Abstract
Humic substances (HS) and preparations created on their basis are known to be effective in growth stimulation and improvement of resistance and productivity of different plant species. We have revealed that the presowing semidry treatment of wheat seeds with preparation HUMI (NVP ‘BashIncom’, Ufa, Bashkortostan, Russia) promoted seedling growth and increased wheat yield. This effect is likely to be due to the shifts induced by HUMI in the hormonal system of plants associated with a 1.5-fold persistent accumulation of cytokinins (CKs) without any changes in the contents of indoleacetic acid (IAA) and abscisic acid (ABA). Meanwhile, presowing treatment with HUMI had a stabilizing effect on the state of the hormonal system in the course of ontogenesis of wheat inoculated with teliospores Tilletia caries. Obtained data about prevention of the decline in the cytokinin content in HUMI-treated infected wheat is fundamental in the implementation of immuno-stimulatory effect of HUMI resulting in the suppression of the growth and development of pathogens.
Introduction
The problem of stress-resistance is most important for plant growing and much attention has been paid to this problem all over the world. This is indeed the case, since information concerning the chain of reactions taking place in plants in response to extreme external conditions may really contribute to an increase in plant resistance and productivity. These goals are achieved not only by means of selection of stress tolerant cultivars, but also through the purposeful manipulation of adaptation with the help of plant growth regulators (PGR) (Shakirova Citation2007; Bajguz & Hayat Citation2009; Shakirova et al. Citation2010). These substances combine the properties of growth activators and inductors of unspecific resistance which reveal a perspective for their practical application in plant growth. Since plant growing demands an intensification of not only plant resistance, but of productivity also in the first place, purposeful application of regulators of plant growth and development capable of increasing both of them is of high priority. It is obvious that not all PGRs may be used, but only those, which are characterized by a wide spectrum of their protective action (Shakirova et al. Citation2010).
In relation to this, preparations based on humic substances (HS) are of great practical interest. HS constitute the end product of decomposition of organic residues and represent a major part of organic matter in water, soil, and sediment. HS participate in the majority of reactions that take place in the environment, controlling transport, and transformation of chemical compounds, formation of complexes by chelation of metals and other mineral elements, thus influencing the availability of nutrients, and maintenance of soil structure and fertility (Mackowiak et al. Citation2001; Nardi et al. Citation2002; Quaggiotti et al. Citation2004; Muscolo et al. Citation2007; Canellas et al. Citation2008).
HS is most widely used in different branches of agriculture (Pena-Mendez et al. Citation2005). There are many data on the effectiveness of humic acids (HA) and preparations created on their basis in growth stimulation and improvement of resistance and productivity of different plant species. Thus, stimulating effect on plant growth has been demonstrated for low-molecular-weight fractions of HS and HA, in particular (Nardi et al. Citation2002; Dobbss et al. Citation2007; Canellas et al. Citation2008; Muscolo & Sidari Citation2009), although it should be mentioned that biological activity of these substances may be better related to their chemical structure than to their molecular weight (Russell et al. Citation2006; Muscolo et al. Citation2007; Canellas et al. Citation2008). It is of interest that the use of different methods, immunoassay being one of them, enabled identification of IAA in the chemical composition of HS (Muscolo et al. Citation1998). Earlier it has been reported that the presence of cytokinins in the preparation of HA, and their application resulted in elevated cytokinin level in the shoots of Agrostis palustris plants subjected to drought, possibly associated with increased plant drought tolerance (Zhang & Ervin Citation2004). However, in other studies, an insignificant content of CKs were found in the HA (Jannin et al. Citation2012), though these data cannot exclude the effect of these HA on cytokinin metabolism (Jannin et al. Citation2012). Thus, HS treatment appears to resemble that of hormones with stimulating activity in contributing to activation of cell metabolism, which is the basis of growth processes, and in total in increasing productivity of different crops (Canellas et al. Citation2002, Citation2008; Carletti et al. Citation2008; El-Ghamry et al. Citation2009). Meanwhile, it is necessary to emphasize the ability of different variants of HS treatment to protect either biotically or abiotically stressed plants contributing significantly to the increase in their yield (Nardi et al. Citation2002; Zhang & Ervin Citation2004; Nurgalieva et al. Citation2006; Muscolo et al. Citation2007; Shakirova et al. Citation2008; Asik et al. Citation2009; El-Ghamry et al. Citation2009).
Due to the fact that the phytohormones have a key role in the regulation of plant growth and development, one can believe that growth-stimulating and protective effects of HS preparations are based on their effect on the state of the hormonal system in plants. The present work is devoted to analysis of changes in hormonal system of wheat plants pre-treated with HUMI preparation under normal conditions and inoculation with teliospores of Tilletia caries.
Materials and methods
Plant material
Wheat seeds (Triticum aestivum L.) cv. Bashkirskaya 24 were obtained from Chishminsky Crop Production, Bashkortostan, Russia. The objects of investigation were 2–5-d-old seedlings, leaves of adult plants during ontogenesis at the wheat growth stages of tillering and booting, as well as kernels from the spikes at the phase of milky ripeness.
Laboratory experiments
In laboratory experiments, we applied presowing semidry treatment of wheat seeds with HUMI (NVP ‘BashIncom’, Ufa, Bashkortostan, Russia), consisting of sodium salts of humic acids and having molecular weight about 6000 Da, recovered from brown coal (South Ural), the dose of application being 300 mg per kg of seeds. Then, seeds untreated and pre-treated with HUMI were grown on filter paper moistened with tap water under illumination of 200 mmol m−2 s−1 at 22–24 °C and a 16-h photoperiod. For analysis of cell division there were used root tips of 2–5-d-old seedlings. For phytohormone analysis, whole 2–4-d-old seedlings isolated from endosperm were collected and fixed in liquid nitrogen.
Field experiments
In field experiments, we investigated the effectiveness of applications of HUMI on hormonal balance and productivity of wheat plants, as well as wheat resistance to T. caries. These experiments were conducted during two seasons in the field of Chishminsky Crop Production (Bashkortostan, Russia) on a leached chernozem soil, which is typical for this region. For this purpose, the seeds were subjected to presowing semidry treatment with HUMI at a dose of 800 mg per kg of seeds. Next, seeds untreated and pre-treated with HUMI were inoculated with teliospores of T. caries (10 g per 1 kg of seeds) and sown on plots (5 m2) using three replicates. Fungus culture was propagated on hothouse plants of wheat, cv. Zhnitsa (Bashkir State Agrarian University, Ufa). Uninoculated plants grown from seeds untreated with HUMI served as control. Biological evaluation of the effectiveness of the HUMI against the causing agent of common bunt was carried out at the heading stage when the disease could be detected in the field. Common bunt-affected plants were determined by the visual inspection of the grains when we could observe the dark colored grains in the infected plants. The infection degree was evaluated as the ratio of the number of affected plants to the total number of plants. For phytohormone analysis, leaves from wheat plants were collected during the ontogenesis stage of tillering and booting, as well as the grains from the spikes in the phase of milky ripeness, and fixed in liquid nitrogen.
Growth and yield analyses
Growth was determined as changes in the length of the seedlings and the activity of cell division in the apical meristem of wheat seedling roots. Three thousand cells from the root meristem were used for the analysis of mitosis (Shakirova et al. Citation2004). Mitotic index (MI) was calculated as percent of the dividing cells. Each variant of experiment included not less than 30 seedlings. Estimation of the influence of presowing treatment with HUMI on characteristics of wheat productivity was carried out according to (Dospekhov Citation1979). The determined parameters of wheat productivity were ear length (cm), seed number per ear, seed weight per ear (g), weight of 1000 seeds (g), grain yield (g m−2).
Quantification of ABA, IAA, and cytokinins
The contents of free ABA, IAA, and CKs were measured by ELISA as described earlier (Shakirova et al. Citation2004). Plant material was homogenized in 80% ethanol (1:10, w/v) and kept at 4 °C for 16 h to extract phytohormones. The homogenate was centrifuged at 18,000g for 10 min, and the supernatant was concentrated to an aqueous residue in vacuo. The total content of zeatin derivatives was determined in an aliquot of the aqueous residue using rabbit antibodies raised against zeatin riboside having high immunoreactivity towards zeatin, its riboside and nucleotide (Shakirova et al. Citation2004). For ABA and IAA purification, the pH of the aqueous residues of ethanol extracts was adjusted to 2.5 and partitioned with diethyl ether (organic to aqueous phase 1:3). Subsequently, ABA and IAA were transferred from the organic phase into 1% sodium hydrocarbonate (pH 7–8), re-extracted with diethyl ether, methylated with diazomethane, and immunoassayed using polyclonal antibodies to ABA and IAA (Shakirova et al. Citation2004).
Statistical analysis
Four samples were collected in each experiment, and all experiments were conducted at least three times. The results were statistically processed using a StatSoft (Statistica 6.0) computer program. Means and standard errors (SEs) were calculated within each experiment.
Results and discussion
Influence of HUMI on growth and hormonal status of wheat seedlings under normal growth conditions
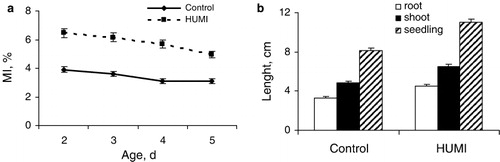
The data presented in demonstrate that treatment of the seeds with HUMI activates seedling growth processes. HUMI-treated seedlings were distinguished by significantly greater mitotic index (MI) of root apical meristem cells () contributing to the increase in length of roots and shoots and whole seedling ().
Hormonal system is well known to play a decisive role in regulation of growth processes. During germination it responds sensitively to the changes in environment and to the treatment with growth regulators as well (Shakirova et al. Citation2010). It is necessary to emphasize that a study of different hormones in the same plants allows to follow a complex pattern of changes in hormonal status and to evaluate if the treatment with a growth regulator is favorable for the plants. Therefore, it was of interest to carry out a comparative analysis of the status of hormonal system during germination of seedlings both treated and untreated with HUMI.
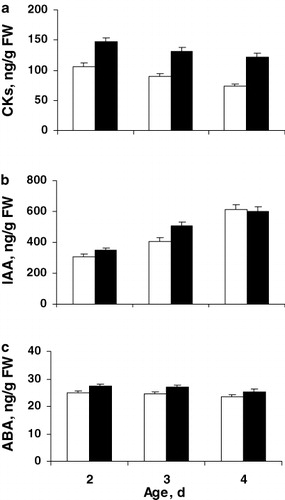
shows that HUMI-treatment resulted in 1.5 fold increase in the concentration of free CKs, being maintained in the seedlings throughout the whole experiment, while there were no significant changes in the content of IAA and ABA as compared to control. The data obtained indicate that, first of all, growth-stimulating effect of HUMI on the wheat plants is due to persistent accumulation of cytokinins known to play the key role in the activation of cell metabolism, which is the basis of plant growth and development during their whole ontogenesis (Argueso et al. Citation2009; Shakirova et al. Citation2010). Recent studies have reported the growth promoting effect of HA in cucumber which is associated with increase in the contents of various forms of cytokinins during incubation of plants on medium with HA (Mora et al. Citation2010). However, it is worth mentioning that these changes in CKs concentration were transient and moderate (Mora et al. Citation2010), whereas our studies have demonstrated 1.5 fold stable accumulation (throughout the whole experiment) of cytokinins, immunoreactive in the test system with rabbit antibodies to zeatin riboside in the wheat plants, after presowing treatment with HUMI.
Growth stimulating effect of HUMI was revealed in field experiments when yield structure was analyzed. As seen from , plants pre-treated with HUMI are characterized by greater size of ears, mass of 1000 seeds and grain yield, indicating prolonged effect of presowing treatment of seeds with HUMI, which produce stimulating effect on productivity of wheat.
Table 1. The effect of HUMI treatment on wheat productivity Triticum aestivum cv. Bashkirskaya 24 (SEs of three assays are indicated).
The significance of hormonal system in the protective action of HUMI on wheat resistance to T. caries
Effectiveness of practical application of preparations created on the basis of HA in increasing productivity of different crops and quality of obtained agricultural products is not doubted. This process is certainly due to the effect of HS as a growth regulator having a hormone-like activity (Nardi et al. Citation2002; Zhang & Ervin Citation2004; Canellas et al. Citation2008). Nevertheless, HS-induced increase in plant productivity is also due to their anti-stress activity to different damaging factors of environment, including causative agents of plant diseases (El-Ghamry et al. Citation2009). In relation to this, it was important to study effectiveness of HUMI application with the aim of increasing wheat resistance against fungal pathogens.
It is necessary to emphasize that common bunt, caused by phytopathogen T. caries is rated among most dangerous wheat diseases having a broad natural habitat in Russia, including the South Urals. This is a highly specialized pathogen, capable of infecting different organs of plant host, although it develops mostly in tissues of meristem, without significantly changing the phenotype of infected plants and their reproductive organs (Karatigin Citation1981).
Nearly 40% of HUMI-untreated plants inoculated with teliospores of T. caries were found to be affected, while only 13% were affected in the case of HUMI-pre-treated plants. These data provide evidence in favor of involvement of HUMI preparation in induction of protective reactions in response to fungus infection. Consequently, application of HUMI preparation as a presowing treatment contributes to both stimulation of growth processes and implementation of plant defense in response to T. caries inoculation. This provides as perspective for effective application of preparations created on the basis of HS to increase productivity of different agricultural crops (Asik et al. Citation2009; El-Ghamry et al. Citation2009).
Hormonal system is an effective mean to regulate vital functions of plants in the course of the whole ontogenesis, responding readily to the slightest changes in environment, including the treatment with growth regulators. In relation to this, it was important to analyze at different stages of development the pattern of changes in the phytohormonal balance of plants pre-treated and untreated with HUMI and infected with T. caries.
Untreated plants were very sensitive to T. caries. This was made apparent by both accumulation of ABA and decline in IAA level, and especially in CKs at all the stages of wheat ontogenesis (). The revealed changes, in total, lead to hormonal imbalance made apparent by analysis of the IAA/ABA and CKs/ABA ratio and especially strong – by relating cytokinins to ABA (). Fungus pathogens are known to cause phytohormonal imbalance in plants, related not only to accumulation of ABA, but also to the parallel decline in auxins and cytokinins (Bari & Jones Citation2009; Grant & Jones Citation2009; Shakirova et al. Citation2010). In this case, the decline in IAA/ABA or CKs/ABA ratios may be also due to a decline in IAA or CKs against the background of the absence of significant changes in concentration of ABA. It is necessary to emphasize that it is to cytokinins that significant role in inhibition of the growth of pathogen fungus is attributed (Argueso et al. Citation2009; Choi et al. Citation2011), and consequently, the decline in cytokinins content in inoculated plants at all the stages of ontogenesis may be considered as an important regulatory impact for successful development of fungus cells in wheat plants.
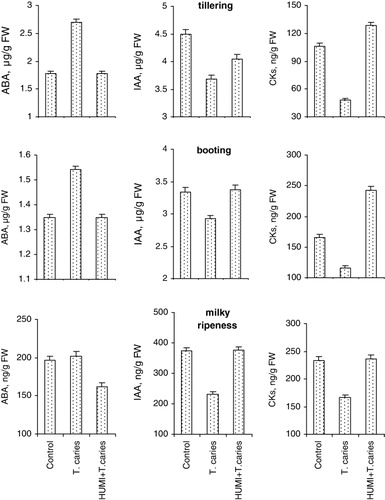
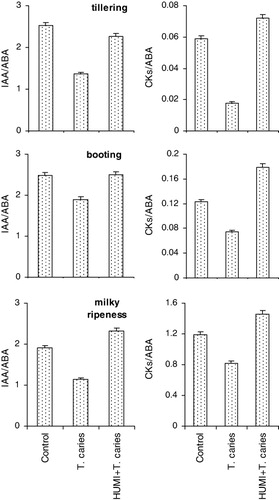
HUMI-pre-treatment accompanied by weak impact on IAA content in plants infected with T. caries contributed to complete prevention of stress-induced decline in CKs content and accumulation of ABA, which, in total was reflected in normalization of the balance of studied phytohormones in the plants ( and ). Shifts in the hormonal balance resulting from HUMI-treatment revealed by us, may serve as a manifestation of protective action of the preparation against T. caries, contributing to significant decline in the percentage of the plants affected by this disease.
Ability of HUMI to induce accumulation of cytokinins revealed by us in the seedlings was apparent also in adult plants in the course of ontogenesis of wheat. Immuno-stimulating activity of HUMI aimed to deter growth and development of fungi may be attributed to its ability to maintain the increased level of cytokinins in infected plants. This may be true not only for T. caries inoculation, but also for other fungi, since it was shown that effectiveness of protective response of plants to fungus agents of different trophity type is related to increased content of hormones of cytokinin type (Yarullina et al. Citation2005; Bari & Jones Citation2009; Choi et al. Citation2011). Cytokinin phytohormones are known to modulate plant immunity and induce multiple defense mechanisms in response to biotic stresses (Grosskinsky et al. Citation2011), and to T. caries inoculation in particular (Maksimov & Khairullin Citation2012). This is generally reflected in a significant decrease in the degree of fungal infection.
Consequently, the mentioned results indicate the important role of endogenous cytokinins in the manifestation of growth-stimulative and protective against fungus agent of common bunt effects of HUMI-treatment on wheat plants.
Conclusion
Accomplishment of the research allowed revealing a long-term growth-stimulating and protective action of HUMI on wheat plants against fungus pathogen. Since HS are acknowledged stimulators of growth processes, it may be expected that growth stimulating effect of HUMI on wheat plants is due to its impact on the hormonal system. Actually, we have shown, for the first time that HUMI applied as a presowing treatment rendered a prolonged effect on phytohormonal balance related to persistent 1.5 fold accumulation of cytokinins against the background of the absence of significant changes in the IAA and ABA content in plants in the course of wheat ontogenesis. Moreover, it was shown that HUMI increases resistance of plants to fungal infection. This protective effect of HUMI is also due to its impact on the status of hormonal system manifested by the reduced stress-induced shifts in the balance of IAA and ABA and prevention of the CKs drop or even by the increase in CKs level in plants infected with T. caries. CKs are known to be characterized by a broad spectrum of regulatory action, including activation of growth processes and implementation of resistance against various stress factors.
This allows us to conclude that combination of growth promoting and protective action against pathogens in HUMI preparation is due to induction of accumulation of endogenous cytokinins in HUMI-treated wheat plants.
Acknowledgments
This research is supported by the Russian Foundation for Basic Research – Povoljie (project no. 11-04-97051).
References
- Argueso C, Ferreira FJ, Kieber JJ. 2009. Environmental perception avenues: the interaction of cytokinin and environmental response pathways. Plant Cell Environ. 32:1147–1160. 10.1111/j.1365-3040.2009.01940.x
- Asik BB, Turan VA, Celik H, Katkat AV. 2009. Effect of humic substance on plant growth and mineral nutrients uptake of wheat (Triticum durum cv. Salihli) under conditions of salinity. Asian J Crop Sci. 1:87–95. 10.3923/ajcs.2009.87.95
- Bajguz A, Hayat S. 2009. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol Biochem. 47:1–8. 10.1016/j.plaphy.2008.10.002
- Bari R, Jones JDG. 2009. Role of plant hormones in plant defence responses. Plant Mol Biol. 69:473–488. 10.1007/s11103-008-9435-0
- Canellas LP, Facxanha AO, Olivares FL, Facxanha AR. 2002. Humic acids isolated from earthworm compost enhance root elongation, lateral root emergence, and plasma membrane H+-ATPase activity in maize roots. Plant Physiol. 130:1951–1957. 10.1104/pp.007088
- Canellas LP, Teixeira Jr. LRL, Dobbss LB, Silva CA, Medici LO, Zandonadi DB, Facanha AR. 2008. Humic acids cross interactions with root and organic acids. Ann Appl Biol. 153:157–166.
- Carletti P, Masi A, Spolaore B, De Laureto PP, De Zorzi M, Turetta L, Ferretti M, Nardi S. 2008. Protein expression changes in maize roots in response to humic substances. J Chem Ecol. 34:804–818. 10.1007/s10886-008-9477-4
- Choi J, Choi D, Lee S, Ryu C-M, Hwang I. 2011. Cytokinins and plant immunity: old foes or new friends? Trends Plant Sci. 16:388–394. 10.1016/j.tplants.2011.03.003
- Dobbss LB, Medici LO, Peres LEP, Pino-Nunes LE, Rumjanek VM, Facxanha AR, Canellas LP. 2007. Changes in root development of Arabidopsis promoted by organic matter from oxisols. Ann Appl Biol. 151:199–211. 10.1111/j.1744-7348.2007.00166.x
- Dospekhov BA. 1979. Technique of field experience. Moscow: Kolos; p. 351.
- El-Ghamry AM, Abd El-Hai KM, Ghoneem KM. 2009. Amino and humic acids promote growth, yield and disease resistance of faba bean cultivated in clayey soil. Austr J Basic Appl Sci. 3:731–739.
- Grant MR, Jones JDG. 2009. Hormone (dis)harmony moulds plant health and disease. Science 324:750–752. 10.1126/science.1173771
- Grosskinsky DK, Naseem M, Abdelmohsen UR, Plickert N, Engelke T, Griebel T, Zeier J, Novák O, Strnad M, Pfeifhofer H, et al. 2011. Cytokinins mediate resistance against Pseudomonas syringae in tobacco through increased antimicrobial phytoalexin synthesis independent of salicylic acid signaling. Plant Physiol. 157:815–830. 10.1104/pp.111.182931
- Jannin L, Arkoun M, Ourry A, Laine P, Goux D, Garnica M, Fuentes M, San Francisco S, Baigorri R, Cruz F, et al. 2012. Microarray analysis of humic acid effects on Brassica napus growth: Involvement of N, C and S metabolisms. Plant Soil. 359:297–319. 10.1007/s11104-012-1191-x
- Karatigin IV. 1981. Smut fungus. Ontogenesis and phylogenesis. Leningrad: Nauka.
- Mackowiak CL, Grossl PR, Bugbee BG. 2001. Beneficial effects of humic acid on micronutrient availability to wheat. Soil Sci Soc Am J. 65:1744–1750. 10.2136/sssaj2001.1744
- Maksimov IV, Khairullin RM. 2012. Activity of trypsin inhibitors in wheat seedlings exposed to pathogenic fungus Tilletia caries and phytohormones. Russ J Plant Physiol. 59:711–716. 10.1134/S1021443712050111
- Mora V, Bacaicoa E, Zamarreno AM, Aguirre E, Garnica M, Fuentes M, Garcia-Mina JM. 2010. Action of humic acid on promotion of cucumber shoot growth involves nitrate-related changes associated with the root-to-shoot distribution of cytokinins, polyamines and mineral nutrients. J Plant Physiol. 167:633–642. 10.1016/j.jplph.2009.11.018
- Muscolo A, Sidari M. 2009. Carboxyl and phenolic humic fractions affect Pinus nigra callus growth and metabolism. Soil Sci Soc Am J. 73:1119–1129. 10.2136/sssaj2008.0184
- Muscolo A, Cutrupi S, Nardi S. 1998. IAA detection in humic substances. Soil Biol Biochem. 30:1199–1201. 10.1016/S0038-0717(98)00005-4
- Muscolo A, Sidari M, Attina E. 2007. Biological activity of humic substances is related to their chemical structure. Soil Sci Soc Am J. 71:75–85. 10.2136/sssaj2006.0055
- Nardi S, Pizzeghello D, Muscolo A, Vianello A. 2002. Physiological effects of humic substances on higher plants. Soil Biol Biochem. 34:1527–1536. 10.1016/S0038-0717(02)00174-8
- Nurgalieva RV, Kildibekova AR, Sakhabutdinova AR, Maslennikova DR, Avalbaev AM, Bezrukova MV, Fatkhutdinova RA, Gilyazetdinov ShYa, Shakirova FM. 2006. Effect of Humi M on the hormonal status of wheat plants under salinity. Agrochemiya (in Russ). 8:25–29.
- Pena-Mendez EM, Havel J, Patocka J. 2005. Humic substances – compounds of still unknown structure: applications in agriculture, industry, environment, and biomedicine. J Appl Biomed. 3:13–24.
- Quaggiotti S, Ruperti B, Pizzeghello D, Francioso O, Tugnoli V, Nardi S. 2004. Effect of low molecular size humic substances on nitrate uptake and expression of genes involved in nitrate transport in maize (Zea mays L.). J Exp Bot. 55:803–813. 10.1093/jxb/erh085
- Russell L, Stokes AR, Macdonald H, Muscolo A, Nardi S. 2006. Stomatal responses to humic substances and auxin are sensitive to inhibitors of phospholipase A2. Plant Soil. 283:175–185. 10.1007/s11104-006-0011-6
- Shakirova FM. 2007. Role of hormonal system in manifestation of growth promoting and antistress action of salicylic acid. In: Hayat S, Ahmad A, editors. Salicylic acid – a plant hormone. The Netherlands: Springer; p. 69–89.
- Shakirova FM, Avalbaev AM, Bezrukova MV, Kudoyarova GR. 2010. Role of endogenous hormonal system in the realization of the antistress action of plant growth regulators on plants. Plant Stress. 4:32–38.
- Shakirova F, Kuznetsov V, Lubyanova A, Fatkhutdinova R, Bezrukova M, Nurgalieva R. 2008. The role of hormonal system in protection of wheat plants induced by HUMI. Proceedings 14th Meeting of International Humic Substances Society; 2008 Sep 14–19; Moscow – S.-Petersburg, Russia; p. 469–472.
- Shakirova FM, Kildibekova AR, Bezrukova MV, Avalbaev AM. 2004. Wheat germ agglutinin regulates cell division in wheat seedling roots. Plant Growth Regul. 42:175–180. 10.1023/B:GROW.0000017481.50472.e9
- Yarullina LG, Troshina NB, Maksimov IV, Khairullin RM. 2005. The effect of pathogens and phytohormones on the rate of oxidation of phenols by oxalate oxidase in wheat seedlings. Biol Bull. 32:143–146. 10.1007/s10525-005-0021-6
- Zhang X, Ervin EH. 2004. Cytokinin-containing seaweed and humic acid extracts associated with creeping bentgrass leaf cytokinins and drought resistance. Crop Sci. 44:1737–1745. 10.2135/cropsci2004.1737
