Abstract
The majority of WRKY transcription factors (TFs) play a role in the regulation of defense response in plants. Three WRKY genes, PtrWRKY1, PtrWRKY2 and PtrWRKY3, were previously identified in Poncirus and their expressions were characterized in response to cold and drought in Poncirus and Citrus. In this study, expressions of these WRKY genes were studied in response to infection with two major pathogens of Citrus, Citrus tristeza virus (CTV) and Phytophthora citrophthora, in resistant Poncirus and susceptible pummelo (Citrus grandis) plants. Northern blot analysis showed that the expression of the PtrWRKY1 gene was induced earlier and stronger in Poncirus than in pummelo in response to CTV infection. On the other hand, the expression of the PtrWRKY1 gene was not altered in response to P. citrophthora infection neither in Poncirus nor in pummelo. When the expression of the PtrWRKY2 gene was analyzed, it was repressed by CTV inoculation in Poncirus plants, whereas the expression was not changed in response to CTV infection in pummelo or in response to P. citrophthora inoculation in Poncirus or pummelo. Similarly, the expression of the PtrWRKY3 gene was repressed in Poncirus, but not changed in pummelo by CTV inoculation; however, the expression of the PtrWRKY3 gene was induced in Poncirus, but it was repressed in pummelo in response to P. citrophthora inoculation. The expression analysis of three different WRKY genes revealed that they are differentially expressed in response to CTV and P. citrophthora infection in resistant Poncirus and susceptible pummelo suggesting that they may play a role in disease resistance in Poncirus.
1. Introduction
WRKY transcription factors (TFs) constitute one of the major groups of transcription factor families in plants. After the identification of the first WRKY protein, SPF1, from sweet potato (Ishiguro & Nakamura Citation1994), a number of WRKY genes were identified and characterized from different plant species, including Arabidopsis thaliana (Chen & Chen Citation2002), barley (Sun et al. Citation2003), tobacco (Hara et al. Citation2000), rice (Liu et al. Citation2007), parsley (Rushton et al. Citation1996), sugarcane (Saccharum hybrid cultivar) (Lambais Citation2001), canola (Yang et al. Citation2009), pepper (Oh et al. Citation2006), tomato (Hofmann et al. Citation2008), grapevine (Marchive et al. Citation2007) and Poncirus trifoliata (Şahin-Çevik Citation2012; Şahin-Çevik & Moore Citation2012; Şahin-Çevik et al. Citation2012). In addition, among plant species with completed genome sequences, A. thaliana has 74, rice (Oryza sativa) contains 109, and woody plant poplar (Populus spp.) includes 104 WRKY genes (Eulgem & Somssich Citation2007; Ross et al. Citation2007; Pandey & Somssich Citation2009).
WRKY TFs play roles in multiple developmental and physiological processes of plants, including senescence (Ulker et al. Citation2007), dormancy (Rohde et al. Citation2007), root development (Zhang et al. Citation2008), oxidative stress (Miller et al. Citation2008), metabolic pathways (Li et al. Citation2004), germination of seeds (Jiang & Yu Citation2009) and biotic and abiotic stresses (Mzid et al. Citation2007; Zheng et al. Citation2007; Zhou et al. Citation2008). Therefore, transcriptional regulation of WRKY genes in response to various biotic and abiotic stresses has been explored for a better understanding of their roles in adaptation and tolerance to these stresses and their involvement in the regulation of stress-responsive genes and pathways in Arabidopsis and other plants (Eulgem et al. Citation2000; Narusaka et al. Citation2004; Wu et al. Citation2005). Expression analysis of WRKY genes from different plants indicated that the majority of WRKY genes were regulated by abiotic stresses, such as cold, drought and salinity (Ramamoorthy et al. Citation2008; Jiang & Deyholos Citation2009) and biotic stresses, especially pathogen infection. Due to the economic importance of pathogen defense in plants, the majority of known well-characterized WRKY genes from Arabidopsis and other agricultural crops were studied in response to pathogen infection (Ryu et al. Citation2006). It has been shown that the expression of various WRKY genes from different plants were induced in response to infection with bacterial (Dellagi et al. Citation2000; Zheng et al. Citation2007), fungal (Dellagi et al. Citation2000; Zheng et al. Citation2006; Marchive et al. Citation2007) and viral (Yoda et al. Citation2002) plant pathogens. The results overall showed that WRKY genes are involved in the activation and regulation of the basal as well as pathogen-induced defense pathways in plants, and that while some are regulated by both abiotic and biotic stresses, others are only involved in biotic stress.
Citrus is one of the most widely grown and economically important fruit crops in tropical and subtropical regions of the world. Production of citrus is limited mostly by cold and diseases, such as tristeza and Phytophthora. The tristeza disease caused by Citrus tristeza virus (CTV) is one of the most economically important viral diseases of Citrus. CTV is a single-stranded positive-sense RNA virus belonging to Closterovius genus in the Closterovridae family (Bar-Joseph & Lee Citation1990). It has long thread-like, flexuous, filamentous particles about 2000 nm by 11 nm consisting of a 20 kb single-stranded positive-sense RNA molecule and the major and minor capsid proteins (Febres et al. Citation1996). Depending on the citrus host and scion-rootstock combinations, CTV causes various symptoms ranging from mild vein clearing to quick decline, killing all citrus varieties grafted in sour orange rootstock (Lee & Bar-Joseph Citation2000). CTV has caused severe epidemics in various citrus growing regions and killed millions of citrus trees in the South-American and the Mediterranean countries (Bar-Joseph et al. Citation1989; Moreno et al. Citation2008). The disease is still a major problem in all citrus growing regions of the world (Moreno et al. Citation2008). Phytophthora species are also among the most common and serious pathogens of citrus. They cause diseases in young plants, as well as in older trees and fruits. Damping-off in the seedbeds, foot and root rot and gummosis in the nurseries, and foot rot, gummosis, feeder root rot and brown rot in the orchards are the main diseases caused by Phytophthora species resulting in serious crop losses (Timmer & Menge Citation1988). P. citrophthora is the most important species in the Phytophthora complex, infecting citrus under moderate temperatures (Erwin & Ribeiro Citation1996).
Most commercial Citrus species are susceptible to tristeza disease caused by CTV, and foot, root and brown rot diseases caused by Phytophthora ssp., especially P. citrophthora. On the other hand, Poncirus trifoliata, a close relative of Citrus, is not only cold-hardy, but also resistant to CTV and Phytophthora ssp. Therefore, P. trifoliata has been used in citrus breeding programs for improving abiotic and biotic stress tolerance of Citrus. Hybrids between Citrus and Poncirus enabled generation of stress-tolerant rootstocks, however, the mechanisms of abiotic and biotic stress tolerance in Poncirus have not been explored in depth. Although CTV resistance in P. trifoliata is known to be controlled by a single dominant gene (Gmitter et al. Citation1996; Fang et al. Citation1998), the resistance gene has not been cloned and the mechanisms of the resistance are not known in this species. On the other hand, resistance to Phytophthora ssp. has not been studied extensively and the genetic and molecular bases of Phytophthora resistance in P. trifoliata are not known.
We have previously identified and isolated three different WRKY TFs, PtrWRKY1, PtrWRKY2 and PtrWRKY3, from two-day cold-acclimated P. trifoliata and the expression of these genes in response to cold and drought stresses was analyzed in Poncirus and pummelo (Citrus grandis) (Şahin-Çevik Citation2012; Şahin-Çevik & Moore Citation2012; Şahin-Çevik et al. Citation2012). Since, some WRKY TFs have roles in both abiotic and biotic stresses, the expression patterns of these three genes were analyzed in this study in response to infection with CTV and P. citrophthora in P. trifoliata which is resistant, and pummelo which is susceptible to both pathogens.
2. Materials and methods
2.1. Plant materials
To study the changes in the expression of three WRKY genes in disease-resistant and susceptible plants, we selected P. trifoliata cv. Rubidoux (trifoliate orange) which is resistant to both CTV and P. citrophthora and C. grandis cv. Reinking which is considered susceptible to both CTV and P. citrophthora. Seeds of P. trifoliata and C. grandis were obtained from the National Clonal Germplasm Repository for Citrus and Dates in Riverside, CA, USA, or extracted from fruits produced in the Çukurova University Subtropical Fruits Research and Application Center in Adana, Turkey. The seeds were planted in a soilless medium in pots and seedlings were grown and maintained in a growth chamber at 28°C and 16-h photoperiod provided by cool white fluorescent light (100 µmol m–2 s–1).
2.2. Pathogen inoculation
2.2.1. Citrus tristeza virus (CTV) inoculation
A previously described Citrus tristeza virus (CTV) isolate, EG-5 (EK-1), recovered from Satsuma mandarins in the Edremit Gulf region of Turkey (Korkmaz et al. Citation2008), and maintained on Madam Vinous sweet orange (Citrus sinensis cv. Madam Vinous) was used for CTV inoculation of trifoliate orange and pummelo seedlings. Ten seedlings with a stem diameter of about 1 cm were selected from 18-month-old trifoliate orange and pummelo seedlings maintained in a growth chamber at 28°C and 16-h photoperiod. Five seedlings from each species were graft inoculated at 10 cm above the soil line with buds taken from a Madam Vinous seedling infected with CTV isolate. The remaining five seedlings were grafted with buds from a healthy sweet orange as controls for wounding (mock inoculation). At least three leaves at different positions above the inoculation sites were collected from each CTV- and mock-inoculated seedlings just before graft inoculation, and 1, 2, 4, 8 and 15 days after graft inoculation. Leaf samples at each time point were bulked and immediately frozen in liquid nitrogen and stored at –80°C until used for total RNA isolation. CTV- and mock-inoculated P. trifoliata and pummelo plants were tested for the presence of CTV in 15 weeks post-inoculation (wpi) using the RT-PCR method as previously described (Korkmaz et al. Citation2008).
2.2.2. Phytophthora citrophthora inoculation
A P. citrophthora isolate recovered from rotting orange fruit was obtained from DSMZ (German Collection of Microorganisms and Cell Cultures) and grown on CMA (Corn Meal Agar, Fluka Analytical, Switzerland) amended with antibiotics (pimaricin 10 µg/ml, penicillin 50 µg/ml, polymyxin 50 µg/ml). Ten seedlings with a stem diameter greater than 0.5 cm were selected from 18-month-old trifoliate orange and pummelo seedlings maintained in a growth chamber at 28°C and 16-h photoperiod. Five seedlings from each species were inoculated with mycelial inoculums of P. citrophthora grown on solid CMA medium (Ippolito et al. Citation1992). The stems of seedlings were inoculated by attaching a 4 mm diameter agar plug with mycelia removed from the edge of an actively growing 5-day-old culture into the wound made by cutting a small bark piece with a razor 10 cm above the soil line. The wounds were wrapped with parafilm to prevent desiccation (Ippolito et al. Citation1992). The remaining five seedlings were mock-inoculated with agar discs taken from the CMA solid medium without P. citrophthora. At least three leaves at different positions above the inoculation sites were collected from each P. citrophthora and mock-inoculated seedlings, just before inoculation, and 1, 2, 4, 8 and 15 days after inoculation. Leaf samples taken at each time point were bulked and immediately frozen in liquid nitrogen and stored at –80°C until use, for total RNA isolation.
2.3. Total RNA isolation
Total RNA was isolated from the leaf samples of inoculated and mock-inoculated P. trifoliata and pummelo seedlings using Trizol Reagents (Invitrogen, USA) according to the manufacturer's instructions. Total RNA concentration was measured by a UV-visible spectrophotometer (BioRad, USA) and the samples were stored at –80°C until use.
2.4. Northern blot hybridizations
The regions for gene-specific probes for the PtrWRKY1, PtrWRKY2, PtrWRKY3 and 18S ribosomal RNA (rRNA) genes were first amplified from a cDNA clone by PCR with the Advantage II DNA polymerase using a gene-specific forward primer and a gene-specific reverse primer with T7 promoter (Şahin-Çevik Citation2012; Şahin-Çevik & Moore Citation2012; Şahin-Çevik et al. Citation2012). The PCR-amplified gene fragments with T7 promoter sequence at 3′ end of the antisense strand were purified by Qiaquick PCR purification kit (Qiagen, Germany), denatured and used as the template for synthesis of DIG-labeled antisense riboprobes specific to each WRKY gene and 18S rRNA using a DIG RNA labeling kit (Roche, Germany) according to the manufacturer's instructions.
About 3 µg of total RNA samples isolated from inoculated plants and mock-inoculated control plants of Poncirus and pummelo were separated on denaturing agarose gels and transferred to nylon membranes. The membranes were pre-hybridized at 68°C for 30 min and hybridized with DIG-labeled antisense RNA probes specific to the 3′ half of the three WRKY genes, PtrWRKY1, PtrWRKY2 and PtrWRKY3 isolated from Poncirus, prepared using a DIG RNA labeling kit (Roche, Germany) at 68°C for 16 h. The membranes were washed two times with 2X SSC and 0.1% SDS at room temperature for 5 min, followed by two washes with 0.1X SSC and 0.1% SDS at 68°C for 15 min. The membranes were then subjected to detection of DIG-labeled RNA probes to detect RNA targets on northern blots using the DIG chemiluminescent detection kit (Roche, Germany) with the CSPD-Star substrate. After hybridizations with the gene specific probe, the membranes were re-hybridized with an 18S ribosomal RNA (rRNA) probe for loading and transfer control. The hybridization reactions were visualized by the ChemiDoc-It (UVP, England) chemiluminescent imaging system. Northern blot experiments were repeated two times for each gene. The expressions of WRKY and 18S rRNA genes were measured and quantified by the LabWorks (UVP, England) image analysis software.
2.5. Expression analysis
To analyze the quantitative expression data for WRKY genes in response to pathogen infection, the measured expression values of each WRKY gene were first normalized by dividing the transcript amount of the 18S rRNA gene at all time points. Then, the changes in the expression analysis defined as percent induction or repression for all three WRKY genes were calculated by subtraction of the determined average expression value of the control (0 h) from the average expression values of different time points of stress treatment and multiplication by 100. The calculated expression level of WRKY genes in response to pathogen and mock inoculations were graphically presented with northern blot images. The normalized expression data were tested for significance of changes in the gene expression at different day post inoculations (dpi) for CTV, Phytophthora and mock inoculations by comparing the means using Duncan's multiple range test (at P ≤ 0.05) for each gene and each application in P. trifoliata and C. grandis independently. An unpaired t-test was conducted to compare the expression of WRKY genes in response to pathogen and mock inoculations for each time point in P. trifoliata and C. grandis separately. The differences of gene expression were considered statistically significant at P ≤ 0.05.
3. Results
3.1. Expression analysis of the WRKY genes in response to CTV infection
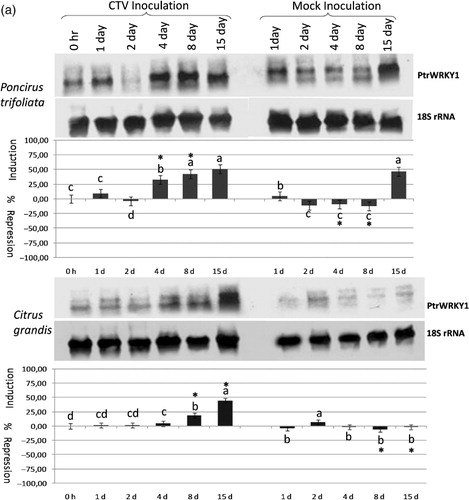
Quantification and statistical analysis of the PtrWRKY1 gene expression data showed that the expression was significantly increased at 4–15 dpi in CTV-inoculated Poncirus plants. However, no significant changes in the expression of the PtrWRKY1 gene were observed until 15 dpi in mock-inoculated Poncirus plants (, upper panel; Table S1Footnote 1 ). On the other hand, the expression of this gene was induced significantly only at 8 dpi and 15 dpi in CTV-infected pummelo plants, but no significant changes were observed in the expression of this gene in mock-inoculated pummelo plants (, lower panel; Table S1). The results demonstrated that the PtrWRKY1 gene is responsive to both virus infection and wounding in Poncirus, but only to virus infection in pummelo. These results also implied that the expression of the PtrWRKY1 gene was induced earlier and stronger in Poncirus compared to pummelo.
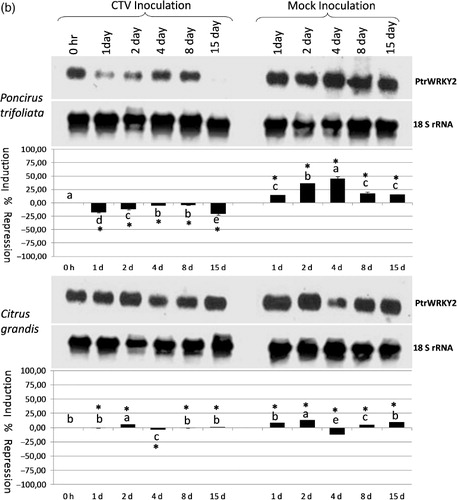
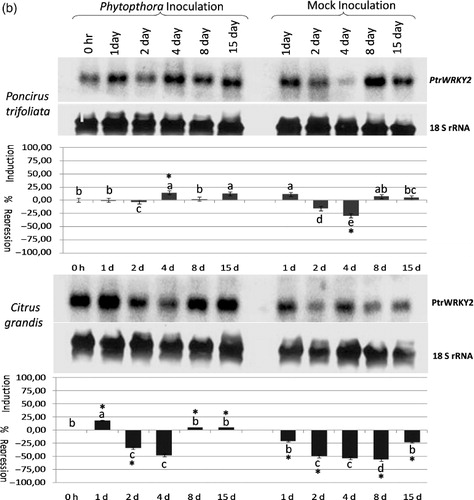
Figure 1a. The expression analysis of the PtrWRKY1(a), PtrWRKY2 (b), and PtrWRKY3 (c) gene in response to Citrus tristeza virus infection in Poncirus trifoliata and Citrus grandis (pummelo) detected by antisense DIG-labeled riboprobe specific to each gene shown on the right. The type and duration of inoculation are indicated on the top and inoculated plant species are shown on the left. The expression of the 18S rRNA gene was used as a loading and transfer control and is shown below the expression of each gene. Quantification of the expression data was presented graphically under the northern blots for each gene. The error bar indicates the standard deviation of percent change in gene expression for each time point. Letters indicate groups determined by Duncan's multiple rage test (at P ≤ 0.05) used for comparisons of the mean expression values of each gene at different time points for CTV inoculation and mock inoculation independently in P. trifoliata and C. grandis. *Statistically significant changes in the expression of the WRKY genes between CTV-inoculated and mock-inoculated plants.When the expression of the PtrWRKY2 gene was analyzed in response to CTV infection, this gene was constitutively expressed in non-inoculated Poncirus and pummelo control plants. While the expression of this gene was repressed at 1–15 dpi in CTV-infected Poncirus plants, it was induced in mock-inoculated Poncirus plants at all time points (, upper panel; Table S1). These results demonstrated that the expression of the PtrWRKY2 gene was increased in response to wounding, whereas it was repressed in response to CTV infection in Poncirus (). Although expression of the PtrWRKY2 gene was induced slightly at 2 dpi, no significant changes in the expression were observed at other time points in CTV-infected pummelo plants (, lower panel; Table S1). In contrast, the expression was induced very slightly at 1, 2, 8, 15 dpi, but repressed only at 4 dpi in mock-inoculated pummelo plants (, lower panel; Table S1) suggesting a wounding responsive gene expression. The result of the expression analyses showed a differential response of PtrWRKY2 to CTV infection and wounding in both Poncirus and pummel, while the expression was increased in mock-inoculated ones, but decreased in CTV-inoculated Poncirus.
Table 1. Summary of expressional response of three inoculations and wounding in Poncirus trifoliata and Citrus grandis.
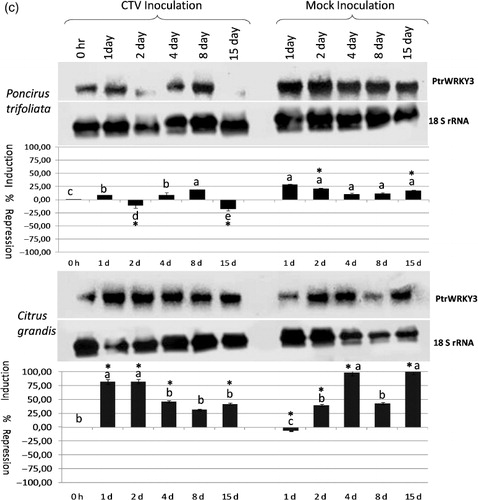
The PtrWRKY3 gene was weakly induced at 1, 4 and 8 dpi, but it was repressed to the same degree at 2 and 15 dpi in CTV-infected Poncirus plants. However, the expression of the PtrWRKY3 gene was induced at all time points in mock-inoculated Poncirus plants (, upper panel; Table S1) suggesting that this gene was induced in response to wounding in Poncirus. The statistical analysis of the expression in CTV and mock-inoculated Poncirus plants showed that changes were significant only at 2 and 15 dpi where the expression was repressed by CTV inoculation. On the other hand, it was significantly induced throughout the experiment in both CTV and mock-inoculated pummelo plants, except 1 d after mock inoculation (, lower panel; Table S1), suggesting that induction of the expression was not in response to CTV inoculation, but in response to wounding in pummelo. Overall expression analyses showed that the PtrWRKY3 gene was induced in response to wounding in both Poncirus and pummelo plants, but it was repressed by CTV inoculation only in CTV-resistant Poncirus.
To determine the occurrence of CTV inoculation in the grafted plants, mock- and CTV-inoculated plants were tested for the presence of CTV at 15 dpi and 15 wpi by RT-PCR. CTV was not detected in any of the mock- or CTV-inoculated Poncirus and pummelo plants tested at 15 dpi, which is considered as the early stage of infection. However, while CTV was detected only in CTV-inoculated pummelo plants, none of the mock-inoculated pummelo plants and none of the mock- and CTV-inoculated Poncirus plants were infected with CTV 15 wpi, which is considered as the late stage of infection (data not shown).
3.2. Expression of the PtrWRKY genes in response to P. citrophthora infection
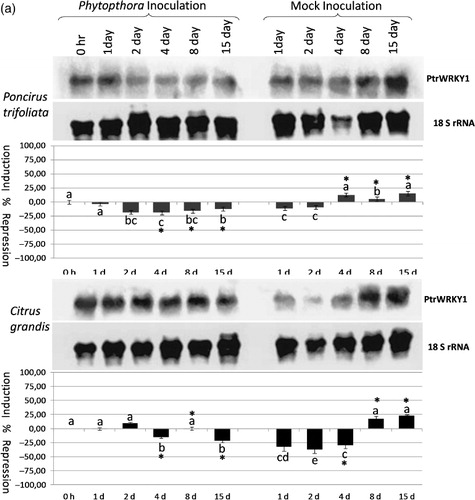
Quantification and statistical analyses of the PtrWRKY1 gene expression in response to P. citropthora infection showed repression at 4–15 dpi in P. citrophthora-inoculated Poncirus. However, it was induced at 4–15 dpi in mock-inoculated Poncirus plants (, upper panel; Table S2). The expression of the PtrWRKY1 gene was decreased significantly at 4 and 15 dpi in response to P. citrophthora infection in pummelo. On the other hand, while the expression of this gene was significantly repressed at 4 dpi, it was significantly induced at 8 and 15 dpi in mock-inoculated pummelo plants. The results suggested that the expression of the PtrWRKY1 gene is repressed by P. citrophthora infection, but it is induced in response to wounding.
Figure 2a. The expression analysis of the PtrWRKY1 (a), PtrWRKY2 (b), and PtrWRKY3 (c) gene in response to Phytophthora citrophthora infection in Poncirus trifoliata and Citrus grandis (pummelo) detected by antisense DIG-labeled riboprobe specific to each gene shown on the right. The type and duration of inoculation are indicated on the top and inoculated plant species are shown on the left. The expression of 18S rRNA gene was used as a loading and transfer control and is shown below the expression of each gene. Quantification of the expression data was presented under the northern blot. The error bar indicates the standard deviation of percent change in gene expression for each time point. Letters indicate groups determined by Duncan's multiple rage test (at P ≤ 0.05) used for comparisons of the mean expression values of each gene at different time points for P. citrophthora inoculation and mock inoculation independently in P. trifoliata and C. grandis. *Statistically significant changes in the expression of WRKY genes in Phytophthora-inoculated and mock-inoculated plants.The expression analyses of the PtrWRKY2 gene in P. citrophthora- and mock-inoculated Poncirus plants revealed no significant changes except at 4 dpi, when the expression was repressed in mock-inoculated Poncirus plants (, upper panel; Table S2). In contrast, the expression of this gene was significantly changed at all time points except at 4 dpi in P. citrophthora and mock-inoculated pummelo plants. The expression was only repressed at 2 and 4 dpi in P. citrophthora-inoculated pummelo plants, whereas it was significantly repressed at 1–15 dpi in mock-inoculated pummelo plants (, lower panel; Table S2). These results suggested that changes in the expression of the PtrWRKY2 gene were mostly due to wounding rather than P. citrophthora infection in Phytophthora-susceptible pummelo plants.
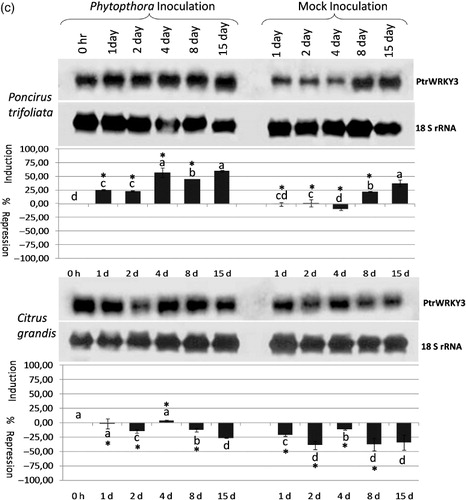
The expression of the PtrWRKY3 gene was induced gradually from 1 to 15 dpi in P. citrophthora-inoculated Poncirus plants; however, it was induced only at 8 and 15 dpi in mock-inoculated Poncirus (, upper panel; Table S2). Statistical analysis showed the PtrWRKY3 gene was induced significantly at 1–8 dpi in response to P. citrophthora inoculation in Poncirus, but it was induced by wounding at 15 dpi in both P. citrophthora and mock-inoculated plants. On the other hand, while the expression of the PtrWRKY3 gene was repressed significantly at 15 dpi in P. citrophthora-inoculated pummelo plants, it was repressed at all time points at different degrees in mock-inoculated pummelo plants (, lower panel; Table S2). Statistical analysis revealed that the PtrWRKY3 gene was repressed significantly at 1–8 dpi only by wounding in pummelo plants (Table S2). These results showed that the PtrWRKY3 gene was induced in response to P. citrophthora inoculation in Phytophthora-resistant Poncirus, but it was repressed by wounding in Phytophthora-susceptible pummelo plants.
4. Discussion
To understand the roles of WRKY TFs during pathogen infection in Poncirus and Citrus, the expressions of three WRKY genes, PtrWRKY1, PtrWRKY2 and PtrWRKY3 from Poncirus were analyzed in response to CTV, and P. citrophthora inoculation in P. trifoliata cv. Rubidoux which is resistant to both pathogens, and Citrus grandis cv. Reinking which is considered susceptible to both pathogens, were selected in this study. Although Mexican lime and sweet orange are the most susceptible Citrus species to CTV, pummelo was selected as susceptible host due to its relative sensitivity to both pathogens, as well as cold and drought stresses used for initial characterization of these WRKY genes. It was previously reported that some Citrus grandis cv. Chandler was resistant to CTV (Fang & Roose Citation1999), but other pummelo cultivars showed differential susceptibility to different CTV isolates (Garnsey et al. Citation1996). Other reports indicated that CTV is able to infect most pummelo cultivars (Xueyuan et al. Citation2002), and our RT-PCR results also showed that the CTV isolate used in this study was able to infect and replicate in pummelo.
The expression analyses of three different WRKY genes showed differential responses to these pathogens in two different hosts (). While the PtrWRKY1 gene was induced in CTV-resistant Poncirus, the PtrWRKY2 and the PtrWRKY3 genes were repressed by CTV infection and induced by wounding in both Poncirus and pummelo plants. The expressions of the PtrWRKY1 and PtrWRKY2 genes were repressed or not changed in response to P. citrophthora inoculation in both Poncirus and pummelo plants, but they were repressed or induced in response to wounding in both Poncirus and pummelo plants. However, the PtrWRKY3 gene was induced in response to P. citrophthora inoculation in resistant Poncirus, but was repressed in response to P. citrophthora infection in susceptible pummelo.
WRKY proteins are grouped into three classes based on the number of WRKY domains. A number of WRKY TFs with a single WRKY domain were classified into Group II, including Arabidopsis WRKY33 (Zheng et al. Citation2006). This group contains a single WRKY domain followed by Cys2His2 motif, as the PtrWRKY1 gene used in this study. For this reason, the PtrWRKY1 gene belongs to the Group II proteins, but phylogenetic analysis showed that the WRKY domain is closely related to N-terminal WRKY domain Group I WRKY TFs (Şahin-Çevik & Moore Citation2012). Unlike PtrWRKY1, PtrWRKY2 and PtrWRKY3 which contain two WRKY DNA binding domains are classified as Group I WRKY TFs (Şahin-Çevik Citation2012; Şahin-Çevik et al. Citation2012). The majority of defense-related WRKY proteins isolated from crop plants, including parsley WRKY1, Arabidopsis ZAP1, pepper CaWRKY2 and tomato LpWRKY1 contain two WRKY domains and belong to Group I WRKY proteins (de Pater et al. Citation1996; Eulgem et al. Citation1999; Oh et al. Citation2006; Hofmann et al. Citation2008). The expression data showing responsiveness of the WRKY genes of the present study to pathogen infection in Poncirus and Citrus, supports our previous findings, indicating that these WRKY genes are phylogenetically related to defense-responsive WRKY proteins from other plants.
The PtrWRKY1 gene was induced upon infection with CTV in Poncirus and to some extent, in pummelo plants. However, the expression was first induced at 4 dpi in CTV-resistant Poncirus plants, but at 8 dpi in CTV-susceptible pummelo plants. In addition, the expression of the PtrWRKY1 gene was also increased in response to wounding in Poncirus, but this change was not detected until 15 dpi. These results indicated that the expression of the PtrWRKY1 gene was induced earlier in the infection period to a greater extent in CTV-resistant Poncirus than in pummelo. On the other hand, the expression of the PtrWRKY3 gene was induced in response to P. citrophthora infection and wounding in Poncirus. Many WRKY TFs, including WRKY3, WRKY4, WRKY18, WRKY22, WRKY29, WRKY25, WRKY33, WRKY40 and WRKY60 from Arabidopsis (Asai et al. Citation2002; Zheng et al. Citation2006; Xu et al. Citation2006; Zheng et al. Citation2007; Lai et al. Citation2008), TIZZ from tobacco (Yoda et al. Citation2002), CaWRKY-a (Park et al. Citation2006) and CaWRKY2 (Oh et al. Citation2006) from pepper, and VvWRKY1 and VvWRKY2 from grapevine (Marchive et. al. 2007) are induced in response to pathogen infections. The involvement of some of the WRKY genes in plant defense and resistance to specific pathogens was experimentally demonstrated, suggesting that the PtrWRKY1 gene induced in response to CTV infection and the PtrWRKY3 gene induced in response to P. citrophthora infection may contribute resistance to these pathogens in Poncirus.
The PtrWRKY2 and PtrWRKY3 genes were both induced in response to wounding in both Poncirus and pummelo, but did not change or were repressed in response to CTV infection in Poncirus and pummelo. However, the timing of repression was much earlier and the degree of repression was much higher in CTV-resistant Poncirus than the CTV-susceptible pummelo, suggesting that they may be negative regulators of CTV resistance in Poncirus. While some WRKY proteins are induced in response to pathogen infection and act as a positive regulator of pathogen defense, others including WRKY11, WRKY17 and WRKY48 from Arabidopsis and OsWRKY62 from rice were repressed, and their involvement in negative regulation of disease resistance in respective plants was demonstrated (Journot-Catalino et al. Citation2006; Xu et al. Citation2006; Zheng et al. Citation2007; Xing et al. Citation2008). Based on the expression data showing repression of the PtrWRKY2 and PtrWRKY3 genes in CTV-resistant Poncirus, but not in CTV-susceptible pummelo, it may be speculated that three TFs may be involved in the negative regulation of CTV resistance in Poncirus.
Most of Arabidopsis WRKY genes having a role in the regulation of the defense response were induced as early as 2 or 4 h after pathogen-infection or application of defense-related hormones, such as salicylic acid (SA), methyl jasmonic acid (JA) or ethylene (ET). Their expression was decreased by 24 h after infection or elicitor treatment suggesting that they are involved in early transcriptional activation (Yoda et al. Citation2002; Dong et al. Citation2003; Oh et. al. 2006). In this study, the first transcriptional response of Poncirus WRKY genes was measured starting at 1 d after inoculation with pathogens or wounding in Poncirus and pummelo plants, and this induction continued over 15 d. In this aspect, this study is the first report examining the expression of WRKY genes in response to wounding and pathogen infection for an extended period. Studying the earlier response of WRKY genes of Poncirus to pathogen infection may provide a better understanding of the physiological function of the corresponding proteins. The difference in induction pattern in response to wounding and pathogen infection in Arabidopsis and Citrus could simply result from the functions of different WRKY genes or differences in the host-pathogen systems.
The expression of Arabidopsis WRKY3 and WRKY4 TFs were rapidly induced after infection with the fungal pathogen Botrytis cinerea, as well as treatment with signal molecules, such as H2O2, SA, JA or 1-aminocyclopropane-1-carboxylic acid (ACC), the immediate precursor of ET biosynthesis (Lai et al. Citation2008). Therefore, the expression of the PtrWRKY1, PtrWRKY2 and PtrWRKY3 genes should also be explored after elicitor treatments.
In this study, changes in the expression of the PtrWRKY1, PtrWRKY2 and PtrWRKY3 genes were determined in response to two different pathogens in Poncirus and Citrus species, pummelo. This is the first report of the expression analysis of WRKY TFs in response to pathogen infection in an economically important perennial crop plant. Since the involvement of these three WRKY TFs in cold and drought stress response has previously been shown, this study extended to the disease response of these genes. It provides a more comprehensive understanding of the involvement of Poncirus WRKY TFs in environmental stress response in Poncirus and Citrus. However, further studies are needed for a better understanding of the functions and specific involvements of each WRKY gene in abiotic and biotic stress response in Poncirus, and their possible use for improving biotic and abiotic stress tolerance in commercial Citrus species.
Supplemental Material.docx
Download MS Word (73.2 KB)Acknowledgments
This study was supported by the Scientific and Technological Research Council of Turkey (TÜBİTAK) project number 104O158. Authors thank Savas Korkmaz for providing the CTV isolate and the National Clonal Germplasm Repository for Citrus and Dates in Riverside, CA, USA and the Subtropical Fruits Research and Application Center at Cukurova University in Adana, Turkey for providing Poncirus and Citrus seeds.
Notes
1. Supplemental Content may be viewed online at http://dx.doi.org/10.1080/17429145.2013.796596
References
- Asai T, Tena G, Plotnikova J, Willmann, MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. 2002. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 415:977–983. 10.1038/415977a
- Bar-Joseph M, Marcus R, Lee RF. 1989. The continuous challenge of Citrus tristeza virus control. Ann Rev Phytopathol. 27:291–316. 10.1146/annurev.py.27.090189.001451
- Bar-Joseph M, Lee RF. 1990. Citrus tristeza virus. Description of Plant Viruses No:353. Surrey: Commonwealth Mycological Institute/Association of Applied Biologist.
- Chen C, Chen Z. 2002. Potentiation of developmentally regulated plant defense response by AtWRKY18, a pathogen-induced Arabidopsis transcription factor. Plant Physiol. 129:706–716. 10.1104/pp.001057
- Dellagi A, Heilbronn J, Avrova AO, Montesano M, Palva ET, Stewart HE, Toth IK, Cooke DEL, Lyon GD, Birch PRJ. 2000. A potato gene encoding a WRKY-like transcription factor is induced in interactions with Erwinia carotovora subspatroseptica and Phytophthora infestans and is coregulated with class I endochitinase expression. Mol Plant-Microbe Interact. 13:1092–1101. 10.1094/MPMI.2000.13.10.1092
- De Pater S, Greco V, Pham K, Memelink J, Kijne J. 1996. Characterization of a zinc-dependent transcriptional activator from Arabidopsis. Nucleic Acids Res. 24:4624–4631. 10.1093/nar/24.23.4624
- Dong J, Chen C, Chen Z. 2003. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol. 5:21–37. 10.1023/A:1020780022549
- Erwin DC, Ribeiro OK. 1996. Phytophthora diseases worldwide. St Paul, MN: APS Press.
- Eulgem T, Rushton PJ, Robatzek S, Somssich IE. 2000. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 5:199–206. 10.1016/S1360-1385(00)01600-9
- Eulgem T, Rushton PJ, Schmelzer E, Hahlbrock K, Somssich IE 1999. Early nuclear events in plant defence signalling: rapid gene activation by WRKY transcription factors. EMBO J. 18:4689–4699. 10.1093/emboj/18.17.4689
- Eulgem T, Somssich IE. 2007. Networks of WRKY transcription factors in defense signaling. Curr Opin Plant Biol. 10:366–371. 10.1016/j.pbi.2007.04.020
- Fang DQ, Federici CT, Roose ML. 1998. A high-resolution linkage map of the citrus tristeza virus resistance gene region in Poncirus trifoliata (L.) Raf. Genetics. 150:883–890.
- Fang DQ, Roose ML. 1999. A novel gene conferring Citrus tristeza virus resistance in Citrus maxima (burm.) merrill. HortSci. 34:334–335.
- Febres VJ, Ashoulin L, Mawassi M, Frank A, Bar-Joseph M, Manjunath KL, Lee RF, Niblett CL. 1996. The p27 protein is present at one end of Citrus tristeza virus particles. Phytopathology. 86:1331–1335.
- Garnsey SM, Su HJ, Tsai MC. 1996. Differential susceptibility of pummelo and Swingle citrumelo to isolates of citrus tristeza virus. In: da Graça JV, Moreno P, Yokomi RK, editors. Proceedings of the 13th conference of IOCV. Riverside: IOCV; p. 138–146.
- Gmitter FG, Xiao SY, Huang S, Hu XL, Garnsey SM, Deng Z. 1996. A localized linkage map of the Citrus tristeza virus resistance gene region. Theor Appl Genet. 92:688–695. 10.1007/BF00226090
- Hara K, Yagi M, Kusano T, Sano H. 2000. Rapid systemic accumulation of transcripts encoding a tobacco WRKY transcription factor upon wounding. Mol Gen Genet. 263:30–37. 10.1007/PL00008673
- Hofmann MG, Sinha AK, Proels RK, Roitsch T. 2008. Cloning and characterization of a novel LpWRKY1 transcription factor in tomato. Plant Physiol Biochem. 46:533–540. 10.1016/j.plaphy.2008.02.009
- Ippolito A, Nigro F, Salerno M, Privitera S. 1992. Influence of the scion on the response of sour orange rootstock to Phytophthora root rot. Proc Int Soc Citruculture. 2:851–853.
- Ishiguro S, Nakamura K. 1994. Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5′ upstream regions of genes coding for sporamin and beta-amylase from sweet potato. Mol Gen Genet. 244:563–571.
- Jiang Y, Deyholos MK. 2009. Functional characterization of Arabidopsis NaCl-inducible WRKY25 and WRKY33 transcription factors in abiotic stresses. Plant Mol Biol. 69:91–105. 10.1007/s11103-008-9408-3
- Jiang W, Yu D. 2009. Arabidopsis WRKY2 transcription factor mediates seed germination and postgermination arrest of development by abscisic acid. BMC Plant Biol. 9:96–109. 10.1186/1471-2229-9-96
- Journot-Catalino N, Somssich IE, Roby D, Kroj T. 2006. The transcription factors WRKY11 and WRKY17 act as negative regulators of basal resistance in Arabidopsis thaliana. Plant Cell. 18:3289–3302. 10.1105/tpc.106.044149
- Korkmaz S, Çevik B, Onder S, Koç NK. 2008. Biological, serological, and molecular characterization of citrus tristeza virus isolates from different citrus cultivation regions of Turkey. Turkish J Agric Forest. 32:369–379.
- Lai Z, Vinod K, Zheng Z, Fan B, Chen Z. 2008. Roles of Arabidopsis WRKY3 and WRKY4 transription factors in plant responses to pathogens. BMC Plant Biol. 8:68–881. 10.1186/1471-2229-8-68
- Lambais MR. 2001. In silico differential display of defense-related expressed sequence tags from sugarcane tissues infected with diazotrophic endophytes. Genet Mol Biol. 24:103–111. 10.1590/S1415-47572001000100015
- Lee RF, Bar-Joseph M. 2000. Tristeza. In: Timmer LW, Garnsey MS, Graham JH, editors. Compendium of citrus diseases. St Paul, MN: APS Press; p. 61–63.
- Li J, Brader G, Palva ET. 2004. The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylatemediated signals in plant defense. Plant Cell. 16:319–331. 10.1105/tpc.016980
- Liu X, Bai X, Wang X, Chu C. 2007. OsWRKY71 a rice transcription factor, is involved in rice defense response. J Plant Physiol. 164:969–979. 10.1016/j.jplph.2006.07.006
- Marchive C, Mzid R, Deluc L, Barrieu F, Pirrello J, Gauthier A, Corio-Costet MF, Regad F, Cailleteau B, Hamd S, Lauvergeat V. 2007. Isolation and characterization of a Vitis vinifera transcription factor, VvWRKY1, and its effect on responses to fungal pathogens in transgenic tobacco plants. J Exp Bot. 58:1999–2010. 10.1093/jxb/erm062
- Miller G, Shulaev V, Mittlera R. 2008. Reactive oxygen signaling and abiotic stress. Physiol Plant. 133:481–489. 10.1111/j.1399-3054.2008.01090.x
- Moreno P, Ambrós S, Albiach-Martí MR, Guerri J, Peña L. 2008. Citrus tristeza virus: a pathogen that changed the course of the citrus industry. Mol Plant Pathol. 9:251–268. 10.1111/j.1364-3703.2007.00455.x
- Mzid R, Marchive C, Blancard D, Deluc L, Barrieu F, Corio-Costet MF, Drira N, Hamd S, Lauvergeat V. 2007. Overexpression of VvWRKY2 in tobacco enhances broad resistance to necrotrophic fungal pathogens. Physiol. Plant. 131:434–447. 10.1111/j.1399-3054.2007.00975.x
- Narusaka Y, Narusaka M, Seki M, Umezawa T, Ishida J, Nakajima M, Enju A, Shinozak K. 2004. Crosstalk in the responses to abiotic and biotic stresses in Arabidopsis: analysis of gene expression in cytochrome P450 gene superfamily by cDNA microarray. Plant Mol Biol. 55:327–342. 10.1007/s11103-004-0685-1
- Oh SK, Yi SY, Yu SH, Moon JS, Park JM, Choi D. 2006. CaWRKY2, a chili pepper transcription factor, is rapidly induced by incompatible plant pathogens. Mol Cell. 22:58–64.
- Pandey SP, Somssich IE. 2009. The role of WRKY transcription factors in plant immunity. Plant Physiol. 150:1648–1655. 10.1104/pp.109.138990
- Park CJ, Shin YC, Lee BJ, Kim KJ, Paek KH. 2006. A hot pepper gene encoding WRKY transcription factor is induced during hypersensitive response to Tobacco mosaic virus and Xanthomonas campestris. Planta, 223:168–179. 10.1007/s00425-005-0067-1
- Ramamoorthy R, Jiang SY, Kumar N, Venkatesh PN, Ramachandran SA. 2008. Comprehensive transcriptional profiling of the WRKY gene family in rice under various abiotic and phytohormone treatments. Plant Cell Physiol. 49:865–879. 10.1093/pcp/pcn061
- Rohde A, Ruttink T, Hostyn V, Sterck L, Van Driessche K, Boerjan W. 2007. Gene expression during the induction, maintenance, and release of dormancy in apical buds of poplar. J Exp Bot. 58:4047–4060. 10.1093/jxb/erm261
- Ross CA, Liu Y, Shen QJ. 2007. The WRKY gene family in rice (Oryza sativa). J Integr Plant Biol. 49:827–842. 10.1111/j.1744-7909.2007.00504.x
- Rushton PJ, Torres JT, Parniske M, Wernert P, Hahlbrock K, Somssich IE. 1996. Interaction of elicitor-induced DNA-binding proteins with elicitor response elements in the promoters of parsley PR1 genes. EMBO J. 15:5690–5700.
- Ryu HS, Han M, Lee SK, Cho JI, Ryoo N, Heu S, Lee YH, Bhoo SH, Wang GL, Hahn TR, Jeon JS. 2006. A comprehensive expression analysis of the WRKY gene superfamily in rice plants during defense response. Plant Cell Rep. 25:836–847. 10.1007/s00299-006-0138-1
- Şahin-Çevik M. 2012. A WRKY transcription factor gene isolated from Poncirus trifoliata show differential responses to cold and drought stresses. Plant Omics J. 5:438–445.
- Şahin-Çevik M, Çevik B, Aşkın MA. 2012. An abiotic stress-responsive WRKY gene is transiently induced in response to cold and drought stresses in Poncirus trifoliata. J Plant Interact. doi: 10.1080/17429145.2012.747006.
- Şahin-Çevik M, Moore GA. 2012. Isolation and expression analysis of a drought and cold stress inducible WRKY gene in the cold-hardy citrus relative Poncirus trifoliata. New Zealand J Crop Hort Sci. iFirst, 1–12. doi: 10.1080/01140671.2012.731654.
- Sun C, Palmqvist S, Olsson H, Boren M, Ahlandsberg S, Jansson C. 2003. A novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to the sugar-responsive elements of the iso1 promoter. Plant Cell. 15:2076–2092. 10.1105/tpc.014597
- Timmer LW, Menge JA. 1988. Phytophthora-induced diseases. In: Whiteside JO, Garnsey SM, Timmer LW, editors. Compendium of citrus diseases. St Paul, MN: APS Press; p. 22–24.
- Ulker B, Mukhtar MS, Somssich IE. 2007. The WRKY70 transcription factor of Arabidopsis influences both the plant senescence and defense signaling pathways. Planta. 226:125–137. 10.1007/s00425-006-0474-y
- Wu, KL, Guo ZJ, Wang HH, Li J. 2005. The WRKY family of transcription factors in rice and Arabidopsis and their origins. DNA Res. 12:9–26. 10.1093/dnares/12.1.9
- Xing DH, Laia ZB, Zhenga ZY, Vinoda KM, Fana BF, Chen ZX. 2008. Stress- and pathogen-induced Arabidopsis WRKY48 is a transcriptional activator that represses plant basal defense. Mol Plant. 1:459–470. 10.1093/mp/ssn020
- Xu X, Chen C, Fan B, Chen Z. 2006. Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. Plant Cell. 18:1310–1326. 10.1105/tpc.105.037523
- Xueyuan Z, Changyong Z, Kezhi T, Yuanhui J, Fangyun Y, Sen H, Quanyou C. 2002. Preliminary evaluation of the tolerance of 18 pummelo cultivars to stem-pitting tristeza. In: Duran-Vila N, Milne RG, da Graça JV, editors. Proceedings of the 15th International Conference of IOCV. Riverside, CA: IOCV; p. 172–175.
- Yang B, Jiang YQ, Rahman MH, Deyholos MK, Kav NNV. 2009. Identification and expression analysis of WRKY transcription factor genes in canola (Brassica napus L.) in response to fungal pathogens and hormone treatments. BMC Plant Biol. 9:68–76. 10.1186/1471-2229-9-68
- Yoda H, Ogawa M, Yamaguchi Y, Koizumi N, Kusano T, Sano H. 2002. Identification of early-responsive genes associated with the hypersensitive response to tobacco mosaic virus and characterization of a WRKY-type transcription factor in tobacco plants. Mol Genet Genom. 267:154–161. 10.1007/s00438-002-0651-z
- Zhang J, Peng Y, Guo Z. 2008. Constitutive expression of pathogen-inducible OsWRKY31 enhances disease resistance and affects root growth and auxin response in transgenic rice plants. Cell Res. 18:508–521. 10.1038/cr.2007.104
- Zheng Z, Mosher SL, Fan B, Klessig DF, Chen Z. 2007. Functional analysis of Arabidopsis WRKY25 transcription factor in plant defense against Pseudomonas syringae. BMC Plant Biol. 7:2–14.
- Zheng Z, Qamar SA, Chen Z, Mengiste T. 2006. Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J. 48:592–605.
- Zhou QY, Tian, AG, Zou HF, Xie ZM, Lei G, Huang J, Wang CM, Wang HW, Zhang JS, Chen SY. 2008. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants Plant Biotech J. 6:486–503.