Abstract
The study highlights the role of sulfur (S) in detoxification of arsenate-induced toxicity and the shift in essential element homeostasis in Zea mays L (SRHM 445). Overall growth of arsenate-treated plants under sulfur starvation (−S) was lower than that in the presence of excess sulfur (+S). Translocation of arsenate from roots to shoots, increased under As(−S) and decreased with As(+S). The level of micronutrients (Cu, Zn, Fe) increased in As(−S) plants. Whereas, the level of K and PO4 was higher in As(−S) plants than in As(+S) plants. Higher malondialdehyde, protein carbonyl, and H2O2 levels in As(−S) plants are indicative of higher oxidative stress. Higher superoxide dismutase (SOD) and ascorbate peroxidase (APX) activities, in As(−S) plants coincided with higher H2O2 levels showing the activity of these enzymes are independent of S availability. Absence of reduced glutathione/oxidized glutathione pool in (−S) plants manifested into failure of ascorbate–glutathione detoxification pathway. Hence, S has dual role of protecting the plant against arsenate-induced toxicity (1) by restricting arsenic (As) translocation to the upper parts and (2) by increasing the activity SOD and APX.
1. Introduction
Sulfur (S) is an essential plant nutrient which ranges between 0.1 and 6% of the dry weight. Besides just being a nutrient, S-containing compounds are also responsible for numerous aspects of crop quality and natural resistance in the plants (De Kok et al. Citation2002). It is also required for the synthesis of glutathione (γGlu-Cys-Gly; GSH), which serves as the major reservoir for nonprotein thiol (NPSH) and also the precursor for phytochelatin (PC) synthesis. Both, PC and reduced glutathione (GSH) are essential for the plants to counteroxidative stress. GSH in Poaceae plant tissue constitutes 1–2% of the total sulfate (Grill et al. Citation2001).
Sulfur deficiency in agricultural soil is increasing due to (1) reduced SO2 atmospheric deposition as a result of strict emission norms and (2) use of low-S fertilizers or declining use of S-containing fungicides under new agronomic practices (McGrath & Zhao Citation1995). Besides, causing loss of plant production, fitness, and resistance to environmental stress and pests, S-deficiency also results into alteration of essential elements composition in plants, the growth and defense mechanisms. Lower Fe in maize and tomato leaves has been reported when grown under S-deficiency (Astolfi et al. Citation2003; Bouranis et al. Citation2003; Zuchi et al. Citation2009). A decrease in Fe and Cu and an increase in Mn concentration in Mulberry (Morus alba L.) leaves grown under low-sulfur condition have been observed by Tewari et al. (Citation2010).
The role of S in heavy metal-induced stress in various plants has been widely investigated. Nocito et al. (Citation2006) showed the abundance of S in the roots increases due to increased expression of S transporters in Zea mays exposed to various heavy metals namely Cd, Cu, Se, and Zn while Tu and Ma (Citation2005) investigated the effects of arsenic (As) on macro (P, K, Ca, and Mg) and micro (Fe, Mn, Cu, Zn, B, and Mo) nutrient status of Pteris vittata. Astolfi et al. (Citation2004) studied the interactions between S nutrition and Cd exposure in Z. mays L. Recently, Zhang et al. (Citation2010) reported that rice plants pretreated with low amounts of S, accumulated less As(III) than those with higher amounts. As(III) level in the shoots of low S pretreated plants were higher than in high S pretreated rice. Srivastava and D'Souza (Citation2009) exposed Hydrilla verticillata to both As(III) and As(V) for 1 d and observed the accumulation of As was twofolds higher in excess S-treated plants than normal + S and − S plants. The excess S plants with As treatment also exhibited significant stimulation of thiol (SH) metabolism. Cao et al. (Citation2004) observed increase (p<0.05) in GSH level in the mature fronds of P. vittata with increase of As levels in the soil. A brief review of all these studies reveals that the elemental level of crop plant under heavy metal(loid) stress in S deficient condition have not been studied in detail.
A large extent of agricultural land in the Indo-Gangetic floodplain (West Bengal, Bihar, Assam, Chhattisgarh, eastern Uttar Pradesh) and deltaic regions of India remains contaminated by As (Chauhan et al. Citation2009), ranging between 9 and 105 mg kg−1 in Chhattisgarh (Patel et al. Citation2005) and 3.34–31.6 mg kg−1 in West Bengal (Roychowdhury et al. Citation2002) with mean of 10.7 mg kg−1. Z. mays L. is a major crop grown both for cash and food in the Indian subcontinent (Mallick et al. Citation2010) and a large extent of maize growing area in India falls within the identified As contaminated zones.
In view of the above and also due to the fact that fewer studies have been undertaken to identify the role of S in the defense mechanism against As-induced toxicity in maize and the shift in elemental homeostasis due to As-induced stress, the present study is carried out to understand the role of S in the detoxification mechanism of Z. mays L. (cv. SRHM 445) under As-induced toxicity and the overall effect on the growth and nutrient uptake. Such knowledge may lead to mitigation of As hazards in crops grown on As contaminated soils.
2. Materials and methods
2.1. Experimental setup and design
Seeds of Z. mays L. var. Ruchi (cv. SRHM 445) were procured from Sri Rama Agri-Genetics (India) Pvt. Ltd., Kurnool, Andhra Pradesh. The disinfected seeds of maize were germinated in plastic trays, placed between blotting sheets moistened by modified 30% Hoagland's nutrient solution (HNS) supplementing with 5 µg ml−1 of Fe-EDTA (Mallick et al. Citation2010). Alternatively, HNS lacking S was prepared by replacing the sulfate salts, i.e. CuSO4, MgSO4.7H2O, MnSO4.7H2O, and ZnSO4.7H2O with equimolar CuCl2, MgCl2.6H2O, MnCl2.4H2O, respectively (Srivastava & D'Souza Citation2009; Tewari et al. Citation2010). After 7 d of growth, four uniform size seedlings (6–8 cm) were transferred to glass beaker (250 ml) containing 100 ml of 30% HNS. The plants were placed in the beaker allowing only the roots to remain submerged in the nutrient solution and the aerial parts of the plants were erect and exposed to light. Under such conditions, the plants were allowed to grow for another 7 d for normalization. The glass beakers were covered with black coating in order to restrict the roots from exposure to light and this condition was maintained throughout the experiment. The experiment consisted of two controls, i.e. control in nutrient medium containing sulfur (C) and control free of sulfate in nutrient medium [C(−S)], in order to differentiate the changes in the plants due to S deficiency or stress due to the presence of chloride. After 7 d of normalization, the plants were exposed to two concentrations of As, i.e. 9 µg ml−1 (0.12 mM) and 12 µg ml−1 (0.16 mM) both in S containing 30% HNS [As(9)(+S) and As(12)(+S), respectively] and S free 30% HNS [As(9)(−S) and As(12)(−S), respectively]. The choice of the concentration of As(V) was based on our earlier study (Mallick et al. Citation2011), where application 12 µg ml−1 of As(V) has caused significant toxic symptoms not suitable for such study. Another set of the plants was exposed to the same two concentrations of As in 30% HNS containing 1 mM of glutathione (GSH), i.e. As(9)(GSH) and As(12)(GSH), respectively, in order to assess the changes in the plant due to excess S supply. Salt of sodium arsenate (NaHAsO4.7H2O) was used to prepare all the As treatments. The pH of the HNS and treatment solutions ranged between 6.8 and 7.0. The treatments were applied after 7 d of normalization after transferring the plants to the glass beakers. During the entire experiment the nutrient solution were replaced after every second day. Plants were harvested for analysis after 7 d (first harvest) and 14 d (second harvest) of treatment. The plants have been particularly exposed to As for longer period (7 and 14 d) in order to allow the plants to regain its nutritional homeostasis preceding the initial shock due to As. All exposure and treatments were carried out in four replicates using random block design.
2.2. Growth parameters and biochemical analysis
Chlorophyll content in the fresh leaves of the plant was determined according to Arnon (Citation1949). Carotenoid content was determined using Duxbury and Yentsch (Citation1956). Protein content in the leaves of the plants was measured (Lowry et al. Citation1951) using bovine serum albumin as a standard. The level of lipid peroxidation was measured in leaves in terms of malondialdehyde (MDA) content using the thiobarbituric acid reaction (Heath & Packer Citation1968). NPSH content was measured following the method by Ellman (Citation1959). Protein oxidation was measure as total carbonyl content in the leaves using Castegna et al. (Citation2003). H2O2 level in the leaves was determined colorimetrically following Velikova et al. (Citation2000) using H2O2 as standard.
2.3. Enzyme extraction and assays
Fresh leaves were used for deriving plant extract following Mallick et al. (Citation2010). Superoxide dismutase (SOD) (EC 1.15.1.1) activity was measured spectrophotometrically at 560 nm following Beauchamp and Fridovich (Citation1971) and presented as U mg−1 protein. About 1 U of SOD activity is the amount of protein required to inhibit 50% initial reduction of nitro-blue tetrazolium under light. The activity of ascorbate peroxidase (APX) (EC 1.11.1.11) was measured by estimating the rate of ascorbate oxidation (ϵ=2.8 mM−1 cm−1 following Nakano and Asada Citation1981) and the enzyme activity was expressed as µmoles of ascorbate oxidized min−1 mg−1 protein. Catalase (CAT) (EC 1.11.1.6) by Scandalios et al. (Citation1983) and the enzyme activity was expressed as mM min−1 mg−1 protein. Guaiacol peroxidase (POD) (EC 1.11.1.7) activity was assayed at 470 nm following Kato and Shimizu (Citation1987) using ϵ=26.6 mM−1 cm−1 and expressed as µmoles of guaiacol oxidized min−1 mg−1 protein. Glutathione reductase (GR) (EC 1.6.4.2) activity was assayed following Smith et al. (Citation1988) and represented as U mg−1 protein, where 1 U is conversion of 1 mM of oxidized glutathione (GSSG) min−1 to GSH. Ascorbate oxidase (AO) (EC 1.10.3.3) activity was assayed following Oberbacher and Vines (Citation1963) and represented as U mg−1 protein, where 1 U is oxidation of 1 µmol of the ascorbic acid (AsA) min−1.
2.4. Native polyacrylamide gel electrophoresis (PAGE) and activity stain
The enzyme extracts were used for loading in the gels. Protein content was estimated through Lowry et al. (Citation1951) and 120 µg of protein was loaded in the wells for all the gels. PAGE was performed at 4°C, 180 V, following Laemmli (Citation1970). Ten percent of PAG was used for resolving and 4% for stacking, omitting SDS form the PAG ingredients. Activity stain for APX was performed as in Mittler and Zilinskas (Citation1993). SOD activity was determined according to Beauchamp and Fridovich (Citation1971). The localization of different isoforms of SOD was done on the basis of the methodology adopted by Chakrabarty et al. (Citation2007). POD activity was visualized according to Fielding and Hall (Citation1978). After electrophoresis, the gels were incubated in 25 mM potassium phosphate buffer (pH 8.0) for 15 min (POD basic), henceforth, the gels were submerged in freshly prepared solution containing 18 mM guaiacol and 25 mM H2O2 in 25 mM K-phosphate buffer (pH 5.0) (POD acidic) and till the POD containing bands appear.
2.5. Elemental and radical analysis
Plant tissues (leaf ~500 mg and root ~300 mg) were digested following Mallick et al. (Citation2010) where roots of the plants were washed thrice with 4°C distilled water to remove the adsorbed elements before drying. The metal contents (Fe, Cu, Zn, Mn, Na, P, and Ca) were determined (µg mL−1) using atomic absorption spectrophotometer (AAS) (GBC Avanta Σ) and As were analyzed (µg L−1) using Perkin Elmer, AAnalyst 600 (AAS) fitted with a graphite furnace. Phosphate content in the tissue was analyzed colorimetrically (690 nm) using ammonium molybdate and SnCl2 from a suitable aliquot of the digest (Gilbert Citation1926).
2.6. Statistical analysis
The whole experiment was setup in the randomized block design. The data were subjected to Duncan's Multiple Range Test for the analysis of significant difference (p<0.05) between the treatments. All the percentages of increase and decrease are calculated as compared to their respective controls, As(+S) and As(GSH) treatments with C(+S) and As(−S) treatments with C(−S), or otherwise mentioned.
2.7. Analytical quality control
Analytical data quality of the elements was ensured through repeated analysis (n=6) of Environmental Protection Agency, USA, quality control samples and the results were found to be within±3.15% of certified values. For plants, recoveries of metal from the plant tissues were found to be 99% as determined by digesting four samples each from untreated plant with known amount of metal. The blanks were run all the time.
3. Results
3.1. Metalloid and nutrient accumulation
Complying with the general trend, metal(loid) accumulations in the roots were higher than in the leaves ( and ), except for Mn, where the reverse situation was observed (C). The accumulation of As and other elements in the plant leaves was studied under (−S), (+S), and (+GSH) treatments at two dosages of As(V) (9 and 12 µg ml−1) and for two periods of exposure (7 and 14 d). In all the As treatments described above, the accumulation of As increased significantly (p<0.05). The accumulation of As in (+S) condition also showed an increase in all the treatments except where high As (12 µg ml−1) was used with (+1 mM GSH). After 7 d, comparison of the levels of As accumulation among the plants receiving lower rate of As (9 µg ml−1), i.e. As(9)(+S), As(9)(−S), and As(9)(GSH), showed that they increased 356.5 and 328.6% in As(9)(−S) and As(9)GSH leaves, respectively, as compared to As(9)(+S). Whereas, the As accumulation in the leaves of plants receiving higher rate of As (12 µg ml−1) and deprived of S [As(12)(−S)], increased 212.1 and 386% as compared to that in the plants receiving As(+S), i.e. As(12)(+S) and As(12)(GSH), respectively. Similar trend was also observed after 14 d. In roots, overall As accumulation was significantly less in As(−S) than As(+S) plants. In As(9)(−S) plants, it decreased 50.7 and 49.0%, after 7 and 14 d, respectively, as compared to As(9)(+S) (B). Similarly, in As(12)(−S) plants it decreased 19.2 and 62.7% after 7 and 14 d, respectively, as compared to As(12)(+S). On the contrary, not much difference was observed between As(+S) and As(GSH).
Figure 1. Levels of As (µg g−1 dw) in the leaves (A) and roots (B), Cu in the leaves (C) and roots (D), Zn in the leaves (E) and roots (F) of Zea mays treated with arsenate and different levels of sulfur. All values are mean of four replicates±SD. Bars marked with same letters are not significantly different (Duncan's test, p<0.05).
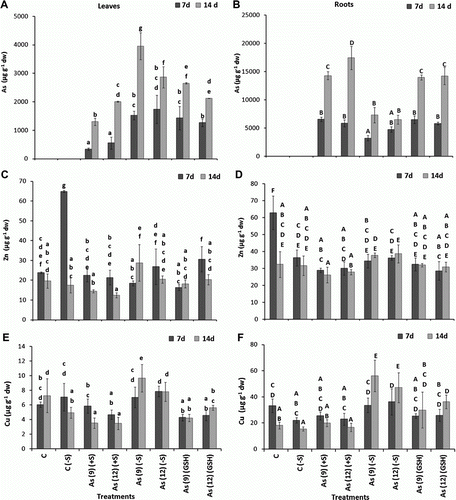
Among essential elements, Zn levels in the leaves decreased in As(+S) plants after both 7 and 14 d, as compared to C(+S), while, no clear trend was observed in As(−S) plants. Although significant difference was observed between the Zn levels of C(−S), As(9)(−S), and As(12)(−S) leaves after 7 d, however, no definite pattern was observed (C,D). While, in roots no significant difference in Zn levels was observed between the treatments. Cu level in the leaves decreased when As was applied with sulfate (+S), whereas, it increased with S starvation (−S) both in leaves and roots. Compared to C(+S), Cu level decreased (p<0.05) 51.65 and 52.5% in As(9)(+S) and As(12)(+S), respectively, after 14 d. On the contrary, in comparison to C(−S) the uptake increased 95.3% in As(9)(−S) and 57.5% in As(12)(−S). Comparison between the Cu levels in the leaves of As(+S) and As(−S) plants, showed a reduction (p<0.05) of 17.06 and 40.6% in As(9)(+S) and As(12)(+S), respectively, after 7 d and 63.6 and 55.39%, respectively, after 14 d (E). In roots, the uptake of Cu increased 261.7% (p<0.05) in As(9)(−S) and 203.5% in As(12)(−S), only after 14 d. Quite similar to the trend observed in leaves, comparison between the Cu levels in roots of As(−S) and As(+S) plants, also showed a higher trend of Cu levels in As(−S) than in As(+S) plants. They increased (p<0.05) in As(9)(−S) and As(12)(−S) by 23.87 and 36.8%, respectively, after 7 d and 64.5 and 64.4%, respectively, after 14 d (F).
Overall, the Fe uptake levels decreased from 7 to 14 d both in leaves and roots of C(+S), C(−S), and As(+S)-treated plants. Compared to C(+S), Fe levels in the leaves, decreased (p<0.05) 20.4% in As(9)(+S) and 34.0% in As(12)(+S) after 7 d (A), whereas, in As(9)(−S) and As(12)(−S) plants, they decreased only 6.5 and 3%, respectively. Whereas, after 14 d, they increased (p<0.05) 630.8% in As(9)(−S) and 538.2% in As(12)(−S), respectively, compared to C(−S). On the contrary, in the roots of As(9)(−S) and As(12)(−S), they increased 47.3 and 52.5% (p<0.05), respectively, after 7 d and 678.0 and 843.0%, respectively, after 14 d (B). Similarly, they also increased (p<0.05) in both As(9)(GSH) and As(12)(GSH) after 7 and 14 d. Comparison between the Fe levels of As(−S) and As(+S) leaves after 14 d showed that it was 788.2% higher (p<0.05) in As(9)(−S) and 800.8% higher in As(12)(−S) as compared to their respective As(+S) plants. Similarly, in roots, Fe content increased 927.4 and 1069.5% in As(9)(−S) and As(12)(−S), respectively, after 14 d. The Fe contents of the As(−S) and As(GSH) plants after 14 d were significantly higher than C, C(−S), and As(+S) plants (A,B).
Figure 2. Levels of Fe (µg g−1 dw) in the leaves (A) and roots (B), Mn in the leaves (C) and roots (D), Ca in the leaves (E) and roots (F) of Zea mays treated with arsenate and different levels of sulfur. All values are mean of four replicates±SD. Bars marked with same letters are not significantly different (Duncan's test, p<0.05).
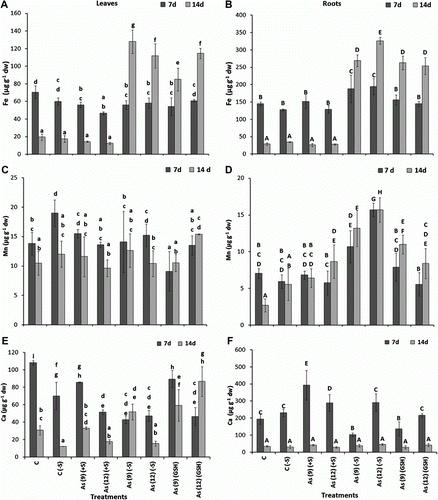
Overall, Mn levels were higher in leaves than in roots across the different treatments after 7 d, and C(−S) plants being the highest (C). In all the treatments with As, both in excess (+S) and (−S) conditions and for both durations (7 and 14 d), the Mn level in leaves at 7 d was 11.8% higher [As 12(−S) relative to As 12(+S)]; while it was 8.9 and 8.2% higher in As(9)(−S) and As(12)(−S) as compared to As (9)(+S) and As (12)(+S), respectively. Significant increase in Mn levels (p<0.05) were also observed in roots of As(9)(−S) and As(12)(−S) both after 7 and 14 d as compared to their respective As(+S) treatments (D).
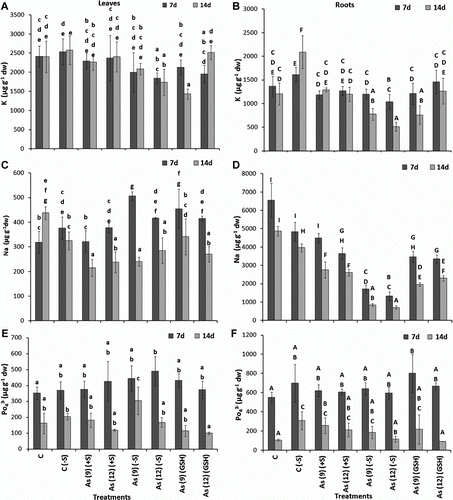
Among the macronutrients, i.e. Ca, K, and Na, no definite trend in accumulation was observed between the treatments and exposure periods. However, Ca and K levels in leaves after 7 d were observed to decrease both in As(9)(+S) and As(12)(+S) as compared to C, on the contrary, the level of Na increased (E and A). After 14 d, the Na level in leaves decreased in all the treatments, however, no definite trend was observed between the Ca levels. When the Ca levels were compared between the As(−S) and As(+S) plants, the levels were observed to be lower in both As(9)(−S) leaves (51.1%) and roots (74.0%) as compared to their respective As(+S) treatments after 7 d (E,F); whereas no clear trend was observed after 14 d. Levels of K decreased in the leaves of As(9)(−S) and As(12)(−S) (p<0.05) both after 7 and 14 d as compared to their respective C(−S) (A,B). However, in the roots, both K and Na levels in As(−S), decreased (p<0.05) both after 7 and 14 d as compared to their respective C(−S) and As(+S) plants (B,D). In As(GSH) treatments, Na levels decreased significantly in As(GSH) roots, with respect to As(+S) plants after 14 d, whereas, no clear trend was observed in the levels of Ca and K. Overall, the level of phosphate (PO4) in As(12) plants was lower, as compared to As(9), in all the S conditions after 14 d. The PO4 level in the leaves of sulfate deprived plants (−S) was higher as compared to (+S) plants, and the levels of PO4 decreased with growth (E,F). However, no significant difference was observed between the PO4 levels of leaves within the treated plants, except in As(9)(−S) after 14 d, which was significantly higher than the other treatments.
Figure 3. Levels of K (µg g−1 dw) in the leaves (A) and roots (B), Na in the leaves (C) and roots (D), PO4 in the leaves (E) and roots (F) of Zea mays treated with arsenate and different levels of sulfur. All values are mean of four replicates±SD. Bars marked with same letters are not significantly different (Duncan's test, p<0.05).
3.2. Growth parameters
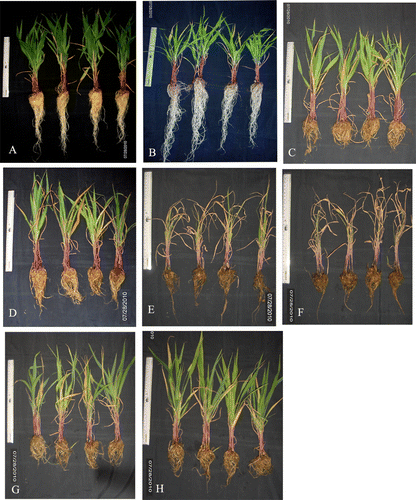
The growth of the plant in the presence of As was impaired in (+S) plants; however, the overall growth of the plants in As(−S) was lower than that in As(+S) after both 7 and 14 d (), where the fresh weight, shoot, and root lengths of As(−S)-treated plants were significantly lower as compared to C, C(−S), As(+S), and As(GSH) (A–C).
Figure 5. (A) The effect of arsenate on fresh weight (g), (B) root length (cm), (C) shoot length, (D) total chlorophyll (mg g−1 fw), (E) carotenoid (mg g−1 fw), and (F) protein contents (mg g−1 fw) of Zea mays after 7 and 14 d treated with As and different levels of sulfur. All values are mean of four replicates ±SD and bars marked with same letters are not significantly different (Duncan's test, p<0.05)
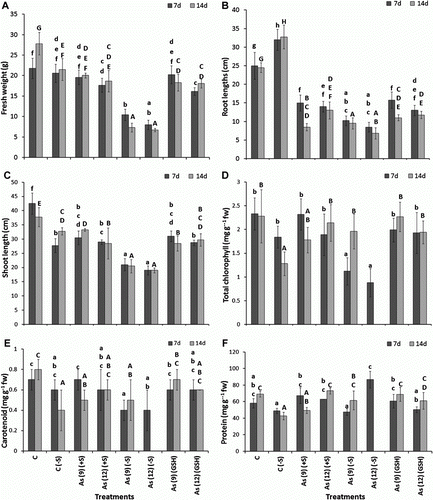
In As(+S) plants after 7 d, the fresh weight decreased 18.7% (p<0.05) in As(12)(+S) and 25.8% in As(12)(GSH) (A). Similarly, both root and shoot lengths decreased (p<0.05) 40 and 28.2%, respectively, in As(9)(+S), and 44.0 and 31.8%, respectively, in As(12)(+S) after 7 d. Similarly after 7 d, the root and shoot lengths decreased (p<0.05) 37.0 and 27.0%, respectively, in As(9)(GSH) and 7.69 and 32.35%, respectively, and in As(12)(GSH) (B,C). Comparison of shoot and root lengths between the As(+S) and As(−S) plants, shows that the decrease was higher in As(−S) than in As(+S) plants. The shoot and root lengths decreased 30.2 and 31.6% (p<0.05), respectively, in As(9)(−S) and 34.5 and 39.3%, respectively, in As(12)(−S) after 7 d. Likewise, comparison of the fresh weights between As(−S) and As(+S) plants after 7 d, shows that they decreased (p<0.05) 46.7 and 54.9%, in As(9)(−S) and As(12)(−S) with respect to their As(+S) treatments. Similar trends were also observed after 14 d. The level of total chlorophyll also decreased in As(−S) as compared to As(+S) plants. After 7 d, they decreased (p<0.05) 51.7 and 53.4% in As(9)(−S) and As(12)(−S), respectively, with respect to their As(+S) treatments (D). The Chl a/b ratio in the As(−S) treatments were higher than As(+S) treatments (). However, no significant difference was observed in carotenoid levels between the treatments both with C(+S) and C(−S) (E). The total soluble protein content of C(−S) was lower than C(+S) plant leaves besides, the protein level in As(12)(GSH) was lower than As(9)(GSH) at both 7 and 14 d (F). However, no clear trend was observed between the two treatments of As(+S) and As(−S).
Table 1. Levels of malondialdehyde (µmol g−1 fw), hydrogen peroxide (µg g−1 fw), carbonyl, glutathione, glutathione oxidized (µg g−1 fw), ascorbate (µM g−1 fw), and Chl a/b ratio in Zea mays leaves treated with arsenate after 7 and 14 d.
3.3. Toxicity and oxidative stress
Level of MDA did not show any significant increase in As(9)(+S) and As(12)(+S) plants both after 7 and 14 d, as compared to C (). Whereas, it increased (p<0.05) 36.8% in As(9)(−S) and 51.5% in As(12)(−S) after 14 d as compared to C(−S). However, no significant difference was observed between As(+S) and As(−S) plants either after 7 or 14 d. H2O2 levels increased (p<0.05) in As(12)(−S) both after 7 and 14 d compared to C(+S). However, after 14 d H2O2 levels of As(9)(−S) and As(12)(−S) plants, increased (p<0.05) 38.4 and 161.6%, respectively, as compared to their respective As(+S) plants (). Overall, the protein carbonyl level decreased in the leaves after 14 d with respect to its 7 d values. In the leaves of As(+S)-treated plants, it increased both after 7 and 14 d as compared to C, but increased (p<0.05) 77.2% in As(12)(+S) after 7 d and 137.6 and 227.3% in As(9)(+S) and As(12)(+S), respectively, after 14 d (). Also, among As(GSH)-treated plants, they increased (p<0.05) 69.9% in As(9)(GSH) and 96.8% in As(12)(GSH), after 7 d and 200.1% in As(12)(GSH) after 14 d. However, no significant difference was observed between As(+S) and As(−S) plants.
3.4. Ascorbate and glutathione pool
Overall, the levels of AsA in the leaves after 7 d did not show any significant difference between C and the treated plants, which ranged between 1509.6 and 2931.4 µM g−1 fw (). However, after 14 d, it increased (p<0.05) as 14.36% in As(9)(+S), 77.39% in As(9)(GSH), and 50.69% in As(12)(GSH) compared to C(+S). Similarly, it also increased (p<0.05) 69.36% in As(9)(−S) and 175.14% in As(12)(−S) after 14 d. When compared between As(+S) and As(−S)-treated plants, it increased (p<0.05) 94.18% in As(12)(−S) after 7 d, 21.53% in As(9)(−S) and 199.24% in As(12)(−S) after 14 d, with respect to their As(+S) treatments. The GSH levels decreased (p<0.05) 34.5% in As(9)(+S) and 17.19% in As(12)(+S) after 7 d. After 14 d, its levels increased (p < 0.05) only in As(12)(GSH) both after 7 d (10.43%) and 14 d (110.6%).
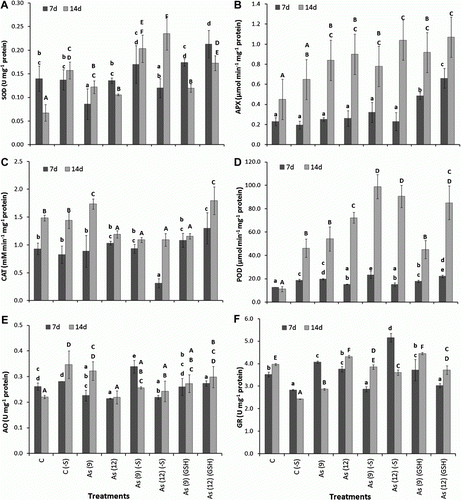
3.5. Antioxidant enzymes
Overall, the activities of the studied antioxidant enzymes were higher after 14 d than 7 d. The activities of SOD (U mg−1 protein) increased in As(GSH)-treated plants after 7 d and in all the sulfate regimes, i.e. As(+S) and As(−S) and As(GSH)-treated plants, after 14 d (A). In As(+S) plants, they increased (p<0.05) 71.4% in As(9)(+S) and 57.1% in As(12)(+S) after 14 d. Similarly, in As(−S) plants, they increased (p<0.05) 25% in As(9)(−S) and 50.0% in As(12)(−S) after 14 d. In As(GSH) plants, they increased (p<0.05) as 50.0% in As(12)(GSH) after 7 d and 71.4% in As(9)(GSH) and 142.8% in As(12)(GSH) after 14 d. Comparison of SOD activities between As(+S) and As(−S), shows that it was 89% higher in As(9) − S) after 7 d, and 67 and 118% higher in As(9)(−S) and As(12)(−S), respectively, over their respective As(+S) plants, after 14 d. APX activities (µmol min−1 mg−1 protein) did not show any significant increase in As(+S)-treated plants after 7 d, except for As(9)(GSH) and As(12)(GSH) where increase (p<0.05) of 130 and 187%, respectively, was observed (B). Whereas, after 14 d they increased (p<0.05) 87 and 100% in As(9)(+S) and as(12)(+S), respectively, and 104 and 138% in As(9)(GSH) and As(12)(GSH), respectively, as compared to C(+S). APX activities also increased (p<0.05) 20 and 60% in As(9)(−S) and As(12)(−S), respectively, after 14 d over its C(−S). Between As(+S) and As(−S) plants, they increased 16.0% in As(12)(−S), compared to As(12)(+S) after 14 d. In contrast, the CAT activity (mM min−1 mg−1 protein) did not show any significant difference in any treatment after 7 or 14 d, except in As(12)(−S) where it decreased (p<0.05) 67% and in As(12)(GSH) where it increased (p<0.05) 40% (C). After 14 d, CAT activities in both As(+S) and As(−S) plants, were lower (p<0.05) with respect to their controls, except for As(9)(+S) and as(12)(GSH). Overall, the POD activities (µmol min−1 mg−1 protein) in the treated plants after 14 d were higher than after 7 d, which were further higher in (−S) than (+S) plants both in C(−S) and As(−S) plants (D). After 14 d, activities of POD increased (p<0.05) 379% in As(9)(+S), 539% in As(12)(+S), 299% in As(9)(GSH) and 652% in As(12)(GSH). Similarly, in As(9)(−S) and As(12)(−S), they increased (p<0.05) as 114.2 and 96.3%, respectively. The POD activities in As(9)(−S) and As(12)(−S) after 14 d were higher by 82.6 and 25.4%, respectively, with respect to their As(+S) treatments. The GR and AO activities of the plants did not show any definite trend within the treatments (E,F).
Figure 6. Effect of arsenate treatment (µg mg−1) with (+S), (−S) and 1 mM (GSH) on the activities of different antioxidant enzymes in leaves of Zea mays after 7 and 14 d. (A) superoxide dismutase (U mg−1 protein); (B) ascorbate peroxidase: (µmol min−1 mg−1 protein); (C) catalase (M min−1 mg−1 protein); (D) guaiacol peroxidase (mmol min−1 mg−1 protein); (E) glutathione reductase (U mg−1 protein); (F) ascorbate oxidase (U mg−1 protein). All the values are means of four replicates ±SD. Bars indicated by same letters are not significantly different (Duncan's Multiple Range Test, p<0.05).
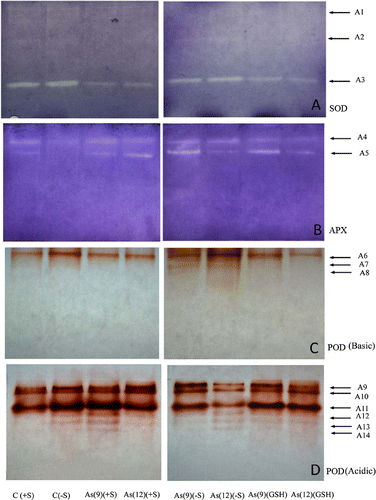
3.6. Native PAGE activity of antioxidant enzymes
Non-denaturing PAGE and activity localization of SOD in the As(−S)-treated leaf extract showed intense activity than As(+S) and As(GSH)-treated plants (A). Analysis of the PAGE revealed that there were three isoforms of SOD, one being major and prominent (A3), whereas, the rest two are minor (A1 and A2). Based on the position, band A3 is speculated to be Cu/ZnSOD and bands A1 and A2 are either FeSOD or MnSOD. Cu/ZnSOD had been observed to be over-expressed in (−S) plants than in (+S). Two isoforms of APX (A4 and A5) can be identified in the PAGE where intensity of the APX bands in the As(+S) plants progressively increased, while the bands corresponding to As(9)(+S) and as(9)(GSH) were more intense than in the corresponding As(12) treatments (B). The POD activity in the acidic PAGE revealed six isoforms (A9–A14) (D), whereas, the basic PAGE revealed three isoforms (A6–A8) (C). The expression of basic POD isoforms (A7 and A8) in As (−S) plants were higher as revealed by their clear bands in comparison to the As(+S) plants, where these bands are invisible. The acidic POD bands of As(9)(+S), As(9)(−S), and As(9)(GSH) plants were relatively more intense than their respective As(12) treatments, however, the intensity of C(−S) was higher than C(+S).
4. Discussion
The present research is a study of the response in terms of nutrient status and its role in antioxidative response in Z. mays toward prolonged As exposure in conditions, where S supply was in adequate, absent, or in surplus. Glutathione, which plays a major role in the detoxification of ROS including those induced by As stress, requires S for its synthesis. Hence, deficiency of S would adversely affect the synthesis of GSH, thereby also affecting As tolerance. In support of this hypothesis, it was observed that the translocation of As from roots to the leaves of As(−S) plants, increased significantly both after 7 and 14 d. This is possibly due to the deficiency of SH rich compounds in the roots of the plant which restricts As translocation to shoot by binding and eventually sequestering it into the root vacuoles (Tripathi et al. Citation2007). On the contrary, the translocation of As in the plants receiving surplus supply of S (GSH), was relatively higher which can be attributed to the greater tolerance in the shoots due to the higher SH pool. In conjunction to higher accumulation of As in the S deprived plants, the growth of the plant measured in terms of chlorophyll, protein, carotenoid contents, and Chl a/b ratio were significantly lower, indicating that the plants were subjected to higher oxidative stress in the absence of S containing compounds.
Among the micronutrients, an increase in the level of Cu, Zn, and Fe in the As(−S) leaves, mostly after 14 d can be either attributed to the higher demand for these elements in the antioxidative defense mechanism, as these elements are co-factors of the antioxidative enzyme (SOD) or possibly S deprivation plays a role in their uptake. On the contrary, the reverse trend of Cu accumulation, which decreased both in leaves and roots in the presence of As and As(GSH) and no difference in As(−S), shows S dependent translocation of Cu. Increase in the Fe translocation in the As(−S) plants with growth, and decrease in the As(+S) plants demonstrates a possible role of Fe in stress under S deficiency. However, the inverse relationship between Fe and S does not hold true in this case because of its higher level in As(GSH) plants, shows translocation of Fe is not dependent upon the S regimes but in the presence of As, the Fe translocation increases. This is contradictory to the results obtained in other studies, where with respect to exposure to other metals in graminaceous plants, lower leaf Fe concentrations have been observed in S-deficient plants as compared to the S-sufficient control (Astolfi et al. Citation2003). Iron–sulfur proteins are found in all life forms such as in Fe − S clusters which plays role in the oxidation–reduction reactions of mitochondrial electron transport, hence, may take part in detoxification of ROS as well. Deletion of genes that encode proteins involved in Fe − S cluster synthesis and export has been reported to manifest into excessive accumulation of mitochondrial Fe (Chen et al. Citation2002). Alternatively, methionine is a S-containing amino acid required for the synthesis of protein and also is a precursor of nicotianamine in strategy II plants, hence S availability plays a role in the Fe-uptake (Zuchi et al. Citation2009). However, the current findings are not in line with this hypothesis, which clearly indicates that the presence of As certainly increases the translocation of Fe in (−S) plants.
The translocation of Mn is irrespective of the presence of As or S, as no change was observed in its accumulation between C and the treated plants. Among the macronutrients, the increase in translocation of K in the shoots of As(−S) plants with As exposure, is indicative of a possible role of K in As stressed plants. The PO4 level in the younger plants (7 d) was higher than the older plants (14 d) and the levels were further higher in As(−S) than As(+S) plants. Lower PO4 level in higher As-treated plants and vice versa in all the S regimes, agrees with the fact which supports that As and PO4 shares common transporters to enter the plant roots, hence a mutual competitive inhibition in uptake exists between them.
The stunted growth of the plants exposed to As, i.e. As, As(−S), and As(GSH) and severe impairment in growth in As(−S) plants in fresh weight, shoot length, and root length elucidates the vitality of S in countering As-induced oxidative stress. On the contrary, same level of growth between C and C(−S) demonstrates little effect of the S deprivation on the growth. Hell and Hillebrand (Citation2001) mentioned that S rarely limits plant growth, hence, the observed unimpeded growth in C(−S) compared to C can be attributed to this underlying fact. Plants receiving As and surplus S (1 mM GSH) exhibited better growth than As(−S) plants. The extra S was able to impart tolerance in the plants toward As-induced stress by virtue of sufficient S pool in the plants. Alternatively, very little difference between the growth of As and As(GSH)-treated plants, indicative of no further enhancement of defense mechanism toward As-induced stress under surplus supply of S. The results observed in the present study are in accordance with Astolfi et al. (Citation2005) where they have also observed increase in S uptake rate (only in + S plants) in Z. mays in comparison to C, which they attributed it toward countermechanism of plant against Cd induced oxidative stress by increasing the binding of the metal to SH compounds.
The observed nonsignificant reduction in the levels of total chlorophyll in As and As(GSH)-treated plants and significant reduction in As(−S)-treated plants, reveals that S plays a role in imparting defense toward degradation or synthesis of vital bio-molecules. Higher Chl a/b ratio in As and As(−S)-treated plants compared to their respective C implicates that the chlorophyll b is more prone to degradation due to As-induced stress and further under S deficiency. The higher protein content in As(−S) plants could be either due to excessive synthesis of stress proteins or weighing of more biomass of the plant leaves during estimation due to loss of moisture in the stressed plant. Carotenoid is essential for the protection of both photosynthetic and nonphotosynthetic tissues from photo-oxidative damage. The decline in the carotenoid level in As(+S) plants can be attributed to the toxicity induced by As, as the presence or deprivation of S had little impact on its content. Sulfur is not known to be a constituent of the carotenoid and hence its absence in the medium did not decrease the level of carotenoid significantly. Total soluble protein content was relatively lower in the (−S) plants than (+S) plants. This could be attributed to, S being constituent of many amino acids, i.e. cysteine and methionine. Alternately, the levels of protein in plants growing in excess S medium were comparable to that of C, suggesting that the supply of S in the nutrient medium was sufficient toward S requirement of the plant, hence, increase in the S did not increase the protein content or any other vital defense mechanism toward As-induced stress.
Measure of MDA content is directly proportional to the oxidative damage of plasma membrane due to ROS. Higher MDA level in As(+S) plants than C, and significantly higher levels in As(−S) plants implies that S has a major role in imparting defense against As-induced oxidative stress. The apparent discrepancy of the presented data in comparison to that reported in other studies, where the MDA levels of the C are observed to be higher than the treated plants, can be attributed to the interference of other compounds, i.e. anthocyanins and carbohydrates (Taulavuori et al. Citation2001; Astolfi et al. Citation2005). In agreement to the observed higher MDA levels in control plants, Astolfi et al. (Citation2005) also observed little difference between the + S and − S receiving Z. mays roots treated with Cd. H2O2 is generated as a product due to dismutation of the ion. Higher levels of the H2O2 in As(−S) than As(+S) plants also corroborates with the higher SOD activity in As(−S) plants. Thus, it can be inferred that the plants exposed to As undergo higher oxidative stress due to generation of H2O2 in the absence of S.
Protein carbonyl content (PCC) has been reported as a biomarker of oxidative stress in the plants (Collen et al. Citation2003; Rodríguez-Serrano et al. Citation2006). Contrary to MDA, the PCC level in the C(−S) plants was higher than C. Furthermore, the values of PCC in As(+S) and As(GSH) plants being significantly higher indicate oxidation of protein was more pronounced than lipid peroxidation, which were further higher in As(+S) than in As(−S) plants. Maize is known to contain lower level of linolenic acid (0.722–1.281%), a polyunsaturated fatty acid (PUFA) containing three double bonds (Orhun & Korkut Citation2011). PUFA are more prone to peroxidation due to ROS. Thus, the observed nonsignificant change in MDA level in leaves can be attributed to the lower peroxidation due to lesser linolenic acid (PUFA) in maize. Alternatively, the generated due to the presence of As in the cell could have led to oxidation of protein which has manifested in the form of significant rise of PCC levels.
In general, the higher level of AsA in the younger plants (7 d) and As(−S) than 14 d plants, can be attributed to the inhibition of the AsA synthesis due to As-induced toxicity at the later stage of growth. SOD is considered as the first line of defense against , which dismutates two
radicals to form H2O2. Higher levels of H2O2 can be correlated to the higher activity of SOD, particularly in As(−S) plants after 14 d, indicative of higher production of H2O2. Thus, it also indicates that in the absence of S, the stressed plants stimulate SOD synthesis as demonstrated by increased activity of SOD. The higher generation of H2O2 in As(−S) plants, can thus be attributed to the toxic manifestation of As in the absence of S. H2O2 is further converted to monodehydroascorbate by APX. The increase in the activity of APX in a dose dependent manner, corresponds with the toxicity due to As in As(−S) plants as well as in As(+S) and As(GSH) plants. Nonsignificant change in the activity of CAT, indicates its lesser role in the conversion of H2O2 into H2O because CAT is specifically located in the peroxisomes, whereas, APX is mostly cytoplasmic, and most of the H2O2 generated due to heavy metal(loid) induced stress is largely cytoplasmic. Hence, most of the H2O2 is speculated to be converted to H2O in the cytoplasm by APX. Higher POD activity in the (−S)-treated plants over the (+S)-treated plants demonstrates the shift in antioxidative defense mechanism of the plant toward antioxidant enzymes which uses non sulfur compounds as substrates for electron donor. In the absence of S in the media, SH rich compounds, i.e. GSH are not being synthesized. In the absence of GSH the entire AsA–GSH detoxification mechanism is being compromised and therefore the ROS defense strategy of the plants shifts from AsA–GSH pathway to a SOD- and POD-mediated detoxification mechanism. GR reduces the GSSG to GSH using an e− from NADPH. Nonsignificant change in the activity of GR is well corroborated with nonsignificant change in GSSG levels. Lower level of GR specific activity in C(−S) compared to C(+S) demonstrates that the activity or synthesis of GR is low in the absence of S, whereas, when the plants are under stress due to As, the synthesis increases independent of the presence or absence of S. Hence, the plants are able to synthesize the GR but in the absence of S the plants are unable to convert GSSG to GSH. The role of AO has been largely attributed to cell elongation and is localized in the extracellular matrix (Kato & Esaka Citation2000); however, some reports reconsidered the role of AO as a possible factor controlling intracellular redox state (Potters et al. Citation2002). According to Potters et al. (Citation2002), AO is the terminal oxidase in electron transport chain in which reducing equivalents are transferred from pyridine nucleotides to oxygen via GSH and AsA cycle. This implies that up- or down-regulation of AO activity should affect the redox state of AsA, GSH, and pyridine nucleotides in opposite ways. Results obtained during this study clearly shows that the activity of AO in (−S) plants were higher than the (+S) plants, however, rise in AO activity does not correspond with decrease of AsA. Alternatively, in the absence of GSH in the As(−S) plants, increased AsA level signifies the shift in antioxidative defense strategy of the plant from GSH to AsA as electron donor.
The native PAGE activity of SOD has also shown higher value in all the (−S) plants, quite similar to the specific activities of these antioxidant enzymes obtained from the in-vitro assays of the leaf extracts. The intense bands of As(−S)-treated plants than the rest, in the basic POD native page, in comparison to relatively less intense bands in acidic POD page, shows that the activity of POD in As(−S) plants was operating in a wide range of pH. The highest specific activity of POD in As(−S) leaves as observed in the in-vitro assays of the leaf extracts is speculated to be operating in the acidic range due the presence of many organic acids, which is also supported by the intense activities of POD bands in acidic PAGE than that of basic native PAGE.
In conclusion, the role of S in restricting the translocation of As from the roots to the shoots of Z. mays is evident as the translocation of As was higher in (−S) plants than in (+S) plants. Among micronutrients, Cu, Zn, and Fe exhibited an increase in the (−S) plants indicative of their possible role in the antioxidant defense mechanism or alteration in their uptake. Overall growth parameters in As(−S) plants were lower than As(+S) plants. Higher level of MDA and H2O2 in As(−S) plants in conjuncture with the higher activities of SOD, APX, and POD shows that in the absence of S, plants undergoes a greater oxidative stress. The higher level of protein oxidation than lipid peroxidation in As(−S) plants has been attributed to the presence of lesser PUFA in maize. Higher H2O2 level in As(−S) plants coincided with higher SOD activity and subsequently higher APX activity with higher H2O2, exhibiting As(−S) plants suffered higher toxicity than As(+S) plants. Higher activity of POD, SOD in As(−S) plants is also attributed to failure of detoxification mechanism by GR due to inability of synthesizing S containing GSH. The role of CAT was minimal suggestive of its localized activity in degrading H2O2. The lower GSH/GSSG pool of As(−S) led to the failure of ascorbate–glutathione cycle due to the absence of S which manifested as higher toxicity in As(−S) plants. Overall, S has shown a dual role in protecting plants from As(V) induced toxicity (1) by restricting As translocation to the upper parts by sequestering in S compounds; (2) and by modulating antioxidant enzymes for As detoxification.
Acknowledgements
The authors are thankful to Director, CSIR-National Botanical Research Institute, for providing required research facilities. A.P. Singh is grateful to UGC, for the award of junior research fellowship. The authors are also thankful to CSIR-New Delhi, for providing the necessary financial support. Special thanks to Dr S. Ranade, Scientist-NBRI for critically checking the manuscript.
Additional information
Notes on contributors
Sarita Sinha
Navin Kumar, Amit Pal Singh, Geetgovind Sinam and Ram Nayan Yadav all contributed equallyReferences
- Arnon , DI . 1949 . Copper enzymes in isolated chloroplast, polyphenol oxidase in Beta vulgaris . Plant Physiol. , 24 : 1 – 15 .
- Astolfi , S , Zuchi , S and Passera , C . 2004 . Role of sulphur availability on cadmium-induced changes of nitrogen and sulphur metabolism in maize (Zea mays L.) leaves . J Plant Physiol. , 161 : 795 – 802 .
- Astolfi , S , Zuchi , S and Passera , C . 2005 . Effect of cadmium on H+ATPase activity of plasma membrane vesicles isolated from roots of different S-supplied maize (Zea mays L.) plants . Plant Sci. , 169 : 361 – 8 .
- Astolfi , S , Zuchi , S , Passera , C and Cesco , S . 2003 . Does the sulfur assimilation pathway play a role in the response to Fe deficiency in maize (Zea mays L.) plants . J Plant Nutr. , 26 : 2111 – 121 .
- Beauchamp , C and Fridovich , Y . 1971 . Superoxide dismutase: improved assays and an assay applicable to acrylamide gels . Anal Biochem. , 44 : 276 – 87 .
- Bouranis , DL , Chorianopoulou , SN , Protonotarios , VE , Siyannis , VF , Hopkins , L and Hawkesford , MJ . 2003 . Leaf response of young iron-inefficient maize plants to sulfur deprivation . J Plant Nutr. , 26 : 1189 – 202 .
- Cao , X , Ma , LQ and Tu , C . 2004 . Antioxidative responses to arsenic in arsenic hyperaccumulator Chinese brake fern (Pteris vittata L.) . Environ Pollut. , 128 : 317 – 325 .
- Castegna A , Drake J , Pocernich C , Butterfield DA . 2003 . Protein carbonyl levels, an assessment of protein oxidation . In : Hensley K , Floyd RA . Apoptosis methods in pharmacology and toxicology: methods in biological oxidation Stress . Totowa , NJ : Human Press ; 161 – 8 .
- Chakrabarty , D , Chatterjee , J and Datta , SK . 2007 . Oxidative stress and antioxidant activity as the basis of senescence in chrysanthemum florets . Plant Growth Regul. , 53 : 107 – 15 .
- Chauhan , VS , Nickson , RT , Chauhan , D , Iyengar , L and Sankararamakrishnan , N . 2009 . Ground water geochemistry of Ballia district, Uttar Pradesh, India and mechanism of arsenic release . Chemosphere. , 75 : 83 – 91 .
- Chen , OS , Hemenway , S and Kaplan , J . 2002 . Inhibition of Fe-S cluster biosynthesis decreases mitochondrial iron export: Evidence that Yfh1p affects Fe-S cluster synthesis. Proc Natl Acad Sci . USA. , 99 : 12321 – 6 .
- Collen , J , Pinto , E , Pedersen , M and Colepicolo , P . 2003 . Induction of oxidative stress in the red macroalga Gracilaria tenuistipitata by pollutant metals . Arch Environ Contam Toxicol. , 45 : 337 – 42 .
- De Kok LJ , Castro A , Durenkamp M , Stuiver CEE , Westerman S , Yang L , Stulen I . 2002 . Sulphur in plant physiology . Proceedings No 500, The International Fertiliser Society ; 2002 Dec 16–17 ; York , 1 – 26 .
- Duxbury , AC and Yentsch , CS . 1956 . Plankton pigment monograph . J Mar Res. , 15 : 92 – 101 .
- Ellman , GL . 1959 . Tissue sulfhydryl group . Arch Biochem Biophys. , 82 : 70 – 77 .
- Fielding , JL and Hall , JL . 1978 . A biochemical and cytological study of peroxidase activity in roots of Pisum sativum . J Exp Bot. , 29 : 969 – 81 .
- Gilbert , BE . 1926 . The adaptation of certain colorimetric methods to the estimation of nitrates, phosphates and potassium in plant solutions . Plant Physiol. , 1 : 191 – 9 .
- Grill , D , Tausz , M and De Kok , LJ . 2001 . Significance of glutathione to plant adaptation to the environment , 262 Dordrecht : Kluwer Academic Publishers .
- Heath , RL and Packer , L . 1968 . Photoperoxidation in isolated chloroplast I. Kinetic and stoichiometry of fatty acid peroxidation . Arch Biochem Biophys. , 125 : 189 – 198 .
- Hell , R and Hillebrand , H . 2001 . Plant concepts for mineral acquisition and allocation . Curr Opin Biotechnol. , 12 : 161 – 8 .
- Kato , N and Esaka , M . 2000 . Expansion of transgenic tobacco protoplasts expressing pumpkin ascorbate oxidase is more rapid than that of wild type protoplasts . Planta. , 210 : 1018 – 22 .
- Kato , M and Shimizu , S . 1987 . Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves; phenolic dependent peroxidative degradation . Can J Bot. , 65 : 729 – 35 .
- Laemmli , UK . 1970 . Cleavage of structural proteins during the assembly of the head of bacteriophage T4 . Nature. , 227 : 680 – 5 .
- Lowry , OH , Rosebrought , NJ , Farr , AL and Randall , RJ . 1951 . Protein measurement with folin phenol reagent . J Bio Chem. , 193 : 265 – 75 .
- Mallick , S , Sinam , G , Mishra , RK and Sinha , S . 2010 . Interactive effects of Cr and Fe treatments on plants growth, nutrition and oxidative status in Zea mays L . Ecotoxicol Environ Saf. , 73 : 987 – 95 .
- Mallick , S , Sinam , G and Sinha , S . 2011 . Study on arsenate tolerant and sensitive cultivars of Zea mays L.: differential detoxification mechanism and effect on nutrients status . Ecotoxicol Environ Saf. , 74 : 1316 – 24 .
- McGrath , SP and Zhao , FJ . 1995 . A risk assessment of sulphur deficiency in cereals using soil and atmospheric deposition data . Soil Use Manag. , 11 : 110 – 14 .
- Mittler , R and Zilinskas , BA . 1993 . Detection of ascorbate peroxidase activity in native gells gels by inhibition of the ascorbate dependent reduction of nitroblue tetrazolium . Anal Biochem. , 212 : 540 – 6 .
- Nakano , Y and Asada , K . 1981 . Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts . Plant Cell Physiol. , 22 : 867 – 80 .
- Nocito , FF , Lancilli , C , Crema , B , Fourcroy , P , Davidian , JC and Sacchi , GA . 2006 . Heavy metal stress and sulfate uptake in maize roots . Plant Physiol. , 141 : 1138 – 48 .
- Oberbacher , MF and Vines , HM . 1963 . Spectrophotometric assay of ascorbic acid oxidase . Nature. , 197 : 1203
- Orhun , GE and Korkut , KZ . 2011 . Interrelationships among the oil and fatty acids in maize . Afr J Agric Res. , 6 : 2115 – 17 .
- Patel , KS , Shrivas , K , Brandt , R , Jakubowski , N , Corns , W and Hoffmann , P . 2005 . Arsenic contamination in water, soil, sediment and rice of central India . Environ Geochem Health , 27 : 131 – 45 .
- Potters , G , De Gara , L , Asard , H and Horemans , N . 2002 . Ascorbate and glutathione guardians of the cell cycle, partners in the crime? . Plant Physiol Biochem. , 40 : 537 – 48 .
- Rodríguez-Serrano , M , Romero-Puertas , MC , Zabalza , A , Corpas , FJ , Gómez , M , Del Río , LA and Sandalio , LM . 2006 . Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo . Plant Cell Environ. , 29 : 1532 – 44 .
- Roychowdhury , T , Uchino , T , Tokunaga , H and Ando , M . 2002 . Arsenic and other heavy metals in soil from an arsenic affected area of West Bengal, India . Chemosphere. , 49 : 605 – 18 .
- Scandalios , JG , Tsaftaris , AS , Chandlee , JM and Skadsen , RW . 1983 . Expression of the developmentally regulated catalase (Cat) genes in maize . Dev Genet. , 4 : 281 – 93 .
- Smith , IK , Vierheller , TL and Thorne , CA . 1988 . Assay of glutathione reductase in crude tissue homogenates using 5, 5'-dithiobis (2-nitrobenzoic acid) . Anal Biochem. , 175 : 408 – 13 .
- Srivastava , S and D'Souza , SF . 2009 . Increasing sulfur supply enhances tolerance to arsenic and its accumulation in Hydrilla verticillata (L.f.) Royle . Environ Sci Technol. , 43 : 6308 – 13 .
- Taulavuori , E , Hellstrom , EK , Taulavuori , K and Laine , K . 2001 . Comparison of two methods used to analyse lipid peroxidation from Vaccinium myrtillus (L.) during snow removal, reacclimation and cold acclimation . J Exp Bot. , 52 : 2375 – 80 .
- Tewari , RK , Kumar , P and Sharma , PN . 2010 . Morphology and oxidative physiology of sulphur-deficient mulberry plants . Environ Exp Bot. , 68 : 301 – 8 .
- Tripathi , RD , Srivastava , S , Mishra , S , Singh , N , Tuli , R , Gupta , DK and Maathuis , FJM . 2007 . Arsenic hazards: strategies for tolerance and remediation by plants . Trends Biotechnol. , 25 : 158 – 65 .
- Tu , C and Ma , LQ . 2005 . Effects of arsenic on concentration and distribution of nutrients in the fronds of the arsenic hyperaccumulator Pteris vittata L . Environ Pollut. , 135 : 333 – 40 .
- Velikova , V , Yordanov , I and Edreva , A . 2000 . Oxidative stress and some antioxidant systems in acid rain-treated plants . Plant Sci. , 151 : 59 – 66 .
- Zhang , J , Zhao , Q-Z , Duan , G-L and Huang , Y-C . 2010 . Influence of sulphur on arsenic accumulation and metabolism in rice seedlings . Environ Exp Bot. , 72 : 34 – 40 .
- Zuchi , S , Cesco , S , Varanini , Z , Pinton , R and Astolfi , S . 2009 . Sulphur deprivation limits Fe-deficiency responses in tomato plants . Planta. , 230 : 85 – 94 .