Abstract
Soil salinization in arid zones is a major factor that resulted in the reduction in the yield and quality of many important crops in Northwestern China. In this study, the potential mechanism of flue gas desulfurization gypsum by-product (FGDB) mediated amendment of alkaline soils was investigated in an oil sunflower model by accessing the Ca2+ distribution and Ca2+-ATPase activity in leaf cells. Our results demonstrated an increased calcium concentration, as well as intact chloroplast structure with increasing calcium precipitates in the cell wall, intercellular space, and vacuole of leaf cells in the plants grown in alkaline soils supplied with FGDB or CaSO4. Additionally, a dose-dependent Ca2+-ATPase activity was detected in the plasma membrane and tonoplast of leaf cells from the plants grown in FGDB or CaSO4 supplemented soils. These results implied that the Ca2+-ATPase activity cause cytosolic Ca2+ efflux. The Ca2+ influx is through the Ca2+-channels, and increasing cytosolic Ca2+ concentration might benefit the stability and integrity of cell membrane and cell wall, sequentially alleviated the injury of oil sunflower against alkali stress.
Introduction
Salinity in arid zones is one of the major abiotic factors limiting agricultural and forestry productivity, rendering an estimated one-third of the world's irrigated land unsuitable for crops (Frommer et al. Citation1999). The amount of salt-affected soil is increasing due to the increase in the evaporation rate and excessive cultivation, which has become a serious issue in Northwestern China. Alkaline soil accumulates salt on its surface, and it is difficult for plants to grow. Salt tolerance of a plant is a complex trait involving responses to cellular osmotic and ionic stresses, as well as their consequent secondary stresses and whole plant coordination. The complexity and polygenic nature of salt stress tolerance are important factors contributing to the difficulties associated with breeding salt-tolerant crop varieties. Hence, the amendment of alkaline soils, as well as a better understanding of the how the plant mechanisms adapt to salinity stress and maintain their growth will ultimately help human with the selection of stress-tolerant plants for exploiting saline soil (Sudhakar et al. Citation2001; Rahnama & Ebrahimzadeh Citation2005; Lecourieux et al. Citation2006).
Calcium is required as a secondary messenger for certain processes in plant defense mechanisms (Messiaen et al. Citation1993; Levine et al. Citation1996; Sanders et al. Citation1999; Ma et al. Citation2009). Increasing number of studies have suggested that Ca2+ widely participated in the processes of signal transduction that is induced by various stimuli and environmental stresses, such as salt, oxidation drought, cold, diseases, and metallic ions, in many plants (Li et al. Citation1997; Kiegle et al. Citation2001; Kadota et al. Citation2004; Kawano et al. Citation2004). Particularly, Ca2+ also participated in many cytosolic signal transduction processes induced by various stimuli outside of cells (Kiegle et al. Citation2001; Kawano et al. Citation2004; Liu et al. Citation2010), which were mainly based on the alteration of the concentration, channel type, spatial distribution, and open mechanism of Ca2+. These studies suggested the important roles of Ca2+ concentrations, Ca2+ distribution, and activity of Ca2+-ATPase in the regulation of plant physiological responses to stress-resistance.
The reclamation of sodic soil involves the replacement of exchangeable Na+ with Ca2+ that could be supplied by the presence or addition of gypsum (Oster & Frenkel Citation1980; Keren & Miyamoto Citation1990). Flue gas desulfurization gypsum by-product (FGDB), 80% of its component is calcium sulfate (CaSO4·2H2O), has shown a capacity for amending alkaline soils. The FGDB is the most common alkaline soil amendment since it has the advantages of being nontoxic to plants, easy to handle, and moderately soluble. Thus the FGDB has been utilized as an efficient and a low cost-effective soil amendment for reclaiming alkaline soil (Keren & Miyamoto Citation1990; Ishak et al. Citation2002). Importantly, recent studies have demonstrated that FGDB was capable of decreasing the soil pH and exchangeable sodium percentage (ESP), improving the physical shape of soil moisture, as well as increasing soil aggregate structure in Northwestern China (Feldhake & Ritchey Citation1996; Sakai et al. Citation2004; Xiao et al. Citation2010). However, limited information about the mechanism of FDGE-mediated soil amendment, as well as the detail effects, and its main component CaSO4 on plants is currently available. Therefore, we hypothesized that the function of FDGE on amendment of alkaline soil might be mediated by a mechanism of alteration of cytosolic calcium distribution and Ca2+-ATPase activity in the plant cells. To test this, hypothesis against alkali stress was investigated in an oil sunflower model. In this study, the effects of the supplementation of FGDB or CaSO4 on the Ca2+ distribution and Ca2+-ATPase activity in leaf cells were evaluated in an oil sunflower model grown in an alkaline soil.
Materials and methods
Experimental soil
Soil used in this study was collected from Pingluo County (38°50′N, 106°32′ E) of Ningxia Province in the Northwest of China, The region is a typical arid zone with a monsoon climate. Mean annual temperature is 8.5°C. The soil texture at this geographic site is classified as mainly clay, with clay 250–450 g kg−1, silt 0–450 g kg−1, and sand 100–550 g kg−1. The total salt content ranges from 2.5 to 6.5 g kg−1. The range of pH is 8.0–10.4. Electrical conductivity (Lecourieux et al. Citation2006) ranges from 0.34 to 2.96 dsm−1.
Plant material and treatment
The experiment was conducted in pots which were filled with 5 kg of soils collected 0–20 depth from Pingluo County in Ningxia Province in the Northwest of China, The components of soil were: total salt 10.80 g kg−1; pH 8.66; total alkalinity 0.60; organic matter 13.00 g kg−1; total phosphorus 0.57 g kg−1; total potassium 21.30 g kg−1; anion 0.60 g kg−1;
11.29 g kg−1; Cl− 6.73 g kg−1; and cation Na+ 14.70 g kg−1; Ca2+ 2.34 g kg−1; Mg2+ g kg−1. FGDB was obtained from Ningxia Maliantai power plant (containing 800.5g Kg−1 of CaSO4·2H2O). A CaSO4 control was included to validate that it was a main and effective compound in the FGDB in this study. The soil and FGDB were mixed at ratios (w/w) of 5 kg:26.66 g (T1) and 5 kg:53.33 g (T2). The soil and CaSO4 were mixed at ratios (w/w) of 5 kg:21.33 g (T3) and 5 kg:42.66 (T4); a control soil (CK) without FGDB or CaSO4 was also included. The plants were grown in a greenhouse at Ningxia University in China, started on 10 April 2009. ‘KWS203’ cultivar oil sunflower was chosen as the experimental plant for this study. Twenty seeds were sown in each pot, and the plants were thinned leaving three of them to grow in the pot after the crops were established. A total of 30 plants grown in 10 pots for 15–20 d were analyzed for each condition in this report.
Preparation of protoplasts
The leaves of oil sunflower were treated with hydrolysate solution (0.2% BSA, 0.5% PVP, 1.0% cellulase R-10, 0.1% pectinase Y-23, and 0.4 M D-sorbitol pH 5.7) in a small beaker by shaking at 55 rpm for 3 h at 27°C in darkness, followed by filtering the solution with a 50 µm nylon mesh. The filtrate was then centrifuged for 5 min at 100× g, and the protoplast pellet was washed twice with the buffer solution containing 5 mM 2-(N-morpholino)ethanesulfonic acid buffer (MES), 1 mM CaCl2, 2 mM MgCl2, 0.4 M D-sorbitol, pH 5.7. Then the protoplasts were suspended in buffer solution and stored at 4°C before use.
Protoplast Fluo-3AM loading
Fluo-3AM stock solution (Sigma, St. Louis, MO, USA) prepared with anhydrous dimethyl sulfoxide (DMSO) was added to protoplast suspension at a final concentration of 5 µM, followed by gentle mixing and incubating at 4°C in darkness for 1 h before it was washed with buffer solution (5 mM MES, 1 mM CaCl2, 2 mM MgCl2, 0.4 M D-sorbitol, and pH 5.7) for three times by centrifugation at 100× g. The protoplasts were then used for experimental analysis.
Confocal scanning laser microscopy
Protoplasts loaded with Fluo-3AM were mounted in a chamber with a clean cover slip attached to the bottom. Fluorescence from the protoplasts loaded with Fluo-3AM was visualized using a LSM 510 confocal microscope. Excitation was at 488 nm and detection was under 510–525 nm.
Fixation and transmission electron microscopy of leaves
The distribution of Ca2+ in the leaf was accessed by a transmission electron microscopy, since Ca2+ was capable of binding potassium pyroantimonate to form dark particles, an indication of the distribution of cellular Ca2+. Briefly, the oil sunflower leaves were cut into approximately 1 mm2 pieces and fixed in a fixative containing 3% glutaraldehyde, 2% potassium pyroantimonate, and 50 mM potassium phosphate (pH 7.6) at 4°C for 4h, followed by washing three times in 50 mM potassium phosphate and 2% potassium pyroantimonate (pH 7.6) for 30 min each. Samples were subsequently fixed in 1% OsO4 containing 2% potassium pyroantimonate and 50 mM potassium phosphate (pH 7.8) for 12–16 h at 4°C, rinsed in 50 mM potassium phosphate buffer for three times (30 min each), dehydrated in a graded acetone series, and embedded in Epon 812 resin (Qin et al. Citation2005). Ultra-thin sections were obtained using a Leica UC6i ultramicrotome and observed using a JEM-1230 transmission electron microscopy.
Ca2+-ATPase activity of plasma membrane and tonoplast extraction
Plasma membrane and tonoplast vesicles of leaves were isolated according to a previously described method with modifications (Wang et al. Citation2001). Briefly, a 30 g of leaves was washed with cold deionized water and homogenized in 60 ml of extraction solution (50 mM Tricine-Tris, 5 mM EGTA, 3 mM MgSO4, 0.5% (w/v) polyvinylpyrrolidone, 5 mM dithiothreitol, 1 mM Phenylmethylsulfonyl fluoride (PMSF), 5% (v/v) glycerol and 10 mM sucrose, and pH 7.8). The homogenate was filtered through four layers of cheesecloth and centrifuged at 10,000× g for 20 min. The pellet was resuspended in suspension solution (250 mM sucrose, 5 mM Tris–MES, 1 mM DTT, PMSF 0.5 mM, and pH 7.0) and ultracentrifuged with 22%, 35%, and 46% (w/w) sucrose of discontinuous gradient density centrifuge at 60,000× g for 2 h. The tonoplast and plasma membrane vesicles were carefully collected at interfaces of 22–36% and 36–45% of sucrose, respectively. The vesicles were frozen in liquid nitrogen and stored at –70°C before further use. All steps of the procedure were performed at 2–4°C. The Ca2+-ATPase hydrolytic activity of plasma membrane or tonoplast was ascertained by determined the amount of inorganic phosphate released in the absence and presence of 3.0 mM Ca(NO3)2, according to a previous method (Chifflet et al. Citation1988). The enzymatic reaction was performed at 37°C for 30 min by mixing 20 µg of membrane vesicle protein in 800 µl of assay buffer (30 mM Tris–MES, pH 7.0, 0.1 mM (NH4)2 MoO4, 1 mM NaN3, 50 mM NaNO3, 0.01% (v/v) Triton X-100, and 3 mM Na2-ATP), followed by adding 50 µl of 55% trichloroacetic acid to terminate the reaction. Spectrophotometer readings were taken at 660 nm.
Total calcium contents assay
The leaves were milled in a pestle after they were dried at 80°C, and the total calcium contents was ascertained essentially according to a previously described method using an atomic absorption spectrophotometry (TSA-900, Beijing, China) (Manual Citation1976).
Statistical analysis
Statistical analyses were conducted using the SPSS for windows (versions 17.0). Duncan test was used to establish differences between the groups. A value of p<0.05 was considered a significant difference.
Results
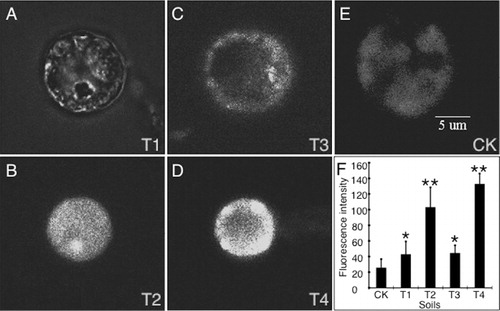
Effects of FGDB and CaSO4 on cytosolic Ca2+ fluorescence intensity
To test whether FGDB or CaSO4 affect the extracellular Ca2+ influx in plant leaves, Fluo-3AM, a Ca2+-specific fluorescence indicator was preloaded into protoplasts, and the changes of cytosolic Ca2+ concentrations were monitored using a laser scanning confocal microscopy (LSCM). showed the effects of FGDB or CaSO4 on the abundance of free cytosolic Ca2+ in protoplasts isolated from sunflower leaves with different treatments. The Ca2+ fluorescence intensities were increased by 1.77-fold and 4.07-fold in those plants grown in T1 and T2 soils in comparison with those CK soil, respectively. Similarly, 1.75-fold and 5.23-fold of increased Ca2+ fluorescence intensities were also observed in the plants grown in T3 and T4 soils that supplied with exogenous CaSO4, as compared to CK soil, respectively. A more enhanced intensity of Ca2+ fluorescence was observed in the groups grown in soils with higher ratio of FGDB or CaSO4 (T2 or T4) than that in lower ratio of FGDB or CaSO4 (T1or T3), and CaSO4 showed to be more effective than that of FGDB (). These data implied a dose-dependent effect of FGDB or CaSO4 on extracellular Ca2+ influx.
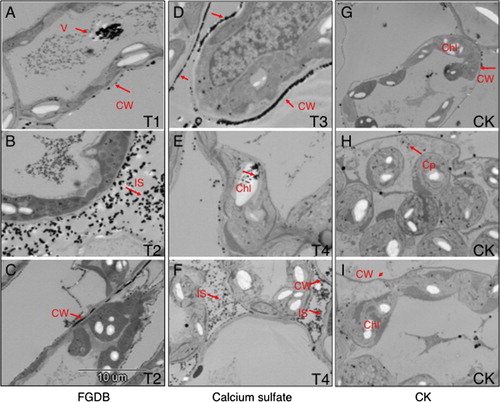
Effects of FGDB and CaSO4 on Ca2+ distribution in leaf cells
As shown in , the damaged structure of deformed chloroplasts (), as well as few calcium precipitates, was observed in the chloroplast and cytoplasm of plants grown in CK soil with alkali stress ( and data not shown). While intact, chloroplasts, as well as increasing abundance of calcium precipitates with increasing ratio of FGDB or CaSO4, were found in the cell wall, intercellular space and vacuole of the plants grown in soils mixed with FGDB or CaSO4 ( and data not shown). Notably, more calcium precipitates in CW were found in plants treated with lower doses of FGDB or CaSO4, while more calcium precipitates in intercellular space and vacuole were observed when a higher dose was added. This result indicated that the alkali stress-induced deformity of chloroplast could alleviate the addition of Ca2+.
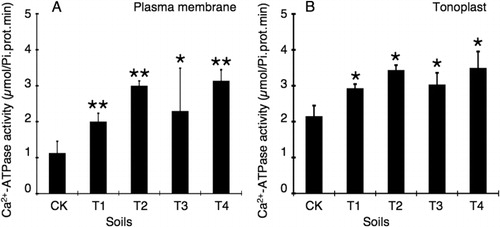
Effects of FGDB and CaSO4 on the activities of plasma membrane and tonoplast Ca2+-ATPase
A dose-dependent increase of Ca2+-ATPase activity was observed in the plasma membrane () and tonoplast () of plants grown in soils supplied with desulfurization gypsum or CaSO4, and the Ca2+-ATPase activity in tonoplast was higher than that in plasma. The Ca2+-ATPase activities were increased by 1.78-fold and 2.67-fold in plasma membrane and by 1.36-fold and 1.60-fold in tonoplast in T1 and T2 soil groups over the CK, respectively. Of note, the Ca2+-ATPase activities were increased by 2.04-fold and 2.79-fold in plasma membrane and by 1.41-fold and 1.48-fold in tonoplast of plants grown in soils of T3 and T4, in comparison with that of CK, respectively. Similar to the cytosolic Ca2+ fluorescence intensity assay, CaSO4 was more effective on Ca2+-ATPase activity in plasma membrane (specially comparing T1 and T3); however, in tonoplast, the Ca2+-ATPase activities were similar to CaSO4 or to FGDB ().
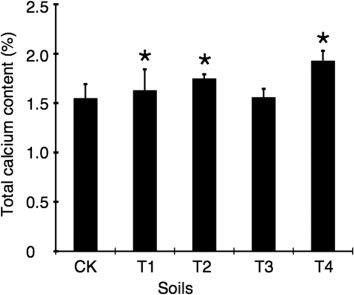
Effects of FGDB and CaSO4 on total calcium contents
Increasing total calcium contents were also found in the plants after applying FGDB or CaSO4 (). The concentrations of total calcium in the plants treated with T1 and T2 soils were significantly increased by 5.16% and 12.9% over that of CK (p<0.05), respectively. And the total contents of calcium in plants treated with T3 and T4 were 0.64% and 24.5% (p<0.05) higher than the control, respectively.
Discussion
Sodic soil contains exchangeable sodium ions on soil colloids and soluble carbonates in the forms of Na2CO3 and NaHCO3. Sodium is responsible for high pH (>8.4), clay dispersion, swelling, and overall physical properties of the soil (Suarez et al. Citation1984; Gupta & Abrol Citation1990). The principle of using FGDB to improve sodic soil involved the replacement of exchangeable Na+ with Ca2+ and discharging Na+ through the leaching of soil. However, there was no direct evidence of whether the FGDB had the capacity to enhance plant sodium resistance through the regulation of cytosolic Ca2+ levels as has been reported. The mechanism of how the Ca2+ in soil was absorbed by the plant under alkali stress has not been established yet either. In this study, cytosolic Ca2+ fluorescence intensity analysis demonstrated that the FGDB or CaSO4 was able to induce Ca2+ elevation, which might be due to the extracellular Ca2+ influx and the participation of Ca2+ in the signal transduction process of alkali stress. However, further study is necessary to test the effect of calcium channel inhibitors on the cytosolic Ca2+ fluorescence intensity.
Calcium is an important nutrient for plants. The role of calcium in the plant life cycle is well established and has been reviewed extensively (Bush Citation1995; White & Broadley Citation2003; Hirschi Citation2004). It is present in three forms: covalently bound calcium, loosely bound calcium that is typically associated with fixed and mobile anions, and cytosolic free calcium (Ge et al. Citation2007). The loosely bound calcium, which could be detected by a potassium antimonite precipitation method, has lower affinity for fixed and mobile anions and functions as an exchangeable form of calcium by transforming into other forms as it is on demand (Wick & Hepler Citation1982; Zhao et al. Citation2002; Zhu et al. Citation2010).
Transient spatial and temporal changes of calcium pools and cytoplasmic Ca2+ concentration were the initial responses to external stimulation, which might trigger a physiological and developmental cascade in plants (Knight Citation1999; Lecourieux et al., Citation2006). In this study, transmission electron microscopy analysis revealed that abundant calcium precipitates were distributed in cell wall, intercellular space, and vacuole of plants treated with FGDB or CaSO4 in a dose-dependent manner. A change of free calcium concentration might alter the loosely bound calcium in the cell wall and intercellular space, together with the evidence of high total calcium content found in the oil sunflower leaves might be loosely bound calcium and free calcium, indicating that the entering calcium was partially stored in vacuole or bound to pectins in the middle lamella and maintains the cell wall stability and integrity (Hanson Citation1984; Palta Citation1996; Hirschi Citation2004).
Ca2+ signal cascade could be directly triggered by extracellular Ca2+ influx and/or released by cytosolic Ca2+. In order to detect the role of Ca2+-ATPase in Ca2+ influx or in discharging cell, the activities of Ca2+-ATPase in plasma membrane and tonoplast were measured. Indeed, increasing activities of Ca2+-ATPase were detected in plasma membrane and in tonoplast of plants grown in soils added with FGDB or CaSO4. This clearly evidenced that the FGDB or CaSO4 induced elevating Ca2+ concentration in cell wall and vacuole and was primarily due to cytosolic Ca2+ efflux by enhancing Ca2+-ATPase activity and re-establishing the low basal free Ca2+ concentration in cytosol; this notion was in agreement with the result of our cytosolic Ca2+ fluorescence intensity and the findings from other groups (Beffagna et al. Citation2000; Sanders et al. Citation2002; Romani et al. Citation2004).
To test whether the effective compound for improving the plant alkali resistance was CaSO4 in desulfurization gypsum, a comparison test between FGDB (containing 80% CaSO4) and CaSO4 (Analytical grade) was included in this study. As expected, FGDB and CaSO4 displayed a similar effect on increasing cytosolic Ca2+ fluorescence intensity, the distribution of calcium, and Ca2+-ATPase activity in the leaves cells of oil sunflower, suggesting that CaSO4 in desulfurization gypsum was the compound that played an essential role in relieving alkali stress in this study.
Conclusions
The results presented in this report demonstrated a dose-dependent effect of FGDB and CaSO4 on cytosolic Ca2+ concentration, Ca2+ distribution, and plasma Ca2+-ATPase activity in oil sunflower. The calcium sulfate of FGDB was the effective compound for plant against saline–alkali stress. The alteration of cytosolic Ca2+ distribution and concentration induced by FGDB or CaSO4 might be partially mediated by enhancing Ca2+-ATPase activity in the leaf cells of this plant. These findings provided an insight into the molecular mechanism of alkaline soil amendment by supplementation of desulfurization gypsum and warranted the potential application of FGDB in the restoration of alkaline soils in Northwestern China.
Acknowledgments
We thank Drs. Liu HH and Jia JZ (College of Biological Sciences, Agricultural University of China) for their helpful suggestions, technical assistance for ultrathin sections, and transmission electron microscopy. This work was supported by grants from the National Basic Research Program of China (973 program) (2012CB723206) and National Key Technology R&D Program of the Ministry of Science and Technology (2013BAC02B04 and 2011BAC07B03).
References
- Beffagna N, Romani G, Sforza M. 2000. H+ fluxes at plasmalemma level: in vivo evidence for a significant contribution of the Ca2+-ATPase and for the involvement of its activity in the Abscisic acid-induced changes in Egeria densa leaves. Plant Biol. 2:168–175. 10.1055/s-2000-9158
- Bush DS. 1995. Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Biol. 46:95–122. 10.1146/annurev.pp.46.060195.000523
- Chifflet S, Torriglia A, Chiesa R, Tolosa S. 1988. A method for the determination of inorganic phosphate in the presence of labile organic phosphate and high concentrations of protein: application to lens ATPases. Anal Biochem. 168:1–4. 10.1016/0003-2697(88)90002-4
- Feldhake C, Ritchey K. 1996. Flue gas desulfurization gypsum improves orchardgrass root density and water extraction in an acid subsoil. Plant and Soil. 178:273–281. 10.1007/BF00011593
- Frommer WB, Ludewig U, Rentsch D. 1999. Taking transgenic plants with a pinch of salt. Science. 285:1222–1223. 10.1126/science.285.5431.1222
- Ge LL, Tian HQ, Russell SD. 2007. Calcium function and distribution during fertilization in angiosperms. Am J Bot. 94:1046–1060. 10.3732/ajb.94.6.1046
- Gupta RK, Abrol I. 1990. Salt-affected soils: their reclamation and management for cop production. Adv Soil Sci. 11:223–288.
- Hanson J. 1984. The functions of calcium in plant nutrition. Adv Plant Nutr. 1:149–208.
- Hirschi KD. 2004. The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol. 136:2438–2442. 10.1104/pp.104.046490
- Ishak C, Seaman J, Miller W, Sumner M. 2002. Contaminant mobility in soils amended with fly ash and flue-gas gypsum: intact soil cores and repacked columns. Water Air Soil Pollut. 134:285–303. 10.1023/A:1014101217340
- Kadota Y, Furuichi T, Ogasawara Y, Goh T, Higashi K, Muto S, Kuchitsu K. 2004. Identification of putative voltage-dependent Ca2+-permeable channels involved in cryptogein-induced Ca2+ transients and defense responses in tobacco BY-2 cells. Biochem Biophys Res Comm. 317:823–830. 10.1016/j.bbrc.2004.03.114
- Kawano T, Kadono T, Fumoto K, Lapeyrie F, Kuse M, Isobe M, Furuichi T, Muto S. 2004. Aluminum as a specific inhibitor of plant TPC1 Ca2+ channels. Biochem Biophys Res Comm. 324:40–45. 10.1016/j.bbrc.2004.09.015
- Keren R, Miyamoto S. 1990. Reclamation of saline, sodic, and boron-affected soils, New York, NY: ASCE; p. 410–431.
- Kiegle E, Moore CA, Haseloff J, Tester MA, Knight MR. 2001. Cell-type-specific calcium responses to drought, salt and cold in the Arabidopsis root. The Plant J. 23:267–278. 10.1046/j.1365-313x.2000.00786.x
- Knight H. 1999. Calcium signaling during abiotic stress in plants. Int Rev Cytol. 195:269–324.
- Lecourieux D, Ranjeva R, Pugin A. 2006. Calcium in plant defence-signalling pathways. New Phytol. 171:249–269. 10.1111/j.1469-8137.2006.01777.x
- Levine A, Pennell RI, Alvarez ME, Palmer R, Lamb C. 1996. Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr Biol. 6:427–437. 10.1016/S0960-9822(02)00510-9
- Li W, Sun ZH, Zhang WC, Ma XT, Liu DH 1997. Role of Ca2+ and calmodulin on freezing tolerance of citrus protoplasts. Acta Phytophysiologica Sinica. 23: 262–266 (in Chinese).
- Liu Z, Ma Z, Guo X, Shao H, Cui Q, Song W. 2010. Changes of cytosolic Ca2+ fluorescence intensity and plasma membrane calcium channels of maize root tip cells under osmotic stress. Plant Physiol Biochem. 48:860–865. 10.1016/j.plaphy.2010.08.008
- Ma YY, Song WY, Liu ZH, Zhang HM, Guo XL, Shao HB, Ni FT. 2009. The dynamic changing of Ca2+ cellular localization in maize leaflets under drought stress. Comptes Rendus Biologies. 332:351–362. 10.1016/j.crvi.2008.12.003
- Manual PE. 1976. Analytical methods for atomic absorption spectrophotometry. Norwalk, Conn: Perkin-Elmer.
- Messiaen J, Read ND, Cutsem PV, Trewavas AJ. 1993. Cell wall oligogalacturonides increase cytosolic free calcium in carrot protoplasts. J Cell Sci. 104:365–371.
- Oster J, Frenkel H. 1980. The chemistry of the reclamation of sodic soils with gypsum and lime. Soil Sci Soc Am J. 44:41–45. 10.2136/sssaj1980.03615995004400010010x
- Palta JP. 1996. Role of calcium in plant responses to stresses: linking basic research to the solution of practical problems. HortScience. 31:51–57.
- Qin Y, Yang J, Zhao J. 2005. Calcium changes and the response to methyl jasmonate in rice lodicules during anthesis. Protoplasma. 225:103–112. 10.1007/s00709-005-0086-6
- Rahnama H, Ebrahimzadeh H. 2005. The effect of NaCl on antioxidant enzyme activities in potato seedlings. Biologia Plantarum. 49:93–97. 10.1007/s10535-005-3097-4
- Romani G, Bonza MC, Filippini I, Cerana M, Beffagna N, Michelis M. 2004. Involvement of the plasma membrane Ca2+-ATPase in the short-term response of Arabidopsis thaliana cultured cells to oligogalacturonides. Plant Biol. 6:192–200. 10.1055/s-2004-817848
- Sakai Y, Matsumoto S, Sadakata M. 2004. Alkali soil reclamation with flue gas desulfurization gypsum in China and assessment of metal content in corn grains. Soil Sediment Contam. 13:65–80. 10.1080/10588330490269840
- Sanders D, Brownlee C, Harper JF. 1999. Communicating with calcium. Plant Cell. Online. 11:691–706.
- Sanders D, Pelloux J, Brownlee C, Harper JF. 2002. Calcium at the crossroads of signaling. The Plant Cell Online. 14:S401–S417.
- Suarez D, Rhoades J, Lavado R, Grieve C. 1984. Effect of pH on saturated hydraulic conductivity and soil dispersion. Soil Sci Soc Am J. 48:50–55. 10.2136/sssaj1984.03615995004800010009x
- Sudhakar C, Lakshmi A, Giridarakumar S. 2001. Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci. 161:613–619. 10.1016/S0168-9452(01)00450-2
- Wang B, Lüttge U, Ratajczak R. 2001. Effects of salt treatment and osmotic stress on V-ATPase and V-PPase in leaves of the halophyte Suaeda salsa. J Exp Bot. 52:2355–2365. 10.1093/jexbot/52.365.2355
- White PJ, Broadley MR. 2003. Calcium in plants. Ann Bot. 92:487–511. 10.1093/aob/mcg164
- Wick SM, Hepler PK. 1982. Selective localization of intracellular Ca2+ with potassium antimonate. J Histochem Cytochem. 30:1190–1204. 10.1177/30.11.6815264
- Xiao G, Luo C, Zhang F, Wang B, Zheng G, Yang J, Mao G, Bai H. 2010. Application amount of desulfurized gypsum from coal fired power plants on improving the quality of alkalized soil. Res Environ Sci. 23:762–767 (in Chinese).
- Zhao J, Yu F, Liang S, Zhou C, Yang H. 2002. Changes of calcium distribution in egg cells, zygotes and two-celled proembryos of rice (Oryza sativa L.). Sex Plant Reprod. 14:331–337. 10.1007/s00497-002-0127-7
- Zhu Z, Xiang-hong M, Shi-ping T. 2010. Effect of preharvest oxalic acid sprays on calcium content and distribution in mango fruit cells. Chinese Bullet Bot. 45:23–28. (in Chinese)