Abstract
Bacterium Pseudomonas spp. olive green (OG) was isolated from marine water, yet, it was characterized as plant growth promoting bacterium (PGPB). Multiple plant growth promoting traits of OG isolate were determined in vitro. It was tested positive for Indole-3-acetic acid (IAA) production with 29 µg ml−1 of IAA yield, phosphate solubilization with 34 µg ml−1 solubilization of Tri-calcium-phosphate and it showed maximum of 32 µg ml−1 of ammonia production. OG isolate was affirming siderophore production, hydrocyanic acid (HCN) production and catalase production. 16S rRNA gene sequence comparison was used to identify the isolate which showed its closest neighbor to be Pseudomonas fluoroscens strain BCPBMS-1. Efficacy of this PGPB was tested on the seedling growth of two test plants chickpea and green gram. Both the test plants treated with OG-based talc bioformulation showed significant growth promotion. Chickpea showed enhanced overall fresh biomass by 24%, overall dry biomass by 27% was observed after 15 days of seeded in pots. Green gram showed enhanced overall dry biomass by 28% was observed after 10 days of seeded in pots.
Introduction
The term plant growth promoting bacteria (PGPB) was first brought in use by Kloepper and Schroth (Citation1978). PGPB can directly or indirectly enhance plant growth. Direct mechanisms include production of plant hormones such as indole acetic acid (IAA), gibberellins and cytokinins (Patten & Glick Citation2002; Dey et al. Citation2004) along with asymbiotic N2 fixation (Kennedy et al. Citation1997), and solubilization of phosphates (Richardson Citation2001; Banerjee & Yasmin Citation2002). On the other hand, indirect mechanisms are the production of iron chelators, siderophores, as well as cyanides (Glick & Pasternak Citation2003; Ahmad et al. Citation2008), since they act as antagonists of plant pathogens. Such plant growth promoting organisms that possess several mechanisms that promote plant growth are known as multi trait Plant Growth Promoting Rhizobacteria (PGPR) (Rana et al. Citation2011; Bhattacharyya & Jha Citation2012). All these traits of PGPB increase seedling emergence, vigor, and yield (Autoun & Kleopper Citation2001).
Inadequate soil management by the use of chemical fertilizers has caused a global problem of nutrition depletion in soil and has made the pH of the soil acidic. Such adverse effects have caused reductions in crop production (Hungria & Vargas Citation2000). There is an immediate need to replace the use of chemical fertilizers by alternative biological fertilizers. The use of microorganisms with the aim of improving soil fertility by maintaining biogeochemical cycles for nutrition management in the soil is necessary for agriculture (Freitas et al. Citation2007). However, during the past couple of decades, the use of PGPR for sustainable agriculture has increased tremendously worldwide (Silva et al. Citation2006). Several PGPB bacterial strains including Bacillus, Pseudomonas, Enterobacter, Azotobacter, Azospirillum, Bradyrhizobium, Actinobacter etc. are being used to develop modern organic biofertilizers (Vessey Citation2003,). Furthermore, Pseudomonas fluorescens and Pseudomonas aeruginosa are two highly potent PGPR strains belonging to Pseudomonas genus and these strains are generally found in the farm soil (Ganeshan & Kumar Citation2005).
In the present study, one such strain named olive green (OG) belonging to Pseudomonas genus was isolated from sea water of Gulf of Khambhat. This strain was identified using 16S rRNA gene sequencing which showed high similarity with P. fluorescens. The research was focused to determine the plant growth promoting efficacy of nonrhizospheric marine bacterium belonging to Pseudomonas genus. To accomplish this aim, marine isolate was in vitro assessed for multiple plant growth promoting traits. The OG isolate was screened and quantified for ammonia production, phosphate solubilization, IAA production, siderophore production, hydrocyanic acid (HCN) production, and catalase production. Further to determine its plant growth promoting traits in vivo; talc-based bioformulation was prepared and its efficacy was tested on seedling growth of green gram (Vigna radiata) and chickpea (Cicer arietinum L.).
Materials and methods
Isolation of bacterial strain
Plant growth promoting bacteria OG was isolated from marine water of Gulf of Khambhat, Gujarat (22°30′N, 72°61′E). For this marine water was serially diluted in the sterile normal saline. 0.1 ml of 104 times diluted sample was inoculated on marine agar 2216 medium (HiMedia) using spread plate technique. All the individual isolates obtained were transferred to nutrient agar medium (HiMedia). The isolate showing characteristic green pigmentation was further used for the experiments and was identified using 16S rRNA gene sequencing. This marine isolate was named OG as it produced characteristic olive green pigmentation in the medium.
Growth parameters of bacterial isolate were studied using marine broth (MB) 2216 and nutrient broth (NB) under various NaCl concentrations and pH. The growth of bacterial isolate was measured by calculating fresh biomass 48 h after inoculation at 27°C with constant agitation of 150 rpm. This bacterial strain was further characterized for its plant growth promotion traits and its effect on germination of chickpea and green gram was studied. Also, pH, electrical conductivity, total dissolved solids (TDS), and salinity of marine water sample was analyzed (Ibekwe et al. Citation2010).
Identification of the organism
16S rRNA gene sequencing technique was used to identify organism along with biochemical characterization of isolate based on the Bergy's Manual of Systematic Bacteriology was performed. For the conformation of marine isolate to be Pseudomonas fluoroscens, pyoverdin, and pyocynin pigment production was tested using Pseudomonas Agar F (HiMedia) and Pseudomonas Agar P (HiMedia).
To start with 16S rRNA gene sequencing, the genomic DNA from OG isolate was extracted and the gene of interest was amplified using polymerase chain reaction. For this universal forward primer (FP) and reverse primer (RP) were procured from 1st BASE (Agile Life Science Technologies India Pvt. Ltd.). Sequence of FP was: 5′-AGAGTTTGATCCTGGCTCAG-3′ and that of RP was: 5′-AAGGAGGTGATCCAGCCGCA-3′. The reaction was carried out in a 50 µl reaction mixture containing 1.5 mM MgCl2, 0.2 mM each dNTP, 25 pmoles of FP and RP, 50 ng DNA template and 5 U Taq DNA polymerase along with reaction buffer. A 34-cycle reaction was performed at 94°C for 45 s, 58°C for 45 s, and 72°C for 105 s followed by a final extension of 10 min at 72°C. The reaction was carried out in a thermocycler. Amplified gene product was sequenced at 1st BASE (Agile Life Science Technologies India Pvt. Ltd.). The BLASTn search program (http://www.ncbi.nlm.nih.gov) was used to look for nucleotide sequence homology. The gene sequences were also submitted to GenBank and accession numbers were assigned. The sequence obtained was then aligned by ClustalW using MEGA 4.0 software (Tamura et al. Citation2007) and a neighbor-joining (NJ) tree with bootstrap value 500 was generated using the software.
Qualitative and quantitative estimation of phosphate solubilization
OG isolate was tested for phosphate solubilization on Pikovskaya's agar plate. Isolate was spot inoculated and incubated at 27±2°C and the size of the halo corresponding to phosphate solubilization was measured after 7 days of incubation. Phosphate solubilization index was calculated (dividing phosphate solubilization zone on Pikovskaya's agar by growth diameter of spot inoculants). The method by Pikovskaya (Citation1948) was used for quantitative estimation of tri-calcium phosphate solubilization by the isolate in the liquid Pikovskaya's medium and the pattern of decrease in the pH was also checked. The concentration of the soluble phosphate was estimated by stannous chloride method (King Citation1932) from the supernatant and estimation was performed at 7th, 10th, and 13th days after inoculation.
Ammonia production
Freshly grown culture was inoculated into peptone water and incubated for 7, 10, and 13 days at 27±2°C. Broth was collected, centrifuged and the amount of ammonia in the supernatant was estimated by means of Nesslerization reaction 1ml Nessler's reagent was added to 1ml of supernatant and volume of this mixture was made up to 10ml by addition of ammonia-free distilled water. Development of brown to yellow color was a positive test for ammonia production and optical density was measured by spectrophotometer at 450 nm (Demutskaya & Kalinichenko Citation2010). The concentration of Ammonia was estimated based on a standard curve of ammonium sulfate ranging from 0.1 to 1 µmol ml−1.
Indole acetic acid production
IAA production was estimated using the method described by Brick et al. (Citation1991). Ten percent of exponentially grown culture of bacterial strain of OG was inoculated in 100 ml NB with varying concentration of L-Tryptophan which ranged from 0 to 500 µg ml−1 in different flasks. The broth was collected at 24, 48, and 72 h and centrifuged at 2700 g for 15 min and was assayed for quantitative measurement of IAA, each of 1 ml cell-free supernatant was mixed vigorously with 1 ml Salkowsky's reagent (1 ml of 0.5M FeCl3 in 50 ml of 35% HClO4) along with two drops of orthophosphoric acid and assay system was kept at room 27±2 in dark for 20 min till pink color developed. Optical density was measured spectrophotometrically at 535 nm. The concentration of IAA in each sample was determined from the standard curve of IAA with the standards prepared in the range of 10–100 µg ml−1 of IAA.
Siderophore estimation
Siderophore produced by OG isolate was quantified using CAS-shuttle assay (Tank & Saraf Citation2010). Cultures were grown in deferated Fiss minimal medium. Ten milliliter sample was withdrawn and centrifuged at 2700 g for 15 min. 0.5 CAS assay solution was added to 0.5 ml of culture supernatant and mixed. This mixture was allowed to stand for 20 min. Siderophore if present will remove the iron from the dye complex, resulting in a loss of blue color of the solution. For absorbance at 630 nm measurements, minimal medium was used as blank and percentage of siderophore units were calculated by following formula:
where, A r=absorbance of reference (minimal media+CAS assay solution), A s=absorbance of sample (culture supernatant+CAS assay solution).
Catalase test and hydrocyanic acid production test
Presence of catalase was checked qualitatively using the method described by Clarke and Cowan (Citation1952). Six percent H2O2 was added on the colonies grown on N-agar plates; effervescences of O2 released from the bacterial colonies indicate the presence of catalase activity. For the qualitative estimation of HCN production, Picrate assay described by Kang et al. (Citation2010) was performed. Briefly, NB was amended with 4.4 g glycine L−1 and bacteria were streaked on modified agar plate. A Whatman filter paper no. 1 soaked in 2% sodium carbonate in 0.5% picric acid solution was placed between the base and lid of the petri dish. Plates were sealed with parafilm and incubated at 27±2°C for 4 days. After incubation, the color change of filter paper from yellow to orange-brown indicates the release of cyanide from bacterial isolate.
Preparation of talc-based bioformulation and seed bacterization
The talc-based formulations of the individual bacterial strains were prepared by following the method described by Soe and De Costa (Citation2012). Briefly, a loopful of bacterial culture was inoculated into NB and incubated in a rotary shaker at 150 rpm for 48 h at 27±2°C. Talc powder was taken in a sterilized metal tray and pH was adjusted to neutral by addition of 15 g of CaCO3 per kg of talc. Ten gram of Carboxymethyl cellulose (CMC) was added per kilogram of neutralized talc, mixed well and autoclaved. Four hundred milliliter of 48-h grown bacterial suspension was mixed with carrier-cellulose mixture under aseptic conditions. After drying (approximately 35% moisture content) overnight under sterile conditions, it was used for seed bacterization. Slurry of talc-based bioformulation was prepared and surface sterilized seeds (seeds dipped in 70% ethanol for 3 min and rinsed with sterile distilled water) were soaked overnight for the bioformulation to get coated on seeds. Efficacy of bioformulation was tested on chickpea and green gram. Seeds which were procured from Anand Agricultural University, Anand 388110, Gujarat. The purpose of using talc-based bioformulation was to allow bacterial cells to adhere seed coat due to the presence of CMC which acts as a binding agent. Second, talc-based bioformulation improves the viability of bacterial cells and shelf life of the biofertilizer. After 24 h, chickpea and green gram seeds treated with bioformulations were sowed in the pots sized 10×6 cm and the growth parameters of seedlings were analyzed. All the pot experiments were carried out during the month of June where the average temperature was 32.0°C and average humidity was 48%. Furthermore, the soil used for the growth for chickpea and green gram was tested for its physical and chemical properties as per the methods described by Ibekwe et al. (Citation2010). Physical attributed Sieve analysis and Atterberg limit (plastic limit, liquid limit, and plasticity index). For chemical properties; Total nitrogen, conductivity, TDS, pH, phosphates, sodium, potassium, calcium, and magnesium were estimated from the soil sample. Physical properties of soil were examined at Birla Vishvakarma Mahavidyalay engineering college, Material testing department, Vallabh Vidhyanagar, Gujarat and chemical properties of soil were examined at Gujarat laboratory, Ahmedabad.
Statistical analysis
After the germination of test plants the significant difference resulted by the bioformulation treatment on the seedling growth was analyzed using statistical analysis. For this analysis of variance (ANOVA) was carried out using triplicate value to identify significant difference in each vegetative parameter between treated and nontreated seeds. Mean values of triplicates were compared at significance levels of 5%, 1%, and 0.1% LSD.
Results
Study of bacterial biomass production
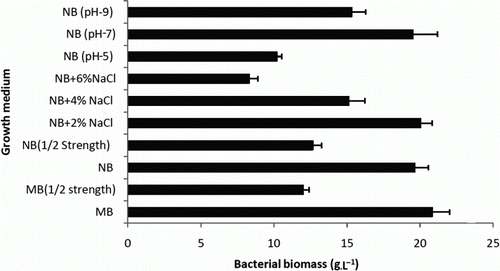
Marine water sample from which OG isolate was obtained had pH 7.7, electrical conductivity of 4.2 ms cm−1, TDS up to 2688 ml L−1, salinity was found to be 2.8 g%. Biomass production of OG isolate in NB and MB 2216 is shown in . MB medium supported maximum growth with fresh biomass of 22.1 gL−1, followed by 20.2 gL−1 in NB supplemented with 2% NaCl at neutral pH. Supplemented 2% NaCl marginally enhanced growth but the organism showed significant growth and biomass production (19.8 gL−1) in NB without NaCl supplementation. Hence, NB was used as a growth medium for further experiments.
Identification of OG isolate
16S rRNA sequencing was performed at 1st BASE (Agile Life Science Technologies India Pvt. Ltd.). After performing BLASTn, 16S rRNA sequences of organisms showing maximum similarity was aligned by using ClustalX and NJ tree was developed using MEGA 4.0 with Bootstrap values based on 500 replications are listed as percentages at the branching points (). Sequence data have been deposited in the GenBank nucleotide sequence database under the accession number JX154204. 16S rRNA of OG isolate showed maximum similarity with Pseudomonas fluoroscens strain BCPBMS-1 under the accession number HQ907732. Results of biochemical characterization are given in . OG isolate was tested positive for Pyocyanin and Pyoverdin production and also with other biochemical tests it can be confirmed that the OG isolate belongs to Pseudomonas fluoroscens spp.
Figure 2. Phylogenetic analysis based on 16S rRNA gene sequences available from European Molecular Biology Laboratory (EMBL) library constructed after multiple alignments of data by ClustalX. Distances and clustering with the neighbor joining method was performed using MEGA 4.0 software package. Bootstrap values based on 500 replications listed as per percentages at the branching points.
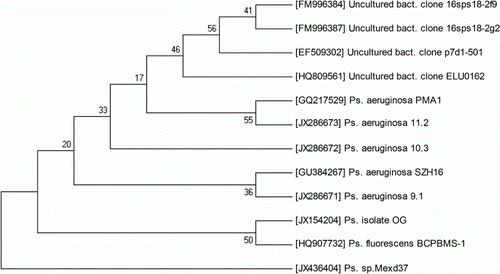
Table 1. Biochemical characterization of Pseudomonas spp. OG.
Phosphate solubilization and ammonia production
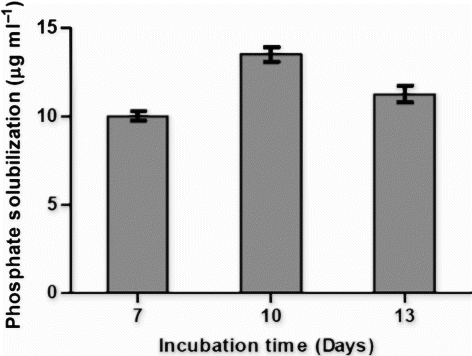
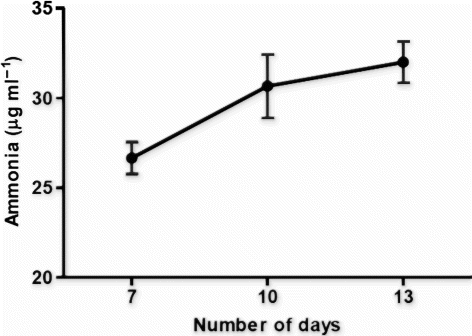
Qualitative estimation of OG isolate of phosphate solubilization showed the zone of 16 mm, diameter of spot inoculant was 7 mm and Solubilization Index so calculated was 2.28. Quantitative estimation of phosphate solubilization was performed on 7th, 10th, and 13th day after inoculation in the liquid Pikovskaya's medium (). OG isolate solubilized maximum of 34 µg ml−1 of tri calcium phosphate after 7 days of incubation. The pattern of phosphate solubilization showed constant increase till 7 days and then gradual decrease after 10 days of incubation. Ammonia production by OG isolate was determined on 7th, 10th, and 13th day from peptone water (). Maximum concentration of ammonia produced by OG isolate was 32 µg ml−1 after 13 days of incubation.
IAA production
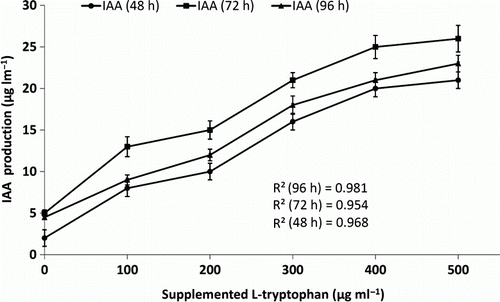
IAA production by OG isolate determined at 48 h, 78 h, and 96 h after incubation (). With 500 µg ml−1 concentration of supplemented L-tryptophan, maximum IAA production obtained was 29 µg ml−1 after 48 h. The pattern of IAA production showed constant increase up to 78 h and thereafter a continuous decrease 94 h after incubation. A correlation between IAA production and supplemented L-tryptophan was determined. Concurrent increase in IAA production was observed with an increase in L-tryptophan supplementation in the medium with correlation coefficient (R 2) being > 0.9 indicated linear relationship ().
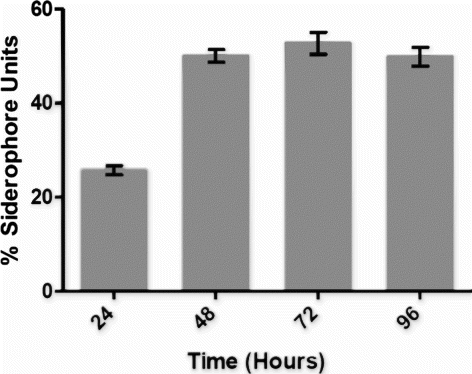
Siderophore, HCN, and catalase production
Siderophore production was determined in Fiss minimal medium at 24, 48, 72, and 96 h of incubation (). Maximum siderophore produced was 56.25% siderophore units at 72 h of incubation. There was gradual increase in the siderophore production till 72 h followed by a slight decline in siderophore after 96 h of incubation. OG was found to be producing HCN at 27±2°C. Filter paper strip impregnated with 0.5% picric acid and 2.0% sodium carbonate changed its color from yellow to orange after 72 h indicates the production of HCN. OG isolate was found to be catalase positive. Strong effervescences of O2 were evolved when 6% H2O2 solution was flooded on the colonies grown on nutrient agar. This indicates a positive qualitative result for catalase production.
Effect of talc-based bacterial bio-formulation on seedling growth of green gram and chickpea
PGPB treated and nontreated seedlings showed significant difference in the germination rate and this was true for both the test plants green gram and chickpea. Results of various growth parameters of treated and nontreated seeds of chickpea and green gram are listed in and , respectively. Various physical and chemical properties of soil used for the pot experiments are listed in . Significant increase in the seedling growth of both the plants when PGPB treated plants were compared with nontreated control is shown in . Difference in the Stem length and root length of chickpea seedlings was observed with an increase of 109% and 69% in, respectively, after 10 days of sowing. Whereas, after 15 days stem length and root length increased by 117% and 70%, respectively. There was increase by 20–24% in overall fresh biomass of chickpea seedling over 10–15 days. Also notable increase in the range of 18–22% of vegetative parameters including root fresh and dry mass, stem wet and dry mass and finally plant dry mass after 10 days was observed. Similarly, after 15 days the increase in the respective parameters was in the range of 25–31%. Moreover, green gram seedling showed increase by 53% and 55% in root length, shoot length, respectively, after 5 days of sowing and after 10 days enhancement in the growth was up to 41 and 52% in the respective vegetative parameters. The overall fresh biomass and dry biomass after 5 and 10 days of sowing were 51%, 48% and 27%, 28%, respectively. Comparing overall growth parameters namely shoot length, root length, fresh and dry biomass of plant showed significant difference in the growth of PGPB treated and nontreated seedlings and this was true for both the test plants under study. All the pot experiments were performed twice and each pot experiment replicated thrice.
Figure 7. Figure (a) showing the significant difference in PGPB treated and nontreated control seedling of green gram after 10 days, Figure (b) showing the significant difference in PGPB treated and nontreated control seedling of chickpea after 15 days.
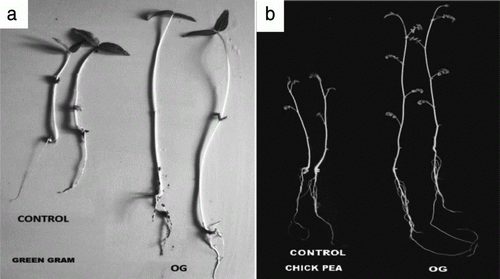
Table 2. Effect of OG-based bioformulation treatment on the seedling growth of chickpea up to at 10 and 15 days after been sown in pots. Values are the mean of triplicates with standard error of mean.
Table 3. Effect of OG-based bioformulation treatment on the germination of green gram at 5 and 10 days after been sown in pots. Values are the mean of triplicates with standard error of mean.
Table 4. Chemical and physical properties of soil used to study growth of chickpea and green gram.
Discussion
The soil bacteria that aggressively colonize the root zone and promote plant growth are generally termed as PGPR and primarily Azotobacter, Azospirillum, Bacillus, Bradyrhizobium, Enterobacter, Pseudomonas, etc. are reported as the potent PGPR strains for their ability to act as biofertilizers (Vessey Citation2003). Importance of biofertilizers has been described by many researchers around the world and their utilization has been increasing over the period of time (Silva et al. Citation2006). Haas and Défago (Citation2005) had reported certain strains of fluorescent Pseudomonas as PGPR as they promote plant growth by secreting auxins, gibberellins, and cytokinins. Fluorescent pseudomonads are nonpathogenic rhizobacteria (Jan et al. Citation2011) and several isolates of P. fluorescens, Pseudomonas putida, P. aeruginosa, and P. aureofaciens suppressed the soilborne pathogens through different proposed mechanisms including rhizosphere colonization, antibiosis, and iron chelation by siderophore production (Jan et al. Citation2011). Furthermore, it has been reported that P. putida strain AKMP7 can produce stress suppressing enzymes such as superoxide dismutase (SOD), ascorbate peroxidase (APX), and catalase under heat stress when inoculated with wheat (Triticum spp.; Ali et al. Citation2011). This shows that strains of Pseudomonas can help in plant protection and enhance plant growth by various mechanisms.
Beneficial effects of PGPR are attributed to the production of diverse metabolites including siderophores, HCN, IAA, and other associated activities such as phosphate solubilization and competition in soil and root colonization (Glick Citation1995). In the present study, marine isolate OG was identified as a species belonging to Pseudomonadaceae family and it showed maximum similarity with P. fluorescens. According to Rana et al. (Citation2011) an organism showing more than one trait of plant growth promotion are known as multi trait PGPR. OG isolate produced IAA, ammonia, siderophores etc. and showed all the possible traits of plant growth promotion.
IAA is the phytohormone known to enhance growth in terms of root and stem length of the plant (Aloni et al. Citation2006). It is reported that P. fluorescens CHAO can produce around 32 µg ml−1 of IAA and P. putida can produce up to 24 µg ml−1 of IAA (Haas & Keel Citation2003). Comparatively, OG isolate can produce 29 µg ml−1 of IAA under present study. In the pot experiments, chickpea and green gram both showed significant increase in the root growth and development which can be attributed due to significant IAA production by OG isolate. Similar findings were vindicated by Ercisli et al. (Citation2000), as they showed enhanced rooting percentage in tea cuttings (Camellia sinensis) using PGPB isolates such as Bacillus RC23, Paenibacillus polymyxa RC05, Bacillus subtilis OSU142, Bacillus RC03, Comamonas acidovorans RC41, Bacillus megaterium RC01, and Bacillus simplex RC19. Furthermore, Jha et al. (Citation2012) showed enhancement in root development of Jatropha curus up to 124.14% by Enterobacter cancerogenus MSA2 which produced up to 26 µg ml−1 of IAA, suggested that IAA had an influential role in the growth of roots. Moreover, enhancement of rooting by IAA produced by PGPR will allow plant to absorb more nutrition from the soil, which in turn causes overall enhancement in growth of the plant.
Nitrogen and phosphorous are the two most limiting nutrients in the soil as well as, two most plant enhancing nutrients (Graham & Vance Citation2000). In this context, phosphate fertilizer represents a high cost to the farmer and most of the soils are poor in available phosphorus content and, therefore, it is of interest to take advantage of soil microorganisms for the mobilization of phosphorus in soils (Richardson Citation2001). In the present study, OG isolate showed significant production of ammonia and strong tri-calcium phosphate solubilization. This infers that the presence of OG isolate in the rhizosphere can provide ammonia and available phosphorous to the plant by with nutritional need of the plant can be fulfilled. Moreover, OG isolate also showed positive tests for HCN production and siderophore production. HCN and Siderophore act as a biocontrol agents and protect plant from biotic stresses. This in turn can indirectly enhance the plant growth by keeping plant healthy and disease free (Glick & Pasternak Citation2003). P. fluorescens which can be considered the closest neighbor of OG isolate is extensively reviewed by Haas and Défago (Citation2005) as a strong biocontrol agent which produces siderophores, several antibiotics and HCN which are known to inhibit several plant pathogens including Fusarium oxysporum, Gaeumannomyces graminis, Rhizoctonia solani, etc.
Green gram and chickpea were selected to test OG talc-based formulation because these plants are easy to handle and rapid growing (Patel et al. Citation2012) OG talc-based formulation has successfully enhanced growth promotion of these two test plants. Increased root length, shoot length, biomass, and various other vegetative parameters of plants treated with OG-based bio-formulation were due to the entire plant growth promoting traits tested positive in vitro. In vitro tests must be reflected under in vivo condition hence; green gram and chickpea were selected to check the efficacy of talc-based formulation. Saravanakumar et al. (Citation2011) showed the enhancement in the growth of green gram under water stress by plant growth promoting bacterium P. fluorescens Pf1. Patel et al. (Citation2012) showed the enhancement in the germination of chickpea under saline stress by salt tolerant P. putida and Pseudomonas pseudoalcaligens PGPR. This study elucidates that the marine isolate OG was tested positive for all the plant growth promoting traits in vitro. Phylogenetic analysis based on 16S rRNA gene sequence homology showed its maximum similarity with P. fluoroscens. Furthermore, pot study showed significant enhancement in the germination of green gram and chickpea. Efficacy of OG as a potent PGPR was at par with other species of Pseudomonas when compared with the reported data.
To harness the potential of OG isolate as bio-inoculant in agriculture future studies should be carried out. Effect of this bio-inoculant in different nature of the soils should be studied and further it should be tested for other test plants. Also, field trials using randomized block design will help to understand the response of bio-inoculant under varying ecological parameters.
Acknowledgements
Authors are thankful to Department of Science and Technology (DST), New Delhi, India for the fellowship and financial aid. Authors are also thankful to Charotar University of Science and Technology (CHARUSAT) management for providing necessary facilities.
References
- Ahmad , F , Ahmad , I and Khan , MS . 2008 . Screening of free living rhizospheric bacteria for their multiple plant growth promoting activities . Microbiol Res. , 263 : 173 – 181 . doi: 10.1016/j.micres.2006.04.001
- Ali , SZ , Sandhya , V , Grover , M , Linga , VR and Bandi , V . 2011 . Effect of inoculation with a thermotolerant plant growth promoting Pseudomonas putida strain AKMP7 on growth of wheat (Triticum spp.) under heat stress . J Plant Interact. , 6 ( 4 ) : 239 – 246 . doi: 10.1080/17429145.2010.545147
- Aloni , R , Aloni , E , Langhans , M and Ulrich , CI . 2006 . Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism . Ann Bot. , 97 : 883 – 893 . doi: 10.1093/aob/mcl027
- Autoun , H and Kleopper , JW . 2001 . “ Plant growth promoting rhizobacteria (PGPR) ” . In Encyclopedia of genetics , Edited by: Brenner , S and Miller , JF . 1477 – 1480 . New York : Academic Press .
- Banerjee MR , Yasmin L. 2002 . Sulfur oxidizing rhizobacteria: an innovative environment friendly soil biotechnological tool for better canola production . Proceeding of AGROENVIRON ; 2002 Oct 26–29 ; Cairo , Egypt , p. 1 – 7 .
- Bhattacharyya , PK and Jha , DK . 2012 . Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture . World J Microbiol Biotechnol. , 28 : 1327 – 1350 . doi: 10.1007/s11274-011-0979-9
- Brick , JM , Bostock , RM and Silverstone , SE . 1991 . Rapid in situ assay for indole acetic acid production by bacteria immobilized on nitrocellulose membrane . Appl Environ Microbiol. , 57 : 535 – 538 .
- Clarke , H and Cowan , ST . 1952 . Biochemical methods for bacteriology . J Gen Microbiol , 6 : 187 – 197 .
- Demutskaya , LN and Kalinichenko , IE . 2010 . Photometric determination of ammonium nitrogen with the nessler reagent in drinking water after its chlorination . J Water Chem Tech. , 32 ( 2 ) : 90 – 94 . doi: 10.3103/S1063455X10020049
- Dey , R , Pal , KK , Bhatt , DM and Chauhan , SM . 2004 . Growth promotion and yield enhancement of peanut (Arachis hypogaea L) by application of plant growth promoting rhizobacteria . Microbiol Res. , 159 : 371 – 394 . doi: 10.1016/j.micres.2004.08.004
- Ercisli , S , Esitken , A and Sahin , F . 2000 . Effect of IBA and bacteria (Agrobacterium rubi) on the rooting of cuttings of sour cherry cv. Kutahya . Bahce. , 29 : 75 – 80 .
- Freitas , ADS , Vieira , CL , Santos , CERS , Stamford , NP and Lyra , MCCP . 2007 . Caracterização derizóbios isolados de Jacatupé cultivado em solo salino no Estado de Pernanbuco, Brasil [Characterization of rhizobia isolated from L. Pachyrhyzus erosus cultivated in saline soil of the State of Pernambuco, Brazil] . Bragantia. , 66 : 497 – 504 . doi: 10.1590/S0006-87052007000300017
- Ganeshan , G and Kumar , MA . 2005 . Pseudomonas fluorescens, a potential bacterial antagonist to control plant diseases . J Plant Interact. , 1 ( 3 ) : 123 – 134 . doi: 10.1080/17429140600907043
- Glick , BR . 1995 . The enhancement of plant-growth by free-living bacteria . Can J Microbiol. , 41 : 109 – 117 .
- Glick , BR and Pasternak , JJ . 2003 . “ Plant growth promoting bacteria ” . In Molecular biotechnology principles and applications of recombinant DNA , 3rd edn , Edited by: Glick , BR and Pasternak , JJ . 436 – 454 . Washington : ASM Press .
- Graham , PH and Vance , CP . 2000 . Nitrogen fixation in perspective: an overview of research and extension needs . Field Crops Res. , 65 : 93 – 106 .
- Haas , D and Défago , G . 2005 . Biological control of soil-borne pathogens by Fluorescent Pseudomonas . Nat Rev Microbiol. , 3 ( 4 ) : 307 – 319 . doi: 10.1038/nrmicro1129
- Haas , D and Keel , C . 2003 . Regulation of antibiotic production in root-colonizing Pseudomonas spp. and relevance for biological control of plant disease . Annu Rev Phytopathol. , 41 : 117 – 153 . doi: 10.1146/annurev.phyto.41.052002.095656
- Hungria , M and Vargas , MAT . 2000 . Environmental factors affecting N2 fixation in grain legumes in 641 the tropics, with an emphasis on Brazil . Field Crops Res. , 65 : 151 – 164 . doi: 10.1016/S0378-4290(99)00084-2
- Ibekwe , AM , Poss , JA , Grattan , SR , Grieve , CM and Suarez , D . 2010 . Bacterial diversity in cucumber Cucumis sativus rhizosphere in response to salinity, soil pH, and boron . Soil Biol Biochem. , 42 ( 4 ) : 567 – 575 . doi: 10.1016/j.soilbio.2009.11.033
- Jan , AT , Azam , M , Ali , A and Haq , Q . 2011 . Novel approaches of beneficial Pseudomonas in mitigation of plant diseases – an appraisal . J Plant Interact. , 6 ( 4 ) : 195 – 205 . doi: 10.1080/17429145.2010.541944
- Jha , CK , Patel , B and Sarf , M . 2012 . Stimulation of the growth of Jatropha curcas by the plant growth bacterium Enterobacter cancerogenus MSA2 . World J Microbiol Biotechnol. , 28 : 891 – 899 . doi: 10.1007/s11274-011-0886-0
- Kang , Y , Cheng , J , Mei , L and Yin , S . 2010 . Screening and identification of plant growth-promoting rhizobacteria . Acta microbiologica Sinica. , 50 ( 7 ) : 853
- Kennedy , IR , Pereg-Gerk , LL , Wood , C , Deaker , R , Glichrist , K and Katupitiya , S . 1997 . Biological nitrogen fixation in nonleguminous field crops: facilitating the evolution of an effective association between Azosirillum and wheat . Plant Soil. , 194 : 65 – 79 . doi: 10.1023/A:1004260222528
- King , JE . 1932 . The colorimetric determination of phosphorus . Biochem J. , 26 : 292 – 297 .
- Kloepper JW , Schroth MN. 1978 . Plant growth promoting rhizobacteria on radishes . In Proceedings of the Fourth International Conference on Plant Pathogen Bacteria (vol. 2) (p. 879–882); Aug 27–Sept 2 ; Angers: INRA .
- Patel , D , Jha , CK , Tank , N and Saraf , M . 2012 . Growth enhancement of chickpea in saline soils using plant growth-promoting rhizobacteria . J Plant Growth Regul. , 31 : 53 – 62 . doi: 10.1007/s00344-011-9219-7
- Patten , CL and Glick , BR . 2002 . Role of Pseudomonas putida indole-acetic acid in development of the host plant root system . Appl Environ Microbiol. , 68 : 3795 – 3801 . doi: 10.1128/AEM.68.8.3795-3801.2002
- Pikovskaya , RI . 1948 . Mobilization of phosphorus in soil in connection with the vital activity of some microbial species . Mikrobiologiya. , 17 : 362 – 370 .
- Rana , A , Saharan , B , Joshi , M , Prasanna , R , Kumar , K and Nain , L . 2011 . Identification of multi-trait PGPR isolates and evaluating their potential as inoculants for wheat . Ann Microbiol. , 61 : 893 – 900 . doi: 10.1007/s13213-011-0211-z
- Richardson , AE . 2001 . Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants . Aust J Plant Physiol. , 28 : 897 – 906 .
- Saravanakumar , D , Kavino , M , Raguchander , T , Subbian , P and Samiyappan , R . 2011 . Plant growth promoting bacteria enhance water stress resistance in green gram plants . Acta Physiol Plant. , 33 : 203 – 209 . doi: 10.1007/s11738-010-0539-1
- Silva , VN , Silva , LESF and Figueiredo , MVB . 2006 . Atuação de rizóbios com rizobactérias promotoras de crescimento em plantas na cultura do caupi (Vigna unguiculata L. Walp) [Rhizobias performance with rhizobacteria growth promoter in plants in the cowpea crop (Vigna unguiculata [L.] Walp)] . Acta Sci Agron. , 28 : 407 – 412 .
- Soe , KT and De Costa , DM . 2012 . Development of a spore-based formulation of microbial pesticides for control of rice sheath blight . Biocontrol Sci Techn. , 22 ( 6 ) : 633 – 657 . doi: 10.1080/09583157.2012.676025
- Tamura , K , Dudley , J , Nei , M and Kumar , S . 2007 . MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0 . Mol Biol Evol. , 24 : 1596 – 1599 .
- Tank , N and Saraf , M . 2010 . Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants . J Plant Interact. , 5 ( 1 ) : 51 – 58 . doi: 10.1080/17429140903125848
- Vessey , JK . 2003 . Plant growth promoting rhizobacteria as biofertilizers . Plant Soil. , 255 : 571 – 586 . doi: 10.1023/A:1026037216893