Abstract
The conversion of the Brazilian savannas for pastures and agricultural use has caused the species Dimorphandra wilsonii (Fabaceae-Caesalpinioideae) to become isolated and restricted to areas occupied by African grasses of Urochloa sp. This highly endangered tree species was cultivated in the presence of Bradyhizobium japonicum (symbiont 1), Glomus etunicatum (symbiont 2), and Urochloa decumbens in low nitrogen (N) availability in order to evaluate its growth in these experimental conditions. Even though the nodulation and mycorrhization was of low occurrence, the inoculated plants with symbionts had the greatest nitrogen and chlorophyll content, photosynthetic radiation use efficiency, and biomass accumulation in relation to the plants which had not been inoculated and/or cultivated in the presence of U. decumbens. The results suggest effective N2 fixation, independent of the localization of bacteria, whether in the root tissue interior or free in the rhizosphere. Therefore, the presence of N2-fixing bacteria can benefit the early growth of D. wilsonii, whereas the occurrence and aggressive persistence of U. decumbens can limit this development, increasing the threat of extinction of this species in their habitat.
Introduction
One of the most important global changes is biodiversity loss because alterations in land use, such as conversion of tropical ecosystems to pastures, is one of the main causes (Rockström et al. Citation2009). In recent decades, the Brazilian savanna biome – Cerrado – vegetation has been removed and replaced with agriculture and animal husbandry and consequently, many endemic species in these areas are critically threatened with extinction, as in the case of Dimorphandra wilsonii Rizz. This tree species with medicinal applications potential – whose fruits contain rutin, quercetin, and rhamnose – were described (Rizzini Citation1969) as occurring in the transition zone between the cerrado forest and semi-deciduous forest and 17 years later it was declared in danger of extinction (Rizzini & Matos Filho Citation1986). Actually, the population of D. wilsonii is isolated and restricted to grassland areas on private properties (Paraopeba, Minas Gerais, Brazil) – in the same region (Fernandes et al. Citation2007).
The Brazilian cerrado is established in naturally low-fertility soils and is maintained due to its equilibrium between vegetation and biochemical cycles. Thus, the introduction of exotic species of Poaceae, used for pastures, alters the ecosystems at the biotic and abiotic level (D'Antonio & Vitousek Citation1992). In tropical America, African grasses such as Urochloa decumbens are the most important of those introduced to Brazil and have increased tremendously, due to rapid adaptation and aggressive growth in Brazilian soil conditions, representing a biological invasion (Pivello et al. Citation1999). This vegetation substitution results in competition for nutrients, water, and light between introduced and remaining native/endemic species (Williams & Baruch Citation2000; Rossiello & Antunes Citation2012).
In reference to nutrient availability in soils after vegetation removal, and grassland plantation and establishment, nitrogen (N) is subject to one of the greatest losses (Vitousek Citation1982). In accordance with this information, Fonseca et al. (Citation2010a) verified that D. wilsonii presents nutritional requirements consistent with soil fertility in its native habitat but can have biological fixation of nitrogen (BFN), uncommon in the subfamily Caesalpinioideae, as an adaptive strategy to establish itself in oligotrophic soils. Fonseca et al. (2010a) also verified the roots of D. wilsonii as being colonized by arbuscular mycorrhizal (AMF) and ectomycorrhyzal (ECM), and these colonizations can also be related with the specie's adaptive strategies to survive in soils with low nutrient availability, and so not only connected to nitrogen (N), but the absorption and transference of phosphorus as well (Cruz et al. Citation2007). The triple symbiosis verified in D. wilsonii can be essential in the nutrient dynamics for this species and needs to be investigated in detail, since ECM are frequently in the Caesalpinioideae subfamily but AMF and BNF are less common in this group (Lavin et al. Citation2005).
According to Heywood and Iriondo (Citation2003), knowledge about established relationships between this species and its environment facilitates more effective conservation actions. With the species on the verge of extinction, the studies of biotic and abiotic factors that characterize the actual habitat of D. wilsonii become more relevant. With the objective of studying the relationships between these factors, D. wilsonii was cultivated in the presence of strain BHCB8.5/Bradyrhizobium sp. (BNF), Glomus etunicatum (Becker & Gerdemann) (AMF), and U. decumbens Stapf (grass). Our hypothesis is that the fungi–bacteria–plant symbiotic relationship can benefit the plant in limited N conditions and, moreover, facilitate the tree species in early growth and development, allowing for its coexistence with the expansion of African grasses in the Brazilian cerrado.
Materials and methods
Plant material
D. wilsonii Rizz. seeds from 10 remaining individuals, which had low genetic variability (Souza & Lovato Citation2010) were mechanically scarred to remove dormancy, disinfected in ethanol 70% (v/v) for one min, then in sodium hypochlorite 2.5 (v/v) for 10 min and washed liberally in sterilized distilled water. Three seeds were planted in 2.5 L pots filled with a sterilized sand and vermiculite mixture 1:1 (v/v). After germination, one healthy seedling 10 cm in height was maintained in each pot. A total of 20 plants were divided in four groups of five plants which received or did not receive inoculants or U. decumbens seedlings (). The fertilization was subdivided in two applications per week using modified Hoagland solution, ¼ ionic strength, and 1.5 mM N concentration in NH4(SO4)2 form. After 120 days of plant cultivation, D. Wilsonii plants were separated into root and shoots and dried at 60°C until constant mass. Dry biomass was used to determine root, shoot, and total mass. U. decumbens Stapf. seeds were scarred in sulfuric acid (96%, 36 N) for 15 min to break dormancy, washed liberally in sterilized distilled water and planted in 2.5 L pots containing a sterilized mixture of sand and vermiculite in proportion (1:1) (v/v). After 10 days, four of these seedlings were transplanted to pots containing D. wilsonii according to treatments (). During the experiment, U. decumbens plants were torn every 15 days at 5 cm above substrate to simulate herbivore grazing.
Table 1. Treatments used to evaluate growth of D. wilsonii.
Molecular identification of N2-fixing bacteria, viability evaluation of mycorrhiza spores and double inoculation
Strain BHCB8.5 (Bradyrhizobium sp.) was previously isolated from nodules extracted from D. wilsonii roots cultivated in pots and purified in YMA medium (Vincent Citation1970). From these pure strain cultures, bacteria gene 16S rDNA was amplified through polymerase chain reaction (PCR) using primer PA/907R. The product of this reaction was purified using Easy Spin commercial kit. The molecular fragment of interest was sequenced by Macrogen, Inc. (Korea) and the sequence obtained was compared with sequences deposited in the GeneBank (Program BLASTN 2.2.25 + ). Inoculation strain BHCB8.5 was grown in YMB culture medium (Vincent Citation1970) at 28°C, with constant agitation for 48 h. Then the microbial cells were washed, centrifuged and suspended in sterilized saline solution (NaCl 0.9%) to ensure absence of nutrients in the culture medium. The adopted treatments () received 1 mL (108 ufc mL−1) of inoculated bacterial suspension on the root zone. At the end of the experiment, D. wilsonii roots of inoculated treatments were evaluated for the occurrence of nodules. From previous identification of AMF root colonizers of D. wilsonii (Fonseca et al. Citation2010b), the AMF species selected for this study was G. etunicatum. The viability evaluation of inoculated spores, obtained by Simbyon (Czech Republic), was performed according to Walley and Germida (Citation1995). From three inoculated 100 g samples, spores were isolated by humidity sifting before centrifuging (Daniels & Skipper Citation1982). Afterwards, three samples of 40 spores were put in INT solution (iodonitrotetrazolium – 1 mg mL−1) at ambient temperature for 48 h and viewed with stereoscopic microscope. INT, in contact with spores, is reduced by dehydrogenases in tissue in vivo to become reddened. Variations in this color are proportional to enzyme activity in the spore and allow degree of viability verification. Thus, the spores colored red or orange were considered to be viable and those that were brown or black unviable. Two hundred spores of G. etunicatum inoculant per pot were used as a fungal inoculation, obtained from Simbyom (Czech Republic), applied on the root zone of adopted treatments (). After growth, D. wilsonii root fragments of 1 cm length, obtained 1–2 cm above root apices, were stained (Koske & Gemma Citation1989) and the colonization evaluated on squared plates in accordance with Giovannetti and Mosse (Citation1980).
Evaluation of double inoculation and presence of U. decembens on growth of D. wilsonii
The experimental design was completely random, performed in a factor scheme of 2×2 [presence or absence of inoculation (strain BHCB8.5 and G. etunicatum)×presence or absence of U. decumbens] with five repetitions being four treatments in total (). The experiment was conducted from autumn to winter in a greenhouse with natural light and mean environmental temperature of 16°C. The root environment temperature was maintained between 20 and 25°C using humid vermiculite heated by a thermal cover over the pots. The vermiculite humidity was controlled daily using a humidity gage (WSM – 101) in the soil and maintained by addition of sterilized distilled water when necessary. The root temperature was monitored by heat control thermostat.
Physiological indicators and chemical analysis
Photochemical reflectance index (PRI) and chlorophyll content index (CHL) were used as physiological indicators of changes in photosynthetic dynamics through light reflectance measurement on intact leaves and allowed for estimation of photosynthetic radiation use efficiency and chlorophyll content. Leaf reflectance measurements were performed with UniSpec-SC (Single channel) Spectral analysis System (Delta T [device]) on three consecutive days and full insolation in the period between 10 and 14 h. Three totally expanded leaflets from each one of the five plants of each treatment were evaluated using a ‘Standard Leaf Clip’ connected to fiber optics, to the halogen light source, and detector. The PRI was estimated using the equation: PRI=(R531 − R570)/(R531 + R570), where R531 and R570 are reflectance at 531 and 570 nm, respectively (Peñuelas et al. Citation1995). The chlorophyll content was estimated with the equation: CHL = R750/R700, where R750 and R700 are reflectance at 750 and 700 nm, respectively. The ‘R’ of each length band corresponds to the ratio between sample leaf reflectance and white reference (Richardson et al. Citation2002).
Nitrogen content estimate and C:N ratio in D. wilsonii plants were performed after the entire roots were dried at 60°C until constant mass, ground into powder (MM 2000), weighed in tin capsules that were then folded and closed. Nitrogen and carbon percentage were determined by the elemental analysis method (EuroVector) through combustion – DCT (Rodrigues et al. Citation2010). The total N content per plant was estimated from the total roots biomass N%.
In vitro evaluation of the allelopathic effect of U. decumbens on inoculated bacteria
Aqueous extracts of U. decumbens were obtained after drying the leaves and roots at 60°C in forced air circulation oven until constant mass was obtained. Leaves and roots were individually ground in water using a refrigerated pulverizing homogenizer (Omni-Mixer Sorval), in the proportion 2:50 and 0.5:50 (g ml−1), respectively. Following this, each one was centrifuged in suspension at 3500 rpm and the supernatant was considered to be 100% extract. The allelopathic effect on BHCB8.5 strain was performed with four repetitions for each of the two extracts tested and the parameter used for evaluation was observation and measurement of the bacterial halo growth inhibition in solid medium (Ferronatto et al. Citation2007). From a pure culture of strain BHCB8.5 in the exponential phase of growth inoculated by spreading the surfaces of the Petri dishes with YMA medium, previously separated in sectors (one repetition per sector). One hundred and fifty microliters of aliquots of each aqueous extract was applied using Pasteur pipette indentations and paper filter disks were placed over respective sectors of Petri dishes. One hundred and fifty microliters of distilled water was applied to the control. All Petri dishes were maintained in a growth chamber at 28°C for five days.
In vitro evaluation of the effects of growth promotion through strain BHCB8.5
Three culture mediums without N: YMA (Vincent Citation1970), Ashby's Manitol M706 (Subba-Rao Citation1977), and LGI (Magalhães et al. Citation1983) were tested. Strain BHCB8.5 was grown in YMA medium and replicated on Petri dishes with the different culture mediums, four repetitions per culture medium, in accordance with the successive dilution method proposed by Oliveira and Magalhães (Citation1999). The Petri dishes were maintained in growth chambers at 28°C and evaluations performed every 5 days, after replication, for 15 days. According to Petri dishes, zone growth points were classified as varying between one (without visible growth) and four (growth in all zones). On the basis of growth in each zone, the strain was classified as having low, moderate, or effective growth in culture medium without N sources. Indole acetic acid (IAA) production by strain BHB8.5 was analyzed by the colorimetric method as proposed by Gordon and Weber (Citation1951). The culture was grown until the exponential phase in YMB medium and then centrifuged at 6000 g for 15 min at 4°C. Subsequently, 2 mL of supernatant was taken and transferred to test tubes in which 2 mL of reagent was then added [1 mL FeCl3 – (0.5 mM) in HClO4 35%]. The tubes were agitated by machine, incubated for 25 min, and absorbance measures were taken at 530 ηm using a spectrophotometer (Camspec M105).
Statistical analysis
Relative growth statistics of D. wilsonii were compared with different treatments for variance analysis (ANOVA), followed by Duncan mean testing (P≤0.05). The existence of linear correlations between the growth parameters studied was examined using Pearson's correlation. In all cases, preliminary analyses proved normal data distribution. All statistical analyses were performed using SPSS program version 19.0.
Results
Strain BHCB8.5 was identified as Bradyrhizobium japonicum bv. glycinearum (USDA 110) and was able to produce nodules. The result of the fungal inoculate viability test for G. etunicatum demonstrated that 72% of the spores were viable. Therefore, both inoculates showed capability to establish symbiosis in inoculate treatments (Symb and Symb + Ud). After 120 days of growth, only one nodule in early development stage was verified in the Symb treatment within the inoculated D. wilsonii plants (Symb and Symb + Ud), having undifferentiated cell mass joined to the emergent region of one of the lateral roots. Mycorrhiza infection was not detected in this treatment's roots. Nodule occurrence was not verified in the Symb + Ud treatment, but the roots of all repetitions presented 1% mycorrhization rate. The Symb treatment plant accumulated the greatest root biomass (), the greatest photosynthetic radiation use efficiency (a), the greatest chlorophyll content (b), and greatest N content (a). When these parameters were compared jointly, some correlations became evident (). Root biomass was positively correlated to shoot biomass (r=0.83), total biomass (r=0.95), and to chlorophyll/CHL (r=0.69). Also, PRI presented positive correlation with shoot (r=0.61) and total biomass (r=0.60), and with chlorophyll/CHL content (r=0.65) and N content (r=0.64). Chlorophyll content also positively correlated with root (r=0.69) and total (r=0.64) biomass.
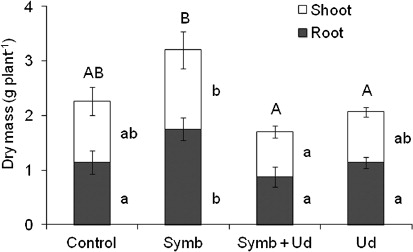
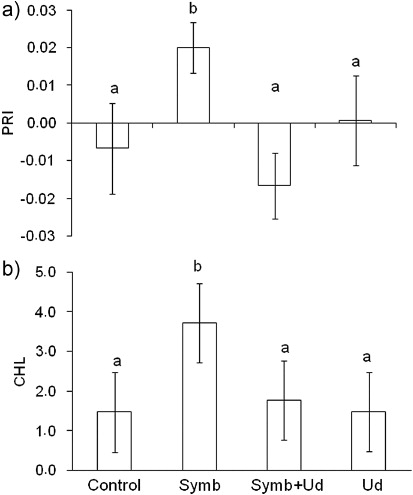
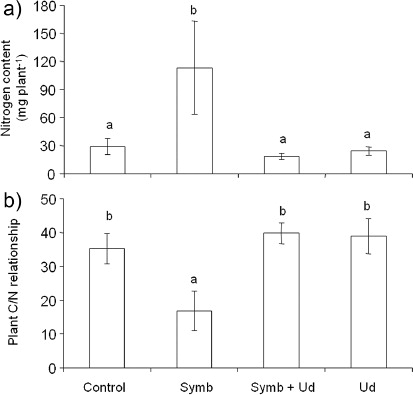
Table 2. Pearson's correlation coefficient between growth parameters of D. wilsonii after 120 days of growth.
Nitrogen content only correlated positively with PRI (r=0.64). ANOVA results showed that the presence of U. decumbens and inoculates (Symb + Ud) resulted in less: (1) root, shoot, and total biomass (); (2) PRI, (3) relative chlorophyll content (a and b), and (4) N content and the greatest C:N ratio (a and b). Results obtained for C:N ratio revealed no significant difference between D. wilsonii and U. decumbens joint treatments (Symb + Ud and Ud) and the control, suggesting that there was no competition for nutrients between these species (b).
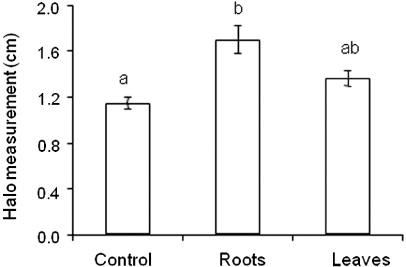
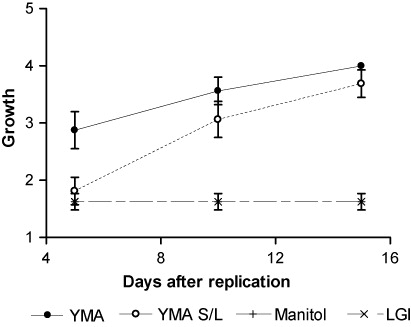
Symb + Ud treatment plants did not present nodules and raised the hypothesis that the presence of Ud hinders symbiosis. Thus, the allelopathic effect of this Poaceae on BHCB8.5 strain was evaluated in vitro and the result demonstrated that the extracts, mainly from roots, induced bacterial growth inhibition in growth medium (). Since AMF fungi are obligate symbionts and did not have root colonization in the Symb treatment, the positive effect observed in this treatment can be related to the bacterial inoculate. Thus, tests were performed to evaluate the growth promotion potential of the strain. To verify whether the bacteria could have free-living N2-fixing capacity, strain BHCB8.5 growth of without N source was evaluated in vitro and the result demonstrated low growth in Ashby's Manitol M706 and LGI medium. In YMA medium without yeast extract, the strain exceeded the average growth reference point, despite having presented low early growth (). The greatest root growth observed in Symb treatment plants could have been related to the plant's hormonal balance and thus a test of IAA production by the strain was conducted in vitro, yet the result was negative.
Discussion
The genetic similarity between strain BHCB 8.5 and B. japonicum bv. glycinearum (USDA 110) is convergent with the history of occupation of the Cerrado for large-scale soya production [Glycine max L. (Merrill)], when this bacteria was introduced, adapted, and naturalized in Brazilian soils (Ferreira & Hungria Citation2002). In the present study, although the Symb treatment had incipient nodulation and mycorrhization had not occurred, the B. japonicum (strain BHCB8.5) was effective, since these plants presented the greatest root biomass, photosynthetic efficiency, chlorophyll, and N content (,a, b and a). The fact that nodulation did not occur in treatment Symb + Ud could also be due to the presence of U. decumbens, suggested by the evaluation test results in vitro of the allelopathic effect of this specie on strain BHCB8.5 (). The allelopathic effect of Urochloa sp. in plants has been well described in the literature (Souza et al. Citation2006), but little is known about the allelopathic effect of these on soil microorganisms, although the effect of U. humidicola as a nitrification inhibitor is known (Subbarao et al. Citation2006).
With reference to the mycorrhiza inoculate, although the AMF fungi colonizers of D. wilsonii roots have been not identified at specie level (Fonseca et al. Citation2010b), the choice of G. etunicatum as an inoculate was due to an abundance in agricultural soils (Douds et al. Citation1995) and, at the same time, being one of the most well-distributed fungi in terms of colonization and positive effect in tropical tree species (Pouyu-Rojas et al. Citation2006). In our study, mycorrhization was exclusively detected in D. wilsonii roots in Symb + Ud treatment, but this was also incipient (1% mycorrhization rate), even though the inoculate presented 72% viability. Since the inoculates used were viable and had detectable effects of symbionts, why were nodulation and mycorrhization so incipient? The infection process of host plant roots by rhizobia and AMF, as much as this is controlled by the plant, is also affected by environmental conditions (Bécard et al. Citation1992; Hartwig Citation1998). In low nitrogen and phosphor concentrations and other nutrients, plants exude phenolic compounds that directly control processes that improve the absorption and assimilation of nutrients in the rhizosphere, mainly to activate chemical signals to attract microorganisms and to promote the growth of free-living micro-organisms in the soil (Cesco et al. Citation2012).
In relation to rhizobia infection, the insufficient exudation of these compounds, mainly flavonoids, interferes in the induction of gene nod and, consequently, in the production and liberation of Nod factors that are fundamental for the infection process and nodule formation (Hartwig Citation1998). Flavonoids also are determinants in the early root colonization process by AMF through inducing spore germination or hypha growth (Dakora & Phillips Citation2002). Flavonoids biosynthesis is initiated by a phenylalanine conversion in cinnamic acid reaction catalyzed by phenylalanine ammonia lyase (PAL), and endogenous factors such as genotype and plant development stage can affect its activity (Cesco et al. Citation2012). Light and temperature are environmental factors that interfere in PAL activity and, consequently in flavonoid biosynthesis. Some studies demonstrated that low temperatures can increase flavonoids production but the accumulation of these is dependent on light (Jaakola & Hohtola Citation2010). Even though the temperature in the plant roots zone was maintained in the range of 20–25°C, favorable for both rhizobia and AMF (Wang et al. Citation2002; Zhang et al. Citation2003), environmental light quality, and greenhouse temperature could have limited photosynthetic activity and therefore affected flavonoids production, resulting in nodulation and incipient rate of mycorrhization. Thus, how can the effect of D. wilsonii growth promotion, verified in treatment Symb (,a, b and a), be explained? Research about the growth promotion capacity of Rhizobium and Bradyrhizobium species, beyond N2 fixation inside the nodule, refers to this benefit associated with non-Fabaceae species, principally in culture rotation systems where a Fabaceae precedes a non-Fabaceae (Hayat et al. Citation2010). These two rhizobacterias genera produce molecules such as phytohormones, phosphate solubilizers, inhibitors of pathogenic micro-organisms, Nod factors, and vitamins that help plant growth. In this study, it was verified that Symb treatment plants accumulated greater biomass, mainly in the roots (). Root growth is directly linked to hormonal balance between IAA and kinetins, and the production or the balance of these phytohormones can be stimulated by rhizobacteria growth promoters (PRGP). Antoun et al. (Citation1998) tested the action of Rhizobium and Bradyrhizobium inoculants in Raphanus sativus L. (Brassicaceae), and observed that 33% of the 18 B. japonicum inoculants, including the genotype similar to strain BHCB8.5 (USDA 110), produce IAA but only 5% of these inoculants solubilized bicalcium phosphate. Results of the IAA production test in vitro by strain BHCB8.5 were negative, but this result might not be conclusive since the concentration of the tryptophan source in the culture medium may not have been sufficient for detectable quantities of IAA synthesis (Spaepen & Vanderleyden Citation2010).
D. wilsonii plants of Symb treatment presented the greatest (PRI) which correlated positively with shoot biomass, total biomass, and chlorophyll content. Rhizobiaceae family bacteria produce molecule signals, Nod factors, which as well as fulfilling a fundamental role in establishing a plant–bacteria symbiosis, can also act in other physiological plant processes as growth promoters (Dakora Citation2003). In soya, Nod factors spray (10−7 M) isolated from B. japonicum increased the photosynthetic rate and total biomass which suggested that photosynthetic rates were stimulated by Nod factors due to an increased meristem activity in a sink organ (Almaraz et al. Citation2007). The greatest root biomass, verified in Symb treatment plants, could have functioned as a sink in the present study, thus stimulating PRI. However, these plants presented the greatest chlorophyll content and, according to Peñuelas and Filella (Citation1998), there is a linear correlation between chlorophyll content and plant N status. Our results demonstrated that not only chlorophyll content but also N content was greatest in Symb treatment plants (b and a) and we allowed this to infer that the B. Japonicum contribution to growth was therefore related to N2 fixation. Nitrogen fixation is considered rare in the Caesalpinioideae subfamily but it has been confirmed in the group Caesalpinieae, which includes Dimorphandra, that all the genera fix N2 inside nodules (Sprent Citation2007). However, the present results suggest that N2 fixation occurred without having formed the necessary nodules. The hypothesis that N supply could have occurred through N2 fixation by free-living BHCB8.5 strain bacteria was not confirmed, since the bacteria presented effective growth only in one of the culture mediums without N source (). Therefore, it was verified in the literature that in two other genera of the Caesalpinieae group, Gleditsia and Peltophorum, roots can be invaded by rhizobia with later infection chain formation, even though N2 fixation occurs inside root cells without having nodule formation (Sprent & James Citation2007). Furthermore, Bryan et al. (Citation1996) detected nitrogenase activity from acetylene reduction in 13 noon-nodulated roots of Fabaceae species. Thus, these authors concluded that N2 fixation could happen in Fabaceae subfamilies even without nodule formation and this mechanism of fixation could be the base of symbiotic Fabaceae–Rhizobiaceae evolution. Based on this, and in the expressive N contributions for plants from Symb treatments (a), the hypothesis that N2 fixation had occurred inside D. wilsonii root cells cannot be eliminated. In the environmental conditions in which this study was performed, competition for nutrients between D. wilsonii and U. decumbens was not verified (b) but it is important to highlight that the presence of this Poaceae (Symb + Ud) can inhibit the plant's establishment and, therefore, the positive effect of the symbiosis between B. japonicum and D. wilsonii (Symb) (,a, b and a). Confronted with a species threatened with extinction, especially through natural habitat degradation, the information obtained in this study could contribute to the management of D. wilsonii in situ, for the establishment of parameters that assist this species’ reintroduction process and, consequently, in the maintenance of its germplasm. However, further studies are necessary with more favorable environmental conditions regarding these biotic interactions, including N availability.
Acknowledgements
The authors thank the Coordination for the Improvement of Higher Education Personnel (CAPES) for foreign research grants (Environmental Biology Center, Science Faculty of the University of Lisbon, Portugal), the Postgraduate program in Plant Biology of the Federal University of Minas Gerais (UFMG), and the Foundation of Zoological Botany in Belo Horizonte, Brazil. The authors also thank Alistair Hayward for the translation and critical review of the original Portuguese text.
References
- Almaraz JJ, Zhou X, Souleimanov A, Smith D. 2007. Gas exchange characteristics and dry matter accumulation of soybean treated with Nod factors. J Plant Physiol. 164:1391–1393. 10.1016/j.jplph.2006.12.007
- Antoun H, Beauchamp CJ, Goussard N, Chabot R, Lalande D. 1998. Potential of Rhizobium and Bradyrhizobium species as plant growth promoting rhizobacteria on non-legumes: effect on radishes (Raphanus sativus L.). Plant Soil. 204:57–67. 10.1023/A:1004326910584
- Bécard G, Douds DD, Pfeffer P. 1992. Extensive in vitro hyphal growth of vesicular-arbuscular mycorrhizal fungi in the presence of CO2 and flavonols. Appl Environ Microbiol. 58:821–825.
- Bryan JA, Berlyn GP, Gordon JC. 1996. Toward a new concept of the evolution of symbiotic nitrogen fixation in the leguminosae. Plant Soil. 186:151–159. 10.1007/BF00035069
- Cesco S, Mimmo T, Tonon G, Tomasi N, Pinton R, Terzano R, Neumann G, Weisskopf L, Renella G, Landi L, Nannipieri P. 2012. Plant-borne flavonoids release into the rhizosphere: impact on soil bio-activities related to plant nutrition: a review. Biol Fert Soil. 48:123–149. 10.1007/s00374-011-0653-2
- Cruz C, Egsgaard H, Trujillo C, Ambus P, Requena N, Martins-Loução MM, Jakobsen I. 2007. Enzymatic evidence for the key role of arginine in nitrogen translocation by arbuscular mycorrhizal fungi. Plant Physiol. 144:782–792. 10.1104/pp.106.090522
- D'Antonio CM, Vitousek PM. 1992. Biological invasions by exotic grasses, the grass/fire cycle, and global change. Ann Rev Ecol Sys. 23:63–87.
- Dakora FD. 2003. Defining new roles for plant and rhizobia molecules in sole and mixed plant cultures involving symbiotic legumes. New Phytol. 158:39–49. 10.1046/j.1469-8137.2003.00725.x
- Dakora FD, Phillips DA. 2002. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil. 245:35–47. 10.1023/A:1020809400075
- Daniels BA, Skipper HA. 1982. Methods for the recovery and quantitative estimation of propagules from soil. In: Schenck NC, editor. Methods and principles of mycorrhizal research. St. Paul, MN: American Phytopathological Society. p. 29–35.
- Douds Junior DD, Galvez L, Janke RR, Wagoner P. 1995. Effect of tillage and farming system upon populations and distribution of vesicular-arbuscular mycorrhizal fungi. Agric Eco Environ. 52:111–118. 10.1016/0167-8809(94)00550-X
- Fernandes FM, Fonseca AG, Kaechele K, Goulart MF, Marinho W, Souza HAV, Queiroz AR, Giorni V, Oliveira G, Rodrigues MJ, et al. 2007. Tentando evitar mais uma extinção: o caso do Faveiro de Wilson (Dimorphandra wilsonii Rizz.) [Trying to prevent another extinction: the case of Wilson faveiro]. In: Investing in Nature editor. Recuperando o verde para as cidades – A Experiência dos Jardins Botânicos Brasileiros [City regreening – the Brazilian experience of botanical gardens]. Rio de Janeiro: Brazilian Network of Botanical Gardens, Botanical Garden Research Institute of Rio de Janeiro; BGCI; p. 87–98.
- Ferreira MC, Hungria M. 2002. Recovery of soybean inoculant strains from uncropped soils in Brazil. Field Crops Res. 79:139–152. 10.1016/S0378-4290(02)00119-3
- Ferronatto R, Marchesan ED, Pezenti E, Bedmarski F, Onofre SB. 2007. Atividade antimicrobiana de óleos essenciais produzidos por Baccharis dracunculifolia D.C. e Baccharis uncinella D.C. (Asteraceae) [Antimicrobial activity of essential oils produced by Baccharis dracunculifolia D.C. and Baccharis uncinella D.C. (Asteraceae)]. Rev Bras Farmacogn. 17:224–230. 10.1590/S0102-695X2007000200016
- Fonseca MB, França MGC, Zonta E, Giorni V. 2010a. Crescimento inicial de Dimorphandra wilsonii (Fabaceae – Caesalpinioideae) em diferentes condições de fertilidade em solo de cerrado [Early development of Dimorphandra wilsonii (Fabaceae – Caesalpinioideae) in Cerrado under different soil fertility conditions]. Acta Bot Bras. 24:322–327. 10.1590/S0102-33062010000200003
- Fonseca MB, Simões JL, Isaías RMS, França MGC, Scotti MR, James EK, Sprent J. 2010b. Rhizobial and arbuscular mycorrhizal fungal symbioses in Dimorphandra wilsonii, a threatened caesalpinioid legume native to Brazilian Cerrado. In: XIII National Meeting of the Spanish Society of Nitrogen Fixation and II Portuguese-Spanish Congress on Nitrogen Fixation (Book Contributions). Zaragoza, Spain (): Manuel Becana; p. 127–128.
- Giovannetti M, Mosse B. 1980. An evaluation of techniques to measure vesicular-arbuscular mycorrhizal infection in roots. New Phytol. 84:489–500. 10.1111/j.1469-8137.1980.tb04556.x
- Gordon SA, Weber RP. 1951. Colorimetric estimation of indoleacetic acid. Plant Physiol. 26:192–195. 10.1104/pp.26.1.192
- Hartwig UA. 1998. The regulation of symbiotic N2 fixation: a conceptual model of N feedback from the ecosystem to the gene expression level. Perspect Plant Ecol Evol Syst. 1:92–120. 10.1078/1433-8319-00054
- Hayat R, Ali S, Ummay A, Khalid R, Ahmed I. 2010. Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol. 60:579–598. 10.1007/s13213-010-0117-1
- Heywood VH, Iriondo JM. 2003. Plant conservation: old problems, new perspective. Biol Conserv. 113:321–335. 10.1016/S0006-3207(03)00121-6
- Jaakola L, Hohtola A. 2010. Effect of latitude on flavonoid biosynthesis in plant space. Plant Cell Environ. 33:1239–1247.
- Koske RE, Gemma JN. 1989. A modified procedure for staining roots to detect V-A mycorrhizas. Mycol Res. 92:486–488. 10.1016/S0953-7562(89)80195-9
- Lavin M, Herendeen PS, Wojciechowski MF. 2005. Evolutionary rates analysis of Leguminosae implicates a rapid diversification of lineages during the tertiary. Syst Biol. 54:575–594. 10.1080/10635150590947131
- Magalhães FM, Baldani JI, Souto SM, Kuykendall JR, Döbereiner J. 1983. A new acid-tolerant Azospirillum species. An Acad Bras Ci. 55:417–430.
- Oliveira LA, Magalhães HP. 1999. Quantitative evaluation of acidity tolerance of root nodule bacteria. Rev Microbiol. 30:203–208. 10.1590/S0001-37141999000300004
- Peñuelas J, Filella I. 1998. Visible and near-infrared reflectance techniques for diagnosing plant physiological status. Trends Plant Sci. 3:151–156. 10.1016/S1360-1385(98)01213-8
- Peñuelas J, Filella I, Gamon JA. 1995. Assessment of photosynthetic radiation-use efficiency with spectral reflectance. New Phytol. 131:291–296. 10.1111/j.1469-8137.1995.tb03064.x
- Pivello VR, Shida CN, Meirelles ST. 1999. Alien grasses in Brazilian savannas: a threat to the biodiversity. Biodiver Conserv. 8:1281–1294. 10.1023/A:1008933305857
- Pouyu-Rojas E, Siqueira JO, Santos JGD. 2006. Compatibilidade simbiótica de fungos micorrízicos arbusculares com espécies arbóreas tropicais [Symbiotic compatibility of arbuscular mycorrhizal fungi between tropical tree species]. Rev Bras Ci Solo. 30:413–424. 10.1590/S0100-06832006000300003
- Richardson AD, Duigan SP, Berlyn P. 2002. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 153:185–194. 10.1046/j.0028-646X.2001.00289.x
- Rizzini CT. 1969. Espécies novas de árvores do planalto central brasileiro [New tree species on the central Brazilian plateau]. An Acad Brasil Ci. 41:239–244.
- Rizzini CT, Matos Filho A de. 1986. Espécies vegetais em extinção [Endangered plant species]. Bol Soc Brasil Cons Nat. 21:99–104.
- Rockström J, Steffen W, Noonel K, Persson A, Chapim FSIII, Lambin EF, Lenton TM, Scheffer M, Folke C, Schellnhuber HJ et al. 2009. A safe operating space for humanity. Nature. 461:472–475. 10.1038/461472a
- Rodrigues CI, Maia R, Máguas C. 2010. Comparing total nitrogen and crude protein content of green coffee beans (Coffea spp.) from different geographical origins. Coffee Sci. 5:1–9.
- Rossiello ROP, Antunes MAH. 2012. Solar radiation utilization by tropical forage grasses: light interception and use efficiency. In: Babatunde EB, editor. Solar radiation, a friendly renewable energy source. Croatia: InTech. p. 221–244.
- Souza HAV, Lovato MB. 2010. Genetic diversity and structure of the critically endangered tree Dimorphandra wilsonii and of the widespread in the Brazilian Cerrado Dimorphandra mollis: implications for conservation. Biochem Systematic Ecol. 38:49–56. 10.1016/j.bse.2009.12.038
- Souza LS, Veline ED, Martins D, Rosolem CA. 2006. Efeito alelopático do capim-braquiária (Brachiaria deecumbens) sobre o crescimento inicial de sete espécies de plantas cultivadas [Allelopathic effects of a grass (Brachiaria decumbens) on the early growth of seven cultivated plant species]. Pl Daninha. 24:657–668. 10.1590/S0100-83582006000400006
- Spaepen S, Vanderleyden J. 2010. Auxin and plant-microbe interactions. Cold Spring Harb Perspect Biol. 1438:1–13.
- Sprent JI. 2007. Evolving ideas of legume evolution and diversity: a taxonomic perspective on the occurrence of nodulation. New Phytol. 174:11–25. 10.1111/j.1469-8137.2007.02015.x
- Sprent JI, James EK. 2007. Where do nodules and mycorrhizas fit in? Plant Physiol. 144:575–581. 10.1104/pp.107.096156
- Subba-Rao NS. 1977. Soil microorganisms and plant growth. New Delhi, India: Oxford and IBH Publishing Co. Pvt. Ltd.; p. 250.
- Subbarao GV, Ishikava T, Ito O, Nakahara K, Wang HY, Berry WL. 2006. A bioluminescence assay to detect nitrification inhibitors released from plant roots: a case study with Brachiaria humidicola. Plant Soil. 288:101–112. 10.1007/s11104-006-9094-3
- Vincent JM. 1970. A practical manual for the study of root-nodule bacteria. Oxford: Blackwell Scientific Pub. Ltd. ( International Biological Program Handbook, 15); p. 164.
- Vitousek PM. 1982. A comparative analysis of potential nitrification and nitrate mobility in forest ecosystems. Ecol Monogr. 52:155–177. 10.2307/1942609
- Walley FL, Germida JJ. 1995. Estimating the viability of vesicular-arbuscular mycorrhizae fungal spores using tertrazolium salts as vital stains. Mycologia. 87:273–279. 10.2307/3760914
- Wang B, Funakoshi DM, Dalpé Y, Hamel C. 2002. Phosphorus-32 absorption and translocation to host plants by arbuscular mycorrhizal fungi at low root-zone temperature. Mycorrhiza. 12:93–96. 10.1007/s00572-001-0150-9
- Williams DG, Baruch Z. 2000. African grass invasion in the Americas: ecosystem consequences and role of ecophysiology. Biol Invas. 2:123–140. 10.1023/A:1010040524588
- Zhang H, Prithiviraj B, Charles TC, Driscoll BT, Smith DL. 2003. Low temperature tolerant Bradyrhizobium japonicum strains allowing improved nodulation and nitrogen fixation of soybean in a short season (cool spring) area. Eur J Agron. 19:205–213. 10.1016/S1161-0301(02)00038-2