertilizer N for 225 kg ha−1 application was not efficiently valorized to gain.
Figure 5. Microscope images of ROS accumulation and callose depositions in wheat peduncles at 16 DAA. (A) ROS in the vascular bundles of wheat peduncles as detected with the fluorescent probe DCFH-DA; images are representative of at least 15 replicate peduncles. The presence of callose is denoted by the green or yellowish green colors. (B) Selected picture representative spatial distribution of callose deposition at least 15 cross sections from different plants. Three millimeter long peduncle segment was taken in the 4±0.5 cm below the ear. The presence of callose is indicated by the bluish green color (white arrows). Bars in (A) and (B) represent 200 µm.
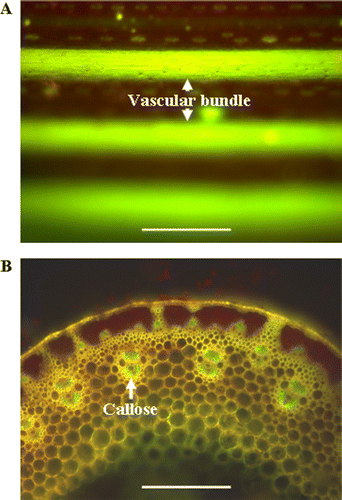
Table 1. Effects of N level on the number of fertile tillers, grain number per ear, and thousand-grain weight.
Temporal changes in peduncle protein concentration and ear grain filling rate
The water soluble protein concentration of peduncles was significantly lower in N− than in N+ wheat at 0 and 8 DAA. However, no difference was observed at 16 DAA and thereafter ().
Table 2. Ear grain weight (g ear−1) and peduncle soluble protein concentration (mg g−1 FW) during grain-filling at two levels of N treatments.
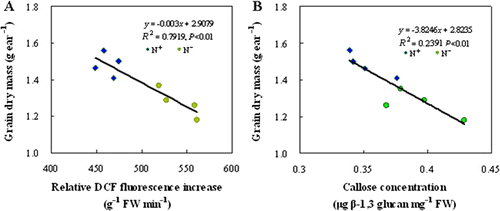
Compared with N+ treatment, ear grain filling rate in N− wheat was similar before 8 DAA but lower thereafter. As a consequence, grain weight for N− treatment was slightly lower at 16 DAA and significantly lower at 24 and 32 DAA than for N+ treatment (). Regression analysis indicated that final ear grain weight was negatively related to the ROS concentration (A) as well as to the callose concentration (B) in peduncles at 16 DAA.
Discussion
Superoxide dismutase (SOD), POD, and CAT are the most effective antioxidant enzymes, and their synergetic effects prevent plant cells from ROS oxidative damage (Gill and Tuteja Citation2010). In this study, we observed that the activities of these enzymes in N− wheat were reduced compared with N+ plants. The differences may be even higher if the activities of these antioxidant enzymes were expressed on a dry weight basis, because the peduncle of wheat grown under low N conditions showed much lower N concentrations (). Consequently, the ROS concentration in N− was higher than N+ wheat (). But it could not be excluded that the differences may be less apparent on a dry weight basis because water content is usually lower at low N. In Arabidopsis, nutrient deprivation caused an increase of ROS concentrations in specific regions of the root (Shin et al. Citation2005), affected the pattern of ROS accumulation in apical parts of roots (Tyburski et al. Citation2009), and triggered an increased production of H2O2 (Chao et al. Citation2012). In sunflower (Helianthus annuus L.), Agüera et al. (Citation2010) found that N deficiency resulted in a decline in the activities of antioxidant enzymes and an increase in H2O2 levels and hence accelerated the plant senescence. The authors postulated that systemic signals, such as a deficit in amino acids or other metabolites, are produced due to the plant N deprivation, and thus leading to a higher oxidation state of the cells and an early plant senescence.
Table 3. Effects of 20 mM H2O2 treatment on the callose concentration (µg β-1,3-glucan mg−1 FW) in N+ wheat.
In this study, we first observed that N deficiency induced a significant increase in callose deposition in the vascular bundles of wheat peduncles, although the difference may be lower on a dry weight basis. In addition, imaging showed that both ROS and callose were mainly produced at the same sites of peduncles (). Verma and Hong (Citation2001) and Bolwell et al. (Citation2002) reported that oxidative conditions induced callose deposition, probably by activating expression of callose synthases. Iwano et al. (Citation2002) showed that in Acidovorax avenae infected rice cells, callose synthesis occurred at the site of H2O2 generation. Exposure of roots to ROS or aluminum that induces ROS accumulation was closely correlated with the spatial patterns of callose deposition (Jones et al. Citation1998, Citation2006). Based on these data, we hypothesize that higher amount of ROS caused by N-deficient stress might at least partially contribute to the callose accumulation. Indeed, we observed subsequently that the ROS concentration was positively related to callose deposition in wheat peduncles.
Callose deposition at the neck region of Pd is one of the cellular control mechanisms regulating Pd permeability and high callose accumulation under either biotic or abiotic stresses restricts macromolecular trafficking (Northcote et al. Citation1989; Zavaliev et al. Citation2011). Recent studies have revealed that ROS metabolism or the cellular redox status is involved in callose regulation and thus in the regulation of Pd permeability for macromolecular trafficking (Benitez-Alfonso and Jackson Citation2009; Stonebloom et al. Citation2009; Benitez-Alfonso et al. Citation2011). The mechanism for those regulations is unclear and is of major interest (Zavaliev et al. Citation2011). In the present study, we found that a lower ear grain filling rate and a significant decrease in grain weight per ear in N-deficient wheat. Bahrani et al. (Citation2011) suggested that the plant retains an amount of N at anthesis that is essential for survival and various biological functions in ear grain filling, while the remainder is available for remobilization. In this study, we indeed observed that the peduncle protein concentration was similar between the two N treatments at 16, 24, and 32 DAA, although it was significantly lower at early stages of grain filling (). Thus, a lower portion of N is available for ear grain filling under N deficient stress.
Interestingly, we observed that ear grain weight is not lower but similar in N− than N+ wheat at 8 DAA. ROS are a common element in plant signal transduction cascades in response to nutrient deprivation (Shin et al. Citation2005) and senescence is needed for reserve remobilization from the vegetable organs to the grains in wheat (Bazargani et al. Citation2011) and sunflower (Helianthus annuus) (Agüera et al. Citation2010). Therefore, it is reasonable to postulate that a proper amount of ROS is needed for initiation of grain filling and increase the grain weight at the early stage of grain filling. However, it can not exclude its adverse action to cause the plant rapid senescence, cell death, and earlier cessation of grain filling. Given a high ROS concentration often causes an oxidative damage to plant cells (Agüera et al. Citation2010; Luna et al. Citation2011), a more effective protection against oxidative stress was needed to protect plant from earlier senescence (Bazargani et al. Citation2011).
In conclusion, our results suggest that N deficiency inhibits the activities of antioxidant enzymes and thus increases the ROS accumulation in wheat peduncles. These processes are closely associated with the callose deposition and spatial distribution in the peduncle vascular bundles. Therefore, it is possible that N deficiency could induce higher ROS production which may, in turn, promote callose accumulation in vascular bundles and hence restricts the grain-filling rate in wheat. Therefore, a proper amount of nitrogen fertilizers is needed for plant requirement of nutrients, increasing plant antioxidant capacity, and promoting cereal ear grain filling. This finding has an important implication for breeding programs on nitrogen use efficiency and guidance of nitrogen management in cereal crop production.
Acknowledgements
Funding for this research was provided by grants from Shandong and the National Earmarked Fund for Modern Agro-industry Technology Research System and from Special Fund for Agro-scientific Research on Public Causes, Minister of Agriculture, China.
References
- Aebi , H . 1984 . Catalase in vitro . Meth Enzymol. , 105 : 121 – 126 .
- Agüera , E , Cabello , P and de la Haba , P . 2010 . Induction of leaf senescence by low nitrogen nutrition in sunflower (Helianthus annuus) plants . Physiol Plant. , 138 : 256 – 267 .
- Bahrani , A , Abad , HHS and Aynehband , A . 2011 . Nitrogen remobilization in wheat as influenced by nitrogen application and post-anthesis water deficit during grain filling . Afr J Biotechnol. , 10 : 10585 – 10594 .
- Barbottin , A , Lecomte , C , Bouchard , C and Jeuffroy , MH . 2003 . Nitrogen remobilization during grain filling in wheat . Crop Sci. , 45 : 1141 – 1150 .
- Bazargani , MM , Sarhadi , E , Bushehri , AAS , Matros , A , Mock , HP , Naghavi , MR , Hajihoseini , V , Mardi , M , Hajirezaei , MR Moradi , F . 2011 . A proteomics view on the role of drought-induced senescence and oxidative stress defense in enhanced stem reserves remobilization in wheat . J Proteomics. , 74 : 1959 – 1973 .
- Benitez-Alfonso , Y and Jackson , D . 2009 . Redox homeostasis regulates plasmodesmal communication in Arabidopsis meristems . Plant Signal Behav. , 4 : 655 – 659 .
- Benitez-Alfonso , Y , Jackson , D and Maule , A . 2011 . Redox regulation of intercellular transport . Protoplasma. , 248 : 131 – 140 .
- Bogard , M , Jourdan , M , Allard , V , Martre , P , Perretant , MR , Ravel , C , Heumez , E , Orford , S , Snape , J Griffiths , S . 2011 . Anthesis date mainly explained correlations between post-anthesis leaf senescence, grain yield, and grain protein concentration in a winter wheat population segregating for flowering time QTLs . J Exp Bot. , 62 ( 10 ) : 3621 – 3636 .
- Bolwell , GP , Bindschedler , LV , Blee , KA , Butt , VS , Davies , DR , Gardner , SL , Gerrish , C and Minibayeva , F . 2002 . The apoplastic oxidative burst in response to biotic stress in plants: a three-component system . J Exp Bot. , 53 ( 372 ) : 1367 – 1376 .
- Bradford , MM . 1976 . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding . Anal Biochem. , 72 : 248 – 254 .
- Chance , B and Maehly , C . 1955 . Assay of catalase and peroxidases . Meth Enzymol. , 11 : 764 – 775 .
- Chao , YY , Chou , TS and Kao , CH . 2012 . Involvement of abscisic acid and hydrogen peroxide in regulating the activities of antioxidant enzymes in leaves of rice seedlings under magnesium deficiency . Plant Growth Regul. , 66 : 1 – 8 .
- Giannopolitis , CN and Ries , SK . 1977 . Superoxide dismutases: I. Occurrence in higher plants . Plant Physiol. , 59 : 309 – 314 .
- Gill , SS and Tuteja , N . 2010 . Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants . Plant Physiol Biochem. , 48 : 909 – 930 .
- Iwano , M , Che , FS , Goto , K , Tanaka , N , Takayama , S and Isogai , A . 2002 . Electron microscopic analysis of the H2O2 accumulation preceding hypersensitive cell death induced by an incompatible strain of Pseudomonas avenae in cultured rice cells . Mol Plant Pathol. , 3 : 1 – 8 .
- Jacobs , AK , Lipka , V , Burton , RA , Panstruga , R , Strizhov , N , Schulze-Lefert , P and Fincher , GB . 2003 . An Arabidopsis callose synthase, GSL5, is required for wound and papillary callose formation . Plant Cell. , 15 ( 11 ) : 2503 – 2513 .
- Jones , DL , Blancaflor , EB , Kochian , LV and Gilroy , S . 2006 . Spatial coordination of aluminium uptake, production of reactive oxygen species, callose production and wall rigidification in maize roots . Plant Cell Environ. , 29 : 1309 – 1318 .
- Jones , DL , Kochian , LV and Gilroy , S . 1998 . Aluminum induces a decrease in cytosolic calcium concentration in BY-2 tobacco cell cultures . Plant Physiol. , 116 : 81 – 89 .
- Kong , LA , Wang , FH , Feng , B , Li , SD , Si , JS and Zhang , B . 2010 . The structural and photosynthetic characteristics of the exposed peduncle of wheat (Triticum aestivum L.): an important photosynthate source for grain-filling . BMC Plant Biol. , 10 : 141
- Luna , E , Pastor , V , Robert , J , Flors , V , Mauch-Mani , B and Ton , J . 2011 . Callose deposition: a multifaceted plant defense response . Mol Plant Microbe Interact. , 24 : 183 – 193 .
- Northcote , DH , Davey , R and Lay , J . 1989 . Use of antisera to localize callose, xylan and arabinogalactan in the cell-plate, primary and secondary cell walls of plant cells . Planta. , 178 : 353 – 366 .
- Shin , R , Berg , RH and Schachtman , DP . 2005 . Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency . Plant Cell Physiol. , 46 ( 8 ) : 1350 – 1357 .
- Stonebloom , S , Burch-Smith , T , Kim , I , Meinke , D , Mindrinos , M and Zambryski , P . 2009 . Loss of the plant DEAD-box protein ISE1 leads to defective mitochondria and increased cell-to-cell transport via plasmodesmata . Proc Natl Acad Sci USA. , 106 ( 40 ) : 17229 – 17234 .
- Thiele , K , Wanner , G , Kindzierski , V , Jurgens , G , Mayer , U , Pachl , F and Assaad , FF . 2009 . The timely deposition of callose is essential for cytokinesis in Arabidopsis . Plant J. , 58 : 13 – 26 .
- Tyburski , J , Dunajska , K and Tretyn , A . 2009 . Reactive oxygen species localization in roots of Arabidopsis thaliana seedlings grown under phosphate deficiency . Plant Growth Regul. , 59 : 27 – 36 .
- Vellosillo , T , Vicente , J , Kulasekaran , S , Hamberg , M and Castresana , C . 2010 . Emerging complexity in reactive oxygen species production and signaling during the response of plants to pathogens . Plant Physiol. , 154 : 444 – 448 .
- Verma , DP and Hong , Z . 2001 . Plant callose synthase complexes . Plant Mol Biol. , 47 : 693 – 701 .
- Voigt , CA , Schäfer , W and Salomon , S . 2006 . A comprehensive view on organ-specific callose synthesis in wheat (Triticum aestivum L.): glucan synthase-like gene expression, callose synthase activity, callose quantification and deposition . Plant Physiol Biochem. , 44 : 242 – 247 .
- Wardlaw , IF . 1990 . The control of carbon partitioning in plants . New Phytol. , 116 ( 3 ) : 341 – 381 .
- Zadoks , JC , Chang , TT and Konzak , CF . 1974 . A decimal code for the growth stages of cereals . Weed Res. , 14 : 415 – 421 .
- Zavaliev , R , Ueki , S , Epel , BL and Citovsky , V . 2011 . Biology of callose (β-1,3-glucan) turnover at plasmodesmata . Protoplasma. , 248 : 117 – 130 .