Abstract
We investigated the effects of prohydrojasmon (PDJ) [propyl (1RS,2RS)-(3-oxo-2-pentylcyclopentyl) acetate] treatment of intact lima bean plants on direct defense against two-spotted spider mites (TSSMs), Tetranychus urticae, and on the production of plant volatiles. Plants were treated with PDJ or water (control) and pieces cut from their leaves were exposed to mated TSSM females. After 3 days, there were no significant differences in TSSM mortality between the treatments, but the females laid significantly fewer eggs on treated disks. Headspace analyses of plants treated with one of three different concentrations of PDJ showed that the treatment induced (Z)-3-hexenyl acetate, (E)-β-ocimene, (Z)-β-ocimene, (E)-4,8-dimethyl-1,3,7-nonatriene, and β-caryophyllene. These compounds were expected to affect the behavioral responses of TSSMs and their natural enemies.
1. Introduction
The jasmonic acid (JA) signaling pathway is involved in the production of volatiles by plants in response to herbivory (herbivore-induced plant volatiles (HIPVs)); these volatiles attract carnivorous natural enemies of the herbivores (e.g. Hopke et al. Citation1994; Ozawa et al. Citation2000; Arimura et al. Citation2009). For example, Hopke et al. (1994) found that treating lima bean (Phaseolus lunatus L.; Fabaceae) with JA increased the emission of volatiles similar to the HIPVs released by leaves infested with either two-spotted spider mites (TSSMs), Tetranychus urticae C.L. Koch (Acari: Tetranychidae) or by beet armyworm, Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae), larvae. Application of methyl jasmonate, methyl linolenate (MeLin, a precursor of JA), and cis-jasmone also attracts natural enemies by inducing volatile production (e.g. Ozawa et al. Citation2004, Citation2008; Bruce et al. Citation2008). Furthermore, herbivore performance is negatively affected by JA and cis-jasmone treatments (e.g. Farmer & Ryan Citation1992; Bruce et al. Citation2003; Heijari et al. Citation2005; Cooper & Rieske Citation2008).
Prohydrojasmon (PDJ) [propyl (1RS,2RS)-(3-oxo-2-pentylcyclopentyl) acetate] has effects similar to endogenous JA (Koshiyama et al. Citation2006). It was developed as a plant growth regulator, particularly for coloring fruits (Koshiyama et al. Citation2006). Among the JA analogs, PDJ was selected for commercial use because of its low environmental load (fast degradation) and low toxicity to animals (Koshiyama et al. Citation2006). This compound was registered as a pesticide (Jasmomate-Ekizai®) in Japan in 2003 to accelerate apple coloration (Koshiyama et al. Citation2006). We recently reported that PDJ treatment of intact corn plants increased their attractiveness to Cotesia kariyai Watanabe (Hymenoptera: Braconidae), a parasitoid of common armyworms, Mythimna separata (Walker) (Lepidoptera: Noctuidae), and decreased armyworm performance on the treated plants (Mandour et al. Citation2013). This was the first study to show that PDJ has the potential to induce plant defenses against leaf-chewing herbivorous arthropods.
The objective of this study was to test whether PDJ treatment of plants affected the performance of sucking herbivores. To do this, we used TSSMs and their host food plant, lima bean. We also conducted headspace analyses of lima bean plants treated with PDJ to test whether PDJ induced the production of the same volatiles that were induced by TSSM damage and JA. Possible use of PDJ for the management of TSSMs was discussed.
2. Materials and methods
2.1. Plant and mites
Lima bean plants were grown in pots (8-cm diam., 7-cm depth) containing soil in a climate-controlled greenhouse (25±2°C, 60–70% RH, 16L–8D). We used lima bean plants with two fully expanded primary leaves (10–13 days old) for all experiments.
Herbivorous TSSMs were obtained from the Laboratory of Ecological Information, Graduate School of Agriculture, Kyoto University, Japan, in 2002, and reared on lima bean plants in an incubator (25±2°C, 60–70% RH, 16L–8D). Adult females of these mites were used for all experiments.
2.2. Chemicals
A commercial formulation of PDJ (5% soluble liquid; Jasmomate-Ekizai®; Meiji Seika Pharma, Tokyo, Japan) was used for all experiments. The absolute concentration of PDJ in Jasmomate-Ekizai® was not opened to the public. Fresh aqueous solutions of PDJ at different concentrations (100-, 200-, and 500-fold dilution) were prepared using distilled water.
2.3. Performance of TSSMs on leaves treated with PDJ
Twenty pots of plants (one plant per pot) were sprayed with 5 mL of a PDJ solution (100-fold dilution) at around 10:00. This amount of PDJ (ca. 0.13 µL/cm2 leaf area) was about one-fourth of the recommended treatment for apple trees (ca. 0.5 µL/cm2 leaf area of a 500-fold dilution). As a control, we sprayed 20 plants with distilled water. One or three days after spraying, we cut a piece of leaf (3×3 cm) from a plant and placed it on moist cotton wool in a Petri dish (9-cm diam.) into which three mated adult females were introduced. We called this dish a leaf disk. TSSMs were introduced to leaf disks at around 10:00, and dead mites and eggs laid on the disks were counted after incubation for 3 days in a chamber (25°C, 16L–8D). We made 10 leaf disks per treatment. One PDJ-treated leaf disk and three control leaf disks after 3 days of the treatment were excluded from the data, because pieces of leaf in these disks were under water.
2.4. Volatile analyses
Uninfested lima bean plants were prepared as described above. We sprayed 1.5 mL of PDJ solution onto each of three (100- and 200-fold dilutions) or four (0- and 500-fold dilutions) plants. One day after treatment and 4–9 h after the onset of the light period, volatiles emitted by the plants were collected and analysed. Each plant was placed in a 2-L glass bottle with two nozzles. One nozzle was connected to an air cylinder and the other to a glass tube packed with Tenax® TA adsorbent (100 mg, mesh 20/35; GL Science, Tokyo, Japan). Pure air from the cylinder entered the bottle at a flow rate of 100 mL/min, and volatile compounds from the headspace were collected with Tenax TA for 1 h. Volatile collections were repeated three to four times.
The volatile compounds collected were analysed by gas chromatography–mass spectroscopy (GC–MS; Agilent 6890 Gas Chromatograph with an HP-5MS capillary column: 30-m long, 0.25-mm i.d., 0.25-µm film thickness; Agilent 5973 Mass Selective Detector, 70 eV; Santa Clara, CA, USA) equipped with a thermal desorption cold trap injector (TCT; CP4010, Chrompack, Bergen op Zoom, The Netherlands). Headspace volatiles were released from the Tenax TA adsorbent by heating in the TCT at 220°C for 8 min under a flow of He gas. The desorbed compounds were collected in the TCT cold trap unit (SIL5CB-coated fused silica capillary) at −130°C. Flash heating of the cold trap unit injected the compounds into the capillary column of the GC. The oven temperature of the GC was programmed to rise from 40°C (5-min hold) to 280°C at 15°C/min. The headspace volatiles were identified by comparing their mass spectra to those of databases (Wiley7N and Wiley275; Agilent) and by comparing their retention times to those of authentic compounds that had previously been analysed.
2.5. Statistical analyses
Percentages of leaf disks on which at least one spider mite died were analysed by Fisher's exact test. The number of eggs laid by TSSMs was analysed with the t-test. Data for disks on which TSSMs died were excluded from analyses. Linear regression analysis using the least squares method was performed to examine the relationship between PDJ concentration and the quantities of leaf volatiles. The amounts of each volatile compound in different PDJ treatments were analysed by Dunnett's test. Volatile compound quantities were Box-Cox transformed before the statistical analyses. When a data-set had values equal to zero, 0.5 was added to all values in the data-set before Box-Cox transformation. All analyses were performed with JMP statistical software (SAS Institute Inc. Citation2010).
3. Results and Discussion
3.1. Performance of TSSMs on leaves treated with PDJ
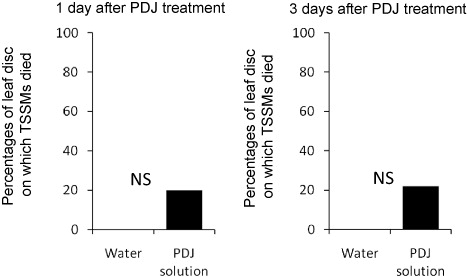
At least one TSSM died after 1 day of treatment on 0% and 20%, respectively, of the PDJ-treated and control leaf disks (Fisher's exact test, P=0.474) (a). Similarly, after 3 days of PDJ treatment, 22.2% of treated leaf disks housed dead TSSMs while 0% of control leaf disks did (Fisher's exact test, P=0.475) (b). Thus, PDJ treatment had no remarkable impacts on the mortality of TSSMs at this concentration, which was one-fourth of the manufacturer's suggested amounts for apple trees (see Section 2). PDJ toxicity to several beneficial insects was not detected (Food and Agricultural Materials Inspection Center, Japan: http://www.acis.famic.go.jp/syouroku/prohydrojasmon/index.htm, in Japanese).
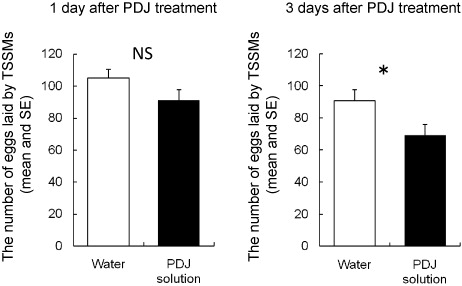
The mean numbers of eggs laid by TSSMs on leaf disks did not differ significantly between the 1-day PDJ treatment and the control (t-test, P=0.124) (a). In contrast, the mean numbers of eggs laid after 3 days differed significantly between the PDJ and control treatments (t-test, P=0.048) (b). These data suggested that PDJ itself did not affect egg production by TSSMs, but that physiological changes in the treated plants caused by PDJ did. Furthermore, changes affecting the performance of TSSMs required at least 3 days (). Choh et al. (2004) reported that JA applied to the soil of potted lima bean plants reduced the numbers of eggs laid on those plants. The data in this study clarified that PDJ treatment of lima bean leaves affected TSSMs similarly to JA treatment.
3.2. Volatile analyses
In total, 10 compounds were recorded in the headspaces of lima bean plants treated with PDJ (). Compounds were identified by comparing their GC–MS retention times and mass spectra with those of authentic samples. The amounts of (Z)-3-hexenyl acetate, (E)-β-ocimene, (E)-4,8-dimethyl-1,3,7-nonatriene, and β-caryophyllene were significantly higher in the headspaces of PDJ-treated (100-fold dilution) plants than in those of control plants (Dunnett's test, α=0.05). Linear regression analysis of the amounts of each compound (ion intensity) and the amounts of PDJ () found significant correlations for (Z)-3-hexenyl acetate (R2=0.3041; P=0.0409), (Z)-β-ocimene (R2=0.4817; P=0.0059), (E)-β-ocimene (R2=0.5462; P=0.0025), (E)-4,8-dimethyl-1,3,7-nonatriene (R2=0.7576; P<0.0001), β-caryophyllene (R2=0.6718; P=0.0003), and all compounds together (R2=0.4662; P=0.0071). These PDJ-induced volatiles were also found in the headspaces of lima bean leaves infested by TSSMs or treated with JA (Dicke et al. Citation1999). In our previous study, volatiles from lima bean leaves heavily-infested with TSSMs repelled TSSMs compared with volatiles from uninfested leaves (Horiuchi et al. Citation2003). In heavily-infested leaf volatiles, the amounts of (Z)-3-hexenyl acetate, (E)-β-ocimene, (Z)-4,8-dimethyl-1,3,7-nonatriene, (E)-4,8-dimethyl-1,3,7-nonatriene, and (E,E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene were significantly higher than those in uninfested leaf volatiles (Horiuchi et al. Citation2003). Linalool is thought to be responsible for the dispersal behavior of TSSMs (Dicke et al. Citation1993) and is induced by TSSMs damage, but this compound was not induced by PDJ treatment. We are currently studying the behavioral responses of TSSMs to PDJ-induced volatiles.
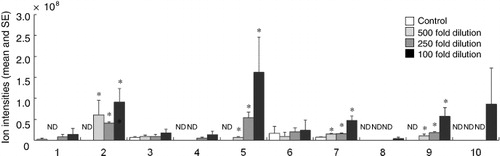
Table 1. Linear regression analysis of the amounts of individual volatile compounds emitted by lima bean plants and the amounts of prohydrojasmon (PDJ) used to treat them.
Volatiles from lima bean leaves infested with TSSMs attracted the carnivorous mite Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseiidae). Among volatiles identified in this study, (E)-β-ocimene and (E)-4,8-dimethyl-1,3,7-nonatriene are known to attract P. persimilis (Dicke et al. Citation1990; Kappers et al. Citation2005; Shimoda et al. Citation2012). Furthermore, the predatory mites Neoseiulus californicus (McGregor) (Acari: Phytoseiidae) were attracted to several volatile compounds from infested leaves that included (Z)-3-hexenyl acetate (Shimoda Citation2010). These data suggested that volatile compounds from PDJ-treated intact lima bean plants also affected the behavior of several predatory mite species.
We previously reported that both JA and SA signaling pathways were activated in lima bean plants in response to TSSM damage (Ozawa et al. Citation2000). Choh et al. (Citation2004) showed that not only JA but also benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester (BTH), a functional analog of SA, applied to the soil of potted lima bean plants reduced the numbers of eggs laid on those plants. Shimoda et al. (2002) reported that females of the predatory Oligota kashmirica benefica (Coleoptera: Staphylinidae) exhibited a significant preference for JA + methyl salicylate (MeSA)-treated lima bean leaves over uninfested leaves, and females predatory Scolothrips takahashii (Thysanoptera: Thripidae) significantly preferred both MeSA− and JA + MeSA-treated lima bean leaves to uninfested leaves. Interestingly, neither predator showed any preference for JA-treated leaves over uninfested leaves (Shimoda et al. Citation2002). Sobhy et al. (Citation2012) reported that BTH increased the attractiveness of host-infested maize plants to a parasitic wasp. Taken together, these findings suggested that the behavior/performance of TSSMs and their carnivorous natural enemies could be manipulated by using PDJ and BTH, which are functional analogs of JA and SA, respectively, as a method of biological control of TSSMs.
Acknowledgements
We thank M.W. Sabelis for comments on statistical analysis. This research was partly supported by a Grant-in-Aid for Scientific Research (S) (No. 19101009) from the Global Centre of Excellence Program ‘Formation of a Strategic Base for Biodiversity and Evolutionary Research: from Genome to Ecosystem’ of Kyoto University, and by the Japan Society for the Promotion of Science (JSPS): Core-to-Core project ‘Studies on ecological interaction networks that promote biodiversity — From gene to ecosystem.’
References
- Arimura G, Matsui K, Takabayashi J. 2009. Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol. 50:911–923. 10.1093/pcp/pcp030
- Bruce TJA, Martin JL, Pickett JA, Pye BJ, Smart LE, Wadhams LJ. 2003. cis-Jasmone treatment induces resistance in wheat plants against the grain aphid, Sitobion avenae (Fabricius) (Homoptera: Aphididae). Pest Manag Sci. 59:1031–1036. 10.1002/ps.730
- Bruce TJA, Matthes MC, Chamberlain K, Woodcock CM, Mohib A, Webster B, Smart LE, Birkett MA, Pickett JA, Napier JA. 2008. cis-Jasmone induces Arabidopsis genes that affect the chemical ecology of multitrophic interactions with aphids and their parasitoids. Proc Natl Acad Sci USA. 105:4553–4558. 10.1073/pnas.0710305105
- Choh Y, Shimoda T, Ozawa R, Dicke M, Takabayashi J. 2004. Exposure of lima bean leaves to volatiles from herbivore-induced conspecific plants results in emission of carnivore attractants: active or passive process? J Chem Ecol. 30:1305–1317. 10.1023/B:JOEC.0000037741.13402.19
- Cooper WR, Rieske LK. 2008. Differential responses in American (Castanea dentata Marshall) and Chinese (C. mollissima Blume) chestnut (Falales: Fagaceae) to foliar application of jasmonic acid. Chemoecology. 18:121–127. 10.1007/s00049-008-0399-y
- Dicke M, Vanbeek TA, Posthumus MA, Bendom N, Vanbokhoven H, Degroot AE. 1990. Isolation and identification of volatile kairomone that affects Acarine predator–prey interactions: involvement of host plant in its production. J Chem Ecol. 16:381–396. 10.1007/BF01021772
- Dicke M, Bruin J, Sabelis MW. 1993. Herbivore-induced plant volatiles mediate plant–carnivore, plant–herbivore and plant–plant interactions; talking plants revisited. In: Schultz J, Raskin I, editors. Plant signals in interactions with other plants. Rockville, MD: American Society of Plant Physiologists. p. 182–196.
- Dicke M, Gols R, Ludeking D, Posthumus MA. 1999. Jasmonic acid and herbivory differentially induce carnivore-attracting plant volatiles in lima bean plants. J Chem Ecol. 25:1907–1922. 10.1023/A:1020942102181
- Farmer EE, Ryan CA. 1992. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell. 4:129–134.
- Heijari J, Nerg AM, Kainulainen P, Viiri H, Vuorinen M, Holopainen JK. 2005. Application of methyl jasmonate reduces growth but increases chemical defence and resistance against Hylobius abietis in Scots pine seedlings. Entomol Exp Appl. 115:117–124. 10.1111/j.1570-7458.2005.00263.x
- Hopke J, Donath J, Blechert S, Boland W. 1994. Herbivore induced volatiles: the emission of acyclic homoterpenes from leaves of Phaseolus lunatus and Zea mays can be triggered by β-glucosidase and jasmonic acid. FEBS Lett. 352:146–150. 10.1016/0014-5793(94)00948-1
- Horiuchi J, Arimura G, Ozawa R, Shimoda T, Takabayashi J, Nishioka T. 2003. A comparison of the responses of Tetranychus urticae (Acari: Tetranychidae) and Phytoseiulus persimilis (Acari: Phytoseiidae) to volatiles emitted from lima bean leaves with different levels of damage made by T. urticae or Spodoptera exigua (Lepidoptera: Noctuidae). Appl Entomol Zool. 38:109–116. 10.1303/aez.2003.109
- Kappers IF, Aharoni A, van Herpen TWJM, Luckerhoff LLP, Dicke M, Bouwmeester HJ. 2005. Genetic engineering of terpenoid metabolism attracts bodyguards to Arabidopsis. Science. 309:2070–2072. 10.1126/science.1116232
- Koshiyama M, Seto H, Kamuro Y, Kateora M. 2006. A jasmonic acid analog, PDJ, comes into practical use as a plant growth regulator (in Japanese). Plant Growth Regul. 38:35–47.
- Mandour NS, Kainoh Y, Ozawa R, Uefune M, Takabayashi J. 2013. Effects of prohydrojasmon-treated corn plants on attractiveness to parasitoids and the performance of their hosts. J Appl Entomol. 137:104–112.
- Ozawa R, Arimura G, Takabayashi J, Shimoda T, Nishioka T. 2000. Involvement of jasmonate- and salicylate-related signaling pathways for the production of specific herbivore-induced volatiles in plants. Plant Cell Physiol. 41:391–398. 10.1093/pcp/41.4.391
- Ozawa R, Shiojiri K, Sabelis MW, Arimura G, Nishioka T, Takabayashi J. 2004. Corn plants treated with jasmonic acid attract more specialist parasitoids, thereby increasing parasitization of the common armyworm. J Chem Ecol. 30:1797–1808. 10.1023/B:JOEC.0000042402.04012.c7
- Ozawa R, Shiojiri K, Sabelis MW, Takabayashi J. 2008. Maize plants sprayed with either jasmonic acid or its precursor, methyl linolenate, attract armyworm parasitoids, but the composition of attractants differs. Entomol Exp Appl. 129:189–199. 10.1111/j.1570-7458.2008.00767.x
- SAS Institute Inc. 2010. JMP version 9.0.2. Cary, NC: SAS Institute Inc.
- Shimoda T. 2010. A key volatile infochemical that elicits a strong olfactory response of the predatory mite Neoseiulus californicus, an important natural enemy of the two-spotted spider mite Tetranychus urticae. Exp Appl Acarol. 50:9–22. 10.1007/s10493-009-9275-x
- Shimoda T, Nishihara M, Ozawa R, Takabayashi J, Arimura G. 2012. The effect of genetically enriched (E)-beta-ocimene and the role of floral scent in the attraction of the predatory mite Phytoseiulus persimilis to spider mite-induced volatile blends of torenia. New Phytol. 193:1009–1021. 10.1111/j.1469-8137.2011.04018.x
- Shimoda T, Ozawa R, Arimura G, Takabayashi J, Nishioka T. 2002. Olfactory responses of two specialist insect predators of spider mites toward plant volatiles from lima bean leaves induced by jasmonic acid and/or methyl salicylate. Appl Entomol Zool. 37:535–541. 10.1303/aez.2002.535
- Sobhy IS, Erb M, Sarhan AA, El-Husseini MM, Mandour NS, Turlings TCJ. 2012. Less is more: treatment with BTH and laminarin reduces herbivore-induced volatile emissions in maize but increases parasitoid attraction. J Chem Ecol. 38:348–360. 10.1007/s10886-012-0098-6