Abstract
In this study, changes in growth, chlorophyll pigments, proline, hydrogen peroxide (H2O2) and malondialdehyde (MDA) contents, and antioxidative enzyme activities were investigated in the seedlings of four different cultivars (cvs) of mustard [Brassica juncea (L.) Czern. & Coss.], i.e. Varuna, RH-30, Rohini, and Vaibhave under potassium (K) nutrition-deficient conditions. K deficiency induced a significant decrease in concentrations of photosynthetic pigments in all four cvs, however, this decrease was higher in cvs. Varuna and RH-30. During K deficiency, proline concentration increased in all mustard cvs, but a maximum increase in this parameter was shown by cvs. Varuna and RH-30. The activity of the key proline metabolizing enzyme γ-glutamyl kinase increased more in cvs. Varuna and RH-30 compared to cvs. Rohini and Vaibhave. The proline oxidase activity showed greater increase in cvs. Vaibhave and Rohini compared to cvs. Varuna and RH-30. K deficiency increased the concentrations of H2O2 and the activities of anti-oxidative defense system enzymes like superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR) in the seedlings of all mustard cvs, but higher activities of these enzymes were observed in cvs. Varuna and RH-30 compared to cvs. Rohini and Vaibhave. A significant lipid peroxidation in terms of MDA contents was also observed in the K-deficient seedlings of all four mustard cvs. This study suggests that K-starvation-induced oxidative stress through the high generation of reactive oxygen species (ROS). All mustard cvs counteracted to some extent the effects of ROS by activation of antioxidant machinery. Overall, the results indicate that of all four mustard cvs, Varuna and RH-30 were tolerant to K deficiency.
Introduction
Plants are known to develop a wide range of adaptive mechanisms under various environmental stress conditions to ensure their survival and maintain growth and productivity (Ahmad & Sharma Citation2010; Hamdia & Shaddad Citation2010; Anjum et al. Citation2011; Shahbaz et al. Citation2012). Mineral nutrients are essential for the growth and development of plants, and are also an important factor in plant-environment interactions. Nutrients affect the plants' response to environment, providing resistance against the changing/adverse environment (Marschner Citation1995; Akram et al. Citation2009; Akram & Ashraf Citation2011). Potassium (K), an important macronutrient plays a vital role in metabolic processes, growth and adaptations to environmental stresses. K is known to be involved in activation of different enzymes and stabilization of protein synthesis of over 60 different enzyme systems in plants (Suelter Citation1970). K plays an active role in different physiological processes like photosynthesis, phloem solute transport of photo-assimilates into sink organs, activation of enzymes, and maintenance of cation:anion balance (Marschner Citation1995; Mengel & Kirkby Citation2001; Akram et al. Citation2008; Akram & Ashraf Citation2011).
Plant movements such as stomatal and leaf movements, and other plant tropisms are driven by K+ to make the surrounding guard cells either turgid or flaccid (Maathuis & Sanders Citation1996; Philippar et al. Citation1999; Franks & Farquhar Citation2007). K accumulation generates osmotic pressure within cells that initiates cellular and leaf expansion (Maathuis & Sanders Citation1996; Elumalai et al. Citation2002). K deficiency also known as potash deficiency is of great agricultural importance because it leads to reduced chlorophyll content and hence decreased photosynthetic activity. The rate of adenosine tri-phosphate (ATP) production is also known to be reduced under K deficiency, slowing down-regulation of the processes dependent on ATP (Hartt Citation1969; Pier & Berkowitz Citation1987; Zhao et al. Citation2001).
Reduced K supply is known to reduce the translocation of nitrates, phosphates, calcium, magnesium, and amino acids. The process of photosynthetic CO2 fixation and utilization of photo-assimilates is strongly dependent on K supply (Cakmak Citation1994, Citation2005). K deficiency severely reduces the photosynthetic CO2 fixation. It also leads to impairment in partitioning and utilization of photosynthates. Such perturbed photosynthetic activity results in accumulation of photosynthetically produced electrons that stimulate the production of reactive oxygen species (ROS) (Cakmak Citation2005; Ashraf & Akram Citation2009). ROS disrupt the normal functioning of the cell and leads to oxidative stress which in turn leads to membrane disruption, protein modifications, inhibition of enzyme activity, damage to nucleic acids, etc. (Shahbaz et al. Citation2008; Ashraf Citation2009; Perveen et al. Citation2010, Citation2011, Citation2012; Akram et al. Citation2012).
Mustard [Brassica juncea (L.) Czern & Coss.] is one of the potential oil-seed crops (Khan et al. Citation2002; Akmal et al. Citation2011). However, its growth and seed-oil-content significantly depends on the K concentration in the growth medium of the crop as K uptake begins when rapid plant growth starts and continues until grain formation (Kuo & Chen Citation1980). Thus, it is necessary to understand how the amount and availability of K could alter crop growth and associated physio-biochemical parameters. Published work on the effect of K deprivation on mustard plants is not much. Keeping this in view, the aim of this study was to investigate the K deficiency-induced modulation in growth, photosynthetic pigments, proline, and activities of anti-oxidant enzymes in the seedlings of four elite mustard cvs.
Materials and methods
Seeds of four B. juncea cvs (Varuna, RH-30, Rohini, and Vaibhave) were surface-sterilized with 2% (w/v) sodium hypochlorite (NaOCl) for 10 min, then washed with distilled water and kept in distilled water for 24 h. The seeds were planted in plastic pots (diameter=30 cm) which contained peat, perlite, and sand (1:1:1, v/v/v). Thinning was done by maintaining one plant per pot after germination. The seedlings were allowed to grow for three weeks under constant temperature 25±4°C for 12–13 h of photoperiod.
After 25 days, plants were transferred to plastic trays containing basic nutrient solution. The composition of the nutrient solution was as follows (mg L−1): 270 N, 31 P, 234 K, 200 Ca, 64 S, 48 Mg, 2.8 Fe, 0.5 Mn, 0.5 B, 0.02 Cu, 0.05 Zn, and 0.01 Mo. The pH of the nutrient solution was monitored daily and maintained at 6.5 with 0.1 mM KOH.
Seven days after transplantation, plastic trays containing seedlings were divided into two groups. Group 1 plants received complete nutrient solution and served as control, while plants in group 2 received the nutrient solution which was deficient in K. The nutrient solution was replaced every alternate day. Symptoms of K deficiency were seen after 27 days of transplantation. After 35 days, the plants were harvested for the analyses. The experiment was laid out in a randomized block design. Each replicate comprised five plants and the treatment was replicated four times (20 plants per treatment).
Determination of chlorophyll content
Hiscox and Israelstam (Citation1979) method was employed for the determination of chlorophyll content. Fresh leaves (100 mg) were put in a test tube containing 10 mL of dimethyl sulphoxide (DMSO). The test tubes were incubated at 65°C for 40 min. Absorbance was read at 480, 510, 645, and 663 nm spectrophotometrically (Beckman 640 D, USA). For the control, DMSO was used.
Proline determination
The method of Bates et al. (Citation1973) was used for the quantification of proline content. Fresh leaves (300 mg) were homogenized in 10 mL 3% aqueous sulfosalicylic acid. After this, the material was subjected to centrifugation for 15 min at 10,000 rpm. The supernatant (2 mL) was mixed with 2 mL of glacial acetic acid and acid ninhydrin and incubated the mixture at 100°C for 1 h. Termination of the reaction took place in an ice bath. Toluene (4 mL) was mixed to the extract and vortexed for 20 s. The chromophore containing toluene was aspirated from the aqueous phase, and absorbance read at 520 nm using toluene as a blank.
Determination of γ-glutamyl kinase activity [ATP: l-glutamate 5-phosphotransferases (EC 2.7.2.11)]
The method of Hayzer and Leisinger (Citation1980) was used for the determination of activity of γ-GK. Fresh leaves (1.0 g) were homogenized in Tris-HCl buffer (50 mM; pH 8.5). The homogenate was centrifuged for 30 min at 40,000 rpm. To the supernatant, ATP (10 mM) was added to prepare the reaction buffer. From this reaction buffer, 0.1 mL was taken and incubated at 37°C for 30 min. Then, 2 mL of the stop buffer were added. Absorbance was read at 535 nm spectrophotometrically (Beckman 640 D, USA). The enzyme activity was expressed in mg−1 protein.
Determination of proline oxidase activity (PROX) [L-proline: O2oxidoreductase (EC 1.4.3.1)]
The PROX activity was determined according to Huang and Cavalieri (Citation1979). Fresh leaves (1 g) were homogenized in 5 mL of Tris-HCl buffer (pH 8.5). After centrifugation of the homogenate at 10,000 rpm for 10 min, the supernatant was collected and again subjected to centrifugation for 20 min at 25,000 rpm. The assay mixture contained reaction extract (0.1 mL), 50 mM Tris-HCl buffer, 5 mM MgCl2, 0.5 mM NADP+, 1 mM KCN, 1 mM phenazine methosulphate (PMS), 0.06 mM 2,6-dichlorophenol indophenols (DCPIP), and distilled water. Then, 3 mL of this assay mixture were used to determine the absorbance at 600 nm monitored continuously for 5 min. The activity of PROX was expressed in units mg−1 protein (1 U=mM DCPIP reduced min−1 mg−1 protein).
Determination of hydrogen peroxide (H2O2) content
The protocol described by Velikova et al. (Citation2000) was followed to determine the H2O2 content. Fresh leaves (500 mg) were homogenized in 5 mL of 0.1% (w/v) trichloro-acetic acid (TCA). Centrifugation of the homogenate was performed at 12,000 rpm for 15 min. To the 5 mL supernatant, 0.5 mL of 10 mM potassium phosphate buffer (pH 7.0) and 1 mL of 1 M potassium iodide (KI) were added. The absorbance of this mixture was read spectrophotometrically at 390 nm. The standard calibration curve was plotted using different concentrations of H2O2 and the content of H2O2 in the samples was calculated by comparison with the standard curve.
Determination of lipid peroxidation
Malondialdehyde (MDA) equivalents were used to determine lipid peroxidation rates following the method of Hodges et al. (Citation1999). Leaf tissue (0.5 g) was crushed in 80% ethanol and the homogenate subjected to centrifugation at 3000 rpm for 10 min at 4°C. The same solvent was used for the extraction of pellet twice. The supernatant (1 mL) was taken in a test tube and equal volumes of solution of 20% TCA, 0.01% butylated hydroxyl toluene (BHT), and 0.65% (w/v) thiobarbituric acid (TBA) were added. This mixture was heated for 25 min at 95°C and then cooled at room temperature. The absorbance was read at 440, 532, and 600 nm spectrophotometrically.
Extraction of the enzymes
Fresh leaves (10 g) were crushed with 50 volumes of 100 mM Tris-HCl (pH 7.5) containing 5 mM DTT, 10 mM MgCl2, 1 mM EDTA, 5 mM magnesium acetate, 1.5% PVP-40, 1 mM PMSF, and 1 µg mL−1 aproptinin. The homogenate was filtered using a cheesecloth and subjected to centrifugation for 15 min at 10,000 rpm. The source of enzyme was the supernatant collected after centrifugation. The extraction buffer also contained serine and cysteine proteinase inhibitors [1 mM phenylmethylsulfonyl fluoride (PMSF)+1µg mL−1 aproptinin]. For the analysis of ascorbate peroxidase (APX) activity, tissues were separately homogenized in the homogenizing medium (as described above for the other enzymes) with 2 mM ascorbate. All experiments were performed at 4°C. The soluble protein content was estimated according to Bradford (Citation1976) with bovine serum albumin as blank.
Enzyme assay
Superoxide dismutase
Activity of superoxide dismutase (SOD, EC 1.15.1.1) was appraised according to Van Rossun et al. (Citation1997) following photoreduction of nitroblue tetrazolium (NBT). The reaction mixture contained supernatant (100 µL), 50 mM potassium phosphate buffer (pH 7.8), 0.1 mM EDTA, 13 mM methionine, 75 µM NBT, 2 µM riboflavin. To initiate the reaction, the mixture was placed in tubes under 15 W fluorescent lamps. The lamps were removed after 10 min to terminate the reaction. Non-illuminated reaction mixture acted as a blank. The absorbance was read at 560 nm spectrophotometerically. The activity of SOD was expressed as unit mg−1 protein. One unit of SOD was defined as the amount of protein causing 50% decrease of the SOD-inhibitable NBT reduction.
Catalase (CAT)
Catalase (CAT, EC 1.11.1.6) activity was determined according to Luck (Citation1974). For this, 20 mM H2O2 (3 mL) and 50 mM phosphate buffer (pH 7.0) were added to the enzyme extract (50 µL). The decrease in absorbance was read at 249 nm. The activity of CAT was calculated using the extinction co-efficient of 36×103 mM−1 cm−1 and expressed as unit mg−1 protein.
Ascorbate peroxidase
The method of Nakano and Asada (Citation1981) was followed for the determination of APX activity. The assay mixture (3 mL) contained: enzyme extract (0.1 mL), 0.1 mM EDTA, 0.5 mM ascorbate, 0.1 mM H2O2, and 50 mM potassium phosphate buffer (pH 7.0). The decrease in absorbance was read at 265 nm. APX activity was assayed by measuring the oxidation of ascorbate at 290 nm. APX activity was expressed as unit mg−1 protein.
Glutathione reductase (GR)
The activity of GR (EC 1.6.4.2) was determined according to Carlberg and Mannervik (Citation1985). The assay mixture (1 mL) contained: potassium phosphate buffer (0.75 µL) (pH 7.0), 2 mM EDTA, 2 mM NADPH (75 µL), and 75 µL (20 mM) oxidized glutathione (GSSG). Addition of enzyme extract (0.1 mL) to the assay mixture initiated the reaction. The decrease in absorbance was read at 340 nm for 2 min. The activity of GR was calculated using the extinction co-efficient of NADPH of 6.2 mM−1 cm−1 and expressed as unit mg−1 protein.
Statistical analysis
One-way analysis of variance (ANOVA) was used for statistical analysis followed by Duncan's Multiple Range Test (DMRT). The values obtained were the mean±S.E. for five samples in each group. P values at 0.05 were considered as significant.
Results
Biomass yield
Analyses of variance and mean data for shoot and root lengths (; ) show that a maximum decrease in shoot length (39.9%) was found to be in cv. Vaibhave and minimum (30%) in cv. Varuna under K-deficient conditions as compared to control conditions. The decrease in root length was observed to be 53.3% in cv. Varuna and 63.6% in cv. Vaibhave. A decrease in shoot dry weight (DW) was also observed which was 51.1% in cv. Varuna, 54.4% in cv. RH-30, 59.3% in cv. Rohini and 63.4% in cv. Vaibhave under K-deficient conditions as compared to control conditions. The decrease in root DW was highest in cv. Vaibhave (66%) and lowest (57.1%) in cv. Varuna under K-deficient conditions (; ).
Table 1. Mean squares from analyses of variance of data for shoot and root lengths, shoot and root dry weights (DW), photosynthetic pigments, proline contents, activities of antioxidant enzymes and levels of non-enzymatic antioxidants of four mustard [B. juncea (L.) Czern & Coss.] cultivars grown under control, or potassium (K)-deficient conditions.
Note: Different letters (a–f) indicate least significant difference between and within means. Means±S.E.; n=3; Cvs, cultivars; K, K-deficiency.
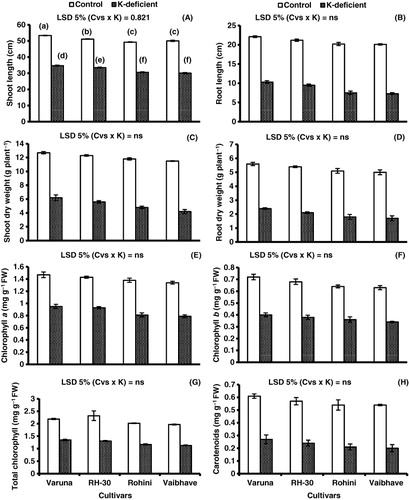
Chlorophyll and carotenoid content
The results for chlorophyll and carotenoid contents in the four mustard cvs under K+ deficiency (; ) show a minimum reduction in chlorophyll a (35.3%) in cv. Varuna and maximum (41%) in cv. Vaibhave as compared to control conditions. The decrease in chlorophyll b in cvs. Varuna, RH-30, and Rohini was 44% and that in cv. Vaibhave 46%. Of all cultivars, cvs. Varuna and RH-30 showed a minimum decrease (38%) in total chlorophyll, while cvs. Rohini and Vaibhave showed a maximum decrease (42%) in total chlorophyll. The carotenoid content was 55.7% in cv. Varuna, 57.8% in cv. RH-30, 61.1% in cv. Rohini, and 62.9% in cv. Vaibhave (; ).
Proline content
Generally, there was a substantial increase in proline content in all four mustard cvs due to K deficiency as compared to control conditions. For example, proline content was found to increase 3 times in cv. Varuna, 2.6 times in cv. RH-30, 2.4 times in both cvs. Rohini and Vaibhave as compared to control under K deficiency (; ).
Note: Different letters (a–f) indicate least significant difference between and within means. Means±S.E.; n=3; Cvs, cultivars; K, K-deficiency.
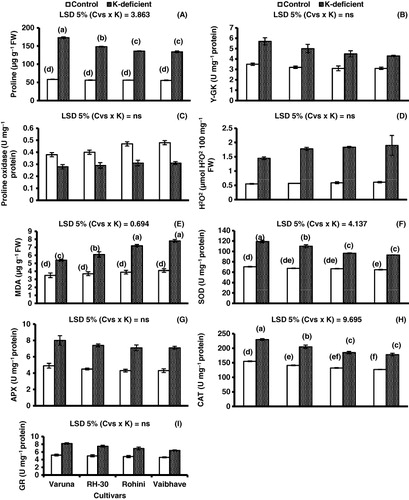
Proline metabolizing enzymes
γ- Glutamyl kinase
Of all four mustard cultivars, cv. Varuna showed 38.5% increase in γ- glutamyl kinase, whereas cv. Vaibhave showed 27.9% increase in this enzyme under K deficiency as compared to control conditions (; ).
Proline oxidase
A maximum increase in proline oxidase was observed in cv. Vaibhave (35.4%) and minimum in cv. Varuna (26.3%) under K deficiency as compared to control conditions (; ).
H2O2 content and lipid peroxidation
K deficiency enhanced the accumulation of H2O2 in all mustard varieties as compared to control conditions, however, the minimum being in cv. Varuna (62%) and maximum in the remaining three cvs (; ).
A significant increase in MDA content was observed due to K deficiency in all mustard cvs as compared to control conditions. The K deficiency induced was 35.1% in cv. Varuna, 39.3% in cv. RH-30, 46.5% in cv. Rohini, and 47.4% in cv. Vaibhave compared with control (; ).
Antioxidant enzymes
The activity of SOD enzyme increased in all mustard cvs due to K deficiency as compared to control conditions (; ). A maximum increase in SOD activity was observed in cv. Varuna (40.8%) and minimum (30%) in cvs. Rohini and Vaibhave.
K deficiency increased the CAT activity in all mustard cvs as compared to control conditions. Cv. Varuna showed a increase of 32.6% in CAT activity, cv. RH-30 31.2%, cv. Rohini and Vaibhave showed 28.6% compared with control under K-deficient regimes (; ).
The APX activity increased in all mustard varieties with K deficiency as compared to control conditions, the highest increase being in cv. Varuna (42.3%) and the minimum (39.1%) in cvs. Rohini and Vaibhave. Of all mustard cultivars, cv. RH-30 showed 40% increase in APX activity under K deficiency as compared to control (; ).
A significant increase (36.5% and 33.3%) in GR activity was observed in cvs. Varuna and RH-30, respectively, due to K deficiency, however, a minimum increase (28.1%) was observed in cv. Vaibhave compared with control (; ).
Discussion
In the present study, K-deficient plants possessed low amount of photosynthetic pigments (chlorophyll a and b), which may have been due to K deficiency-induced enhanced generation of ROS which can frequently damage the organelle membranes (Cakmak Citation2005; Ashraf Citation2009). Long ago, it was reported that photosynthetic components mainly the enzymes involved in Calvin cycle are inhibited by K deficiency (Kaiser Citation1976). K deficiency is also known to induce closing of stomata (Desikan et al. Citation2004), which adds on to the increase in ROS generation by limiting CO2 uptake efficiency. The reduction in the quantity of photosynthetic pigments leads to appearance of chlorosis and necrosis in K-deficient plants, which could be a consequence of enhanced generation of ROS (Cakmak Citation1994; Cakmak Citation2005). K deficiency also reduces ribulose bisphosphate carboxylase activity and increases mesophyll resistance thus subsequently lowering down the rate of photosynthesis (Cakmak & Engels Citation1999; Zhao et al. Citation2001).
Low K accumulation induced high production of ROS. H2O2, one of the important ROS, is known to disrupt the function of cell which in turn reduces growth and development of the plant (Cakmak Citation2005; Ashraf Citation2009; Noreen & Ashraf Citation2009; Noreen et al. Citation2010, Citation2012). For example, in Arabidopsis and maize plants, Shin and Schachtman (Citation2004) observed that K deficiency enhanced H2O2 production in root as well as leaf tissues. Generally, enhanced K deficiency is associated with a marked increase in the activities of antioxidant enzymes and play significant role in detoxification of ROS (Cakmak Citation2005), and plants have developed a complex defense system to combat the oxidative stress-induced damage (Noctor & Foyer Citation1998; Mittler Citation2002; Ashraf Citation2009). For example, SOD regulates the cellular concentration of , H2O2, CAT and several classes of peroxidases play important role to scavenge H2O2 (Scandalios Citation1993; Tsai et al. Citation2005). In the present study, K deficiency enhanced the activities of antioxidant enzymes including SOD, APX, CAT, and glutathione reductase (GR), which might have protected mustard seedlings from oxidative damage caused by K deficiency-induced excessive ROS production. Previously, while working with mulberry plants, Tewari et al. (Citation2004) also observed high activities of antioxidant enzymes but under Mg-deficient conditions.
During environmental stresses, proline accumulation occurs in most plants in large amounts (Akram et al. Citation2007; Ashraf & Foolad Citation2007; Kamran et al. Citation2009; Koyro et al. Citation2011; Shahbaz et al. Citation2011). In an earlier study with maize, salt-induced high proline concentration improved plant growth and development of maize plants (Cha-um & Kirdmanee Citation2009). Recently, transgenic approach to overproduce proline in plants has been adovocated to be an effective means of increasing stress tolerance in plants (Ashraf & Foolad Citation2007; Akram et al. Citation2012). Generally, high proline contents help to protect various vital enzymes, membranes and proteins in the plant cells by scavenging ROS (Banu et al. Citation2010), bringing about osmotic adjustment (Rhodes & Hanson Citation1993), and facilitating water uptake (Flowers Citation2004; Hassine & Lutts Citation2010; Hassine et al. Citation2010). In the present study, proline contents increased significantly in K-deficient mustard seedlings. Synthesis of proline in large amount might serve as a compatible solute that can help plant tissues to tolerate the oxidative stress caused by K deficiency.
Proline oxidase and γ-glutamyl kinase play an important role in controlling the level of proline. Proline oxidase catalyzes the conversion of proline to glutamate and γ-glutamyl kinase plays an important role in the synthesis of proline in plants (Jaleel et al. Citation2007). In the present study, increase in γ-glutamyl kinase and decrease in proline oxidase were observed due to K deficiency in mustard seedlings. Similarly, in an earlier study with sunflower, Manivannan et al. (Citation2007) observed an increase in the activity of γ-glutamyl kinase and decreased activity of proline oxidase underwater deficit conditions.
In conclusion, K deficiency led to decreased growth in terms of shoot and root lengths and DWs and photosynthetic pigments in all four cvs of mustard. Also, K deficiency initiated the generation of ROS, leading to oxidative stress in the seedlings of all mustard cvs. This triggered distinct redox changes in the cellular metabolism in response to the oxidative stress, e.g. activation of antioxidant machinery like SOD, CAT, APX, and GR. The mustard cvs. Varuna and RH-30 showed more accumulation in proline and higher activities of antioxidants, which suggests their enhanced tolerance to K-deficiency stress as compared to cvs. Rohini and Vaibhave.
References
- Ahmad P, Sharma S. 2010. Physio-biochemical attributes in two cultivars of mulberry (M. alba) under NaHCO3 stress. Int J Plant Prod. 4:79–86.
- Akmal M, Nafis T, Mirza KJ, Alam P, Mohammad A, Mujib A, Abdin MZ. 2011. High frequency somatic embryogenesis in mustard crop (B. juncea L. cv. Pusa Jai kisan): microscopic and histological analyses. Aust J Crop Sci. 5:1783–9.
- Akram MS, Ashraf M. 2011. Exogenous application of potassium dihydrogen phosphate can alleviate the adverse effects of salt stress on sunflower (Helianthus annuus L.). J Plant Nutr. 34:1041–57. doi: 10.1080/01904167.2011.555585
- Akram NA, Ashraf M, Al-Qurainy F. 2012. Aminolevulinic acid-induced regulation in some key physiological attributes and activities of antioxidant enzymes in sunflower (Helianthus annuus L.) under saline regimes. Sci Hort. 142:143–8. doi: 10.1016/j.scienta.2012.05.007
- Akram MS, Ashraf M, Shahbaz M, Akram NA. 2009. Role of foliar applied potassium from different sources on physio-biochemical attributes of sunflower (Helianthus annuus L.) under NaCl stress. J Plant Nutr Soil Sci. 172:884–93. doi: 10.1002/jpln.200900102
- Akram NA, Shahbaz M, Ashraf M. 2007. Relationship of photosynthetic capacity and proline accumulation with the growth of differently adapted populations of two potential grasses (Cynodon dactylon (L.) Pers. and Cenchrus ciliaris L.) to drought stress. Pak J Bot. 39:777–86.
- Akram NA, Shahbaz M, Ashraf M. 2008. Nutrient acquisition in differentially adapted populations of Cynodon dactylon (L.) Pers. and Cenchrus ciliaris L. under drought stress. Pak J Bot. 40:1433–40.
- Anjum SA, Xie X, Wang L, Saleem MF, Man C, Lei W. 2011. Morphological, physiological and biochemical responses of plants to drought stress. Afr J Agric Res. 6:2026–32.
- Ashraf M. 2009. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol Adv. 27:84–93. doi: 10.1016/j.biotechadv.2008.09.003
- Ashraf M, Akram NA. 2009. Improving salinity tolerance of plants through conventional breeding and genetic engineering: an analytical comparison. Biotechnol Adv. 27:744–52. doi: 10.1016/j.biotechadv.2009.05.026
- Ashraf M, Foolad MR. 2007. Roles of glycinebetaine and proline in improving plant abiotic stress resistance. Environ Exp Bot. 59:206–16. doi: 10.1016/j.envexpbot.2005.12.006
- Banu NA, Hoque A, Watanabe-Sugimoto M, Islam MM, Uraji M, Matsuoka K, Nakamura Y, Murata Y. 2010. Proline and glycinebetaine ameliorated NaCl stress via scavenging of hydrogen peroxide and methlglyoxal but not superoxide or nitric oxide in tobacco cultured cells. Biosci Biotechnol Biochem. 74:2043–9. doi: 10.1271/bbb.100334
- Bates L, Waldren RP, Teare JD. 1973. Rapid determination of free proline for water stress studies. Plant Soil. 39:205–7. doi: 10.1007/BF00018060
- Bradford MM. 1976. A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–54. doi: 10.1016/0003-2697(76)90527-3
- Cakmak I. 1994. Activity of ascorbate-dependent H2O2-scavenging enzymes and leaf chlorosis are enhanced magnesium and potassium-deficient leaves, but not in phosphorus-deficient leaves. J Exp Bot. 45:1259–66. doi: 10.1093/jxb/45.9.1259
- Cakmak I. 2005. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J Plant Nutr Soil Sci. 168:521–30. doi: 10.1002/jpln.200420485
- Cakmak I, Engels C. 1999. Role of mineral nutrients in photosynthesis and yield formation. In: Rengel Z, editor. Mineral nutrients of crops: mechanisms and implications. New York: The Haworth Press, p. 141–68.
- Carlberg I, Mannervik B. 1985. Glutathione reductase. In: Meister A, editor. Methods in enzymology. San Diego: Academic press, p. 484–90.
- Cha-um S, Kirdmanee C. 2009. Effect of salt stress on proline accumulation, photosynthetic ability and growth characters in two maize cultivars. Pak J Bot. 41:87–98.
- Desikan R, Cheung M, Bright J, Henson D, Hancock JT, Neill SJ. 2004. ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J Exp Bot. 55:205–12. doi: 10.1093/jxb/erh033
- Elumalai RP, Nagpal P, Reed JW. 2002. A mutation in the Arabidopsis KT2/KUP2 potassium transporter gene affects shoot cell expansion. Plant Cell. 14:119–31. doi: 10.1105/tpc.010322
- Flowers TJ. 2004. Improving crop salt tolerance. J Exp Bot. 55:307–19. doi: 10.1093/jxb/erh003
- Franks PJ, Farquhar GD. 2007. The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol. 143:78–87. doi: 10.1104/pp.106.089367
- Hamdia MA, Shaddad MAK. 2010. Salt tolerance of crop plants. J Stress Physiol Biochem. 6:64–90.
- Hartt CE. 1969. Effect of potassium deficiency upon translocation of C-14 in attached blades and entire plants of sugarcane. Plant Physiol. 44:1461–9. doi: 10.1104/pp.44.10.1461
- Hassine AB, Bouzid S, Lutts S. 2010. Does habitat of Atriplex halimus L. affect plant strategy for osmotic adjustment? Acta Physiol Plant. 32:325–31. doi: 10.1007/s11738-009-0410-4
- Hassine AB, Lutts S. 2010. Differential responses of saltbush Atriplex halimus L. exposed to salinity and water stress in relation to senescing hormones abscisic acid and ethylene. J Plant Physiol. 167:1448–56. doi: 10.1016/j.jplph.2010.05.017
- Hayzer DJ, Leisinger TH. 1980. The gene-enzyme relationships of proline biosynthesis in Escherichia coli. J Gen Microbiol. 118:287–93.
- Hiscox JD, Israelstam GF. 1979. A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot. 57:1332–4. doi: 10.1139/b79-163
- Hodges DM, Delong JM, Forney CF, Prange RK. 1999. Improving the thiobarbituric acid-reactive substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–11. doi: 10.1007/s004250050524
- Huang AHC, Cavalieri A. 1979. Proline oxidase and water stress induced proline accumulation in spinach leaves. Plant Physiol. 6:531–5. doi: 10.1104/pp.63.3.531
- Jaleel CA, Gopi R, Sankar B, Manivannan P, Kishorekumar A, Sridharan R, Panneerselvam R. 2007. Studies on germination, seedling vigour, lipid peroxidation and proline metabolism in Catharanthus roseus seedlings under salt stress. South Afr J Bot. 73:190–5. doi: 10.1016/j.sajb.2006.11.001
- Kaiser MW. 1976. The effect of hydrogen peroxide on CO2 fixation of isolated chloroplast. Biochim Biophys Acta. 440:476–82. doi: 10.1016/0005-2728(76)90035-9
- Kamran M, Shahbaz M, Ashraf M, Akram NA. 2009. Alleviation of drought-induced adverse effects in spring wheat (Triticum aestivum L.) using praline as a pre-sowing seed treatment. Pak J Bot. 41:621–32.
- Khan NA, Ansari HR, Khan M, Samiullah RM. 2002. Effect of phytohormones on growth and yield of Indian mustard. Indian J Plant Physiol. 7:75–8.
- Koyro HW, Ahmad P, Geissler N. 2011. Abiotic stress responses: an overview. In: Ahmad P, Prasad MNV, editors. Environmental adaptations and stress tolerance of plants in the era of climatic change. Dordrecht (NY)/Heidelberg (London): Springer, p. 1–28.
- Kuo NC, Chen C. 1980. Response of agronomic characters, seed yield, oil contents and fatty acid composition rape seed to NPK fertilizer treatments. J Agric Assoc China. 11:23–35.
- Luck H. 1974. Catalases. In: Bregmeyer HU, editor. Methods of enzymatic analysis. New York: Academic Press, vol. 2, p. 885.
- Maathuis FJM, Sanders D. 1996. Mechanisms of potassium absorption by higher plant roots. Physiol Plant. 96:158–68. doi: 10.1111/j.1399-3054.1996.tb00197.x
- Manivannan P, Jaleel CA, Sankar B, Kishorekumar A, Somasundaram R, Lakshmanan GMA, Panneerselvam R. 2007. Growth, biochemical modifications and proline metabolism in Helianthus annuus L. as induced by drought stress. Colloids Surf B Biointerf. 59:141–9. doi: 10.1016/j.colsurfb.2007.05.002
- Marschner H. 1995. Mineral nutrition of higher plants. 2nd ed. London: Academic Press Inc.
- Mengel K, Kirkby EA. 2001. Principles of plant nutrition. 5th ed. Dordrecht: Kluwer Academic Publishers.
- Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7:405–10. doi: 10.1016/S1360-1385(02)02312-9
- Nakano Y, Asada K. 1981. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplast. Plant Cell Physiol. 22:867–80.
- Noctor G, Foyer CH. 1998. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 49:249–79. doi: 10.1146/annurev.arplant.49.1.249
- Noreen Z, Ashraf M. 2009. Assessment of variation in antioxidative defense system in salt treated pea (Pisum sativum) cultivars and its putative use as salinity tolerance markers. J Plant Physiol. 166:1764–74. doi: 10.1016/j.jplph.2009.05.005
- Noreen Z, Ashraf M, Akram NA. 2010. Salt-induced regulation of some key physio-biochemical phenomena in five diverse cultivars of turnip (Brassica rapa L.). J Agron Crop Sci. 196:273–85.
- Noreen Z, Ashraf M, Akram NA. 2012. Salt-induced regulation of photosynthetic capacity and ion accumulation in some genetically diverse cultivars of radish (Raphanus sativus L.). J Appl Bot Food Qual. 85:91–6.
- Perveen S, Shahbaz M, Ashraf M. 2010. Regulation in gas exchange and quantum yield of photosystem II (PSII) in salt-stressed and non-stressed wheat plants raised from seed treated with triacontanol. Pak J Bot. 42:3073–81.
- Perveen S, Shahbaz M, Ashraf M. 2011. Modulation in activities of antioxidant enzymes in salt stressed and non-stressed wheat (Triticum aestivum L.) plants raised from seed treated with triacontanol. Pak J Bot. 43:2463–8.
- Perveen S, Shahbaz M, Ashraf M. 2012. Is pre-sowing seed treatment with triacontanol effective in improving some physiological and biochemical attributes of wheat (Triticum aestivum L.) under salt stress? J Appl Bot Food Qual. 85:41–8.
- Philippar K, Fuchs I, Luthen H, Hoth S, Bauer CS, Haga K, Thiel G, Ljung K, Sandberg G, Böttger M et al. 1999. Auxin induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proc Natl Acad Sci. 96:12186–91. doi: 10.1073/pnas.96.21.12186
- Pier PA, Berkowitz GA. 1987. Modulation of water-stress effects on photosynthesis by altered leaf K+. Plant Physiol. 85:655–61. doi: 10.1104/pp.85.3.655
- Rhodes D, Hanson AD. 1993. Quaternary ammonium and tertiary sulphonium compounds in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 44:357–84. doi: 10.1146/annurev.pp.44.060193.002041
- Scandalios JG. 1993. Oxygen stress and superoxide dismutases. Plant Physiol. 101:7–12.
- Shahbaz M, Ashraf M, Akram NA, Hanif A, Hameed S, Joham S, Rehman R. 2011. Salt-induced modulation in growth, photosynthetic capacity, proline content and ion accumulation in sunflower (Helianthus annuus L.). Acta Physiol Plant. 33:1113–22. doi: 10.1007/s11738-010-0639-y
- Shahbaz M, Ashraf M, Al-Qurainy F, Harris PJC. 2012. Salt tolerance in selected vegetable crops. Crit Rev Plant Sci 31:303–20. doi: 10.1080/07352689.2012.656496
- Shahbaz M, Ashraf M, Athar HR. 2008. Does exogenous application of 24-epibrassinolide ameliorate salt induced growth inhibition in wheat (Triticum aestivum L.)? Plant Growth Regul. 55:51–64. doi: 10.1007/s10725-008-9262-y
- Shin R, Schachtman DP. 2004. Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc Natl Acad Sci. 101:8827–32. doi: 10.1073/pnas.0401707101
- Suelter CH. 1970. Enzymes activated by monovalent cations. Science. 168:789–95. doi: 10.1126/science.168.3933.789
- Tewari RK, Kumar P, Tewari N, Srivastava S, Sharma PN. 2004. Macronutrient deficiencies and differential antioxidant responses-influence on the activity and expression of superoxide dismutase in maize. Plant Sci. 166:687–94. doi: 10.1016/j.plantsci.2003.11.004
- Tsai YC, Hong CY, Liu LF, Kao CH. 2005. Expression of ascorbate peroxidase and glutathione reductase in roots of rice seedlings in response to NaCl and H2O2. J Plant Physiol. 162:291–9. doi: 10.1016/j.jplph.2004.06.004
- Van Rossun MWPC, Alberda M, Van Der Plas LHW. 1997. Role of oxidative damage in tulip bulb scale micropropagation. Plant Sci. 130:207–16. doi: 10.1016/S0168-9452(97)00215-X
- Velikova V, Yordanov I, Edreva A. 2000. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. Plant Sci. 151:59–66. doi: 10.1016/S0168-9452(99)00197-1
- Zhao DL, Oosterhuis DM, Bednarz CW. 2001. Influences of potassium deficiency on photosynthesis, chlorophyll content, and chloroplast ultrastructure of cotton plants. Photosynthetica. 39:103–99. doi: 10.1023/A:1012404204910
