Abstract
Bioinvasion has become a serious environmental problem in the world in general and is considered the second biggest threat to biodiversity. Alternanthera philoxeroides is widely distributed and causes the most serious threat to biodiversity in China. The traditional physical or biological control methods are not effective in controlling the invasion and extension of A. philoxeroides. In the present paper, some physiological characteristics of Humulus scandens and A. philoxeroides were investigated in the field and laboratory. The results showed that H. scandens is more competitive than A. philoxeroides, the competitive rate (CR) of H. scandens against A. philoxeroides was 9.834. Additionally, the leaf, stem, and root biomass of A. philoxeroides decreased significantly when the two species co-occurred. Thus, the invasive abilities of these two invasive plants are different and H. scandens strongly inhibited the growth of A. philoxeroides. Moreover, as an annual herb, H. scandens can be easily eliminated by harvesting before its seeds mature. The result suggests that sowing seeds of H. scandens in the habitats invaded by A. philoxeroides could be an ideal biological control method.
Introduction
In recent years, with rapid economic development and increased international trading, bioinvasion has become a serious environmental problem throughout the world in general (Lin et al. Citation2007) and is considered as an important contributor to global change (Vitousek et al. Citation1997; Mooney & Hobbs Citation2000). Moreover, bioinvasion has become the second biggest threat to biodiversity (Cheng et al. Citation2009). Alien invasive species can decrease local biodiversity, change the evolutionary direction of native species, and influence the structure of native communities by competitive exclusion, niche substitution, predation, hybridization, introgression, parasitic/pathogenic organism spread, etc. (Mooney & Cleland Citation2001). Thus, invasive species seriously threaten the biodiversity and natural ecosystems of China (Xie et al. Citation2001), and cause economic losses of more than US $7 billion every year (Fan & Li Citation2001). Alternanthera philoxeroides is an invasive weed. It has the widest distribution and poses the most serious threat in China. And as an amphibious perennial species with perennial roots (Pan et al. Citation2006), it is very difficult to control its invasion and dispersal.
An important mechanism for many invasive species becoming dominant in their new ecosystem is competitive exclusion and niche substitution (Cheng et al. Citation2009). Alien invasive species are usually strong competitors or highly propagative, allowing them to exclude and displace native species, leading to the decrease or even extinction of native populations (Petren & Case Citation1996; Holway Citation1999; Gurevitch & Padilla Citation2004; Bohn et al. Citation2007). Previous studies have investigated competition between alien invasive and native plants (Callaway & Aschehoug Citation2000; Brown et al. Citation2002), insects (Edgerly et al. Citation1993; Juliano Citation1998; Holway & Suarez Citation1999; Braks et al. Citation2004; Zang et al. Citation2005), nematodes (Cheng et al. Citation2009), and other animals (Petren et al. Citation1993; Petren & Case Citation1996; Wauters et al. Citation2002; Wilson et al. Citation2004). However, the competition among different invasive species has been ignored up to date.
In the present paper, some physiological characteristics of A. philoxeroides and Humulus scandens were investigated in the field and greenhouse. H. scandens was chosen as a competitor in this study because it is usually found cohabiting with A. philoxeroides. We focused on three questions: (1) Does an interspecific competitive relationship exist between these two alien invasive plant species when they cohabit? (2) If interspecific competition does occur, which of the two invasive species plant is more competitive? (3) Would it be feasible to control the invasion of A. philoxeroides by sowing seeds of H. scandens in the habitats invaded by A. philoxeroides?
Materials and methods
The field experiment was conducted in abandoned land in Ji'an city, Jiangxi Province (27°06′N, 114°57′E). The average frost-free period in the area is about 285 d. The mean annual temperature is 17.0–18.6 °C. The coldest temperatures are in January, with an extreme minimum temperature of −6 °C, and the hottest temperatures are in July with an extreme maximum temperature of above 40 °C. The mean annual precipitation is 1350–1570 mm, but significantly differs inter-annually, ranging from 1018 mm in dry years to 2130 mm in wet years. The pH is 4.21±0.05 and the moisture is 13.46±0.64% (n = 5). The vegetation is mainly herbaceous species and is dominated by A. philoxeroides and H. scandens.
In the field experiment, nine 1×1 m quadrats in the study site were selected to represent three habitat types, with three replications of each habitat. The first habitat type (CKA) included only A. philoxeroides as an inhabitant. The second habitat type (CKH) included only H. scandens. The two species co-occurred in the third habitat type (CE). All the plant samples in each replicate were sampled. The leaves, roots, and stems of the samples were separated and then dried in an oven at 70 °C, after which they were weighed to determine leaf, root, and stem dry weight.
In the greenhouse experiment, twenty-one 5 kg physicochemically uniform soil samples were collected from a common same and placed in flowerpots. In order to simulate the field study habitats, three treatments (CKA, CKH, and CE), with nine replicated each, were examined. For CKA and CKH treatments, two stems, both of A. philoxeroides or H. scandens, respectively, were planted in one flowerpot. For the CE treatment, one stem of A. philoxeroides and one stem of H. scandens were planted in one flowerpot. A. philoxeroides were planted as two-knot stems without leaf, and H. scandens were planted as seedlings. The numbers of leaves and lateral buds were counted, and the lengths of stems were measured with steel tape every two weeks.
The total relative yield (TRY; Taylor & Aarssen Citation1989) and the competitive rate (CR; Weigelt & Jolliffe Citation2003) were adopted as competition index. A repeated measures ANOVA (RMANOVA) with Turkey's honestly significant difference (HSD) test was performed to examine differences in stem length, biomass, and amount of leaves and lateral buds among different treatments. All data analysis was conducted using the SPSS 15.0, for Windows. Significant differences were determined using P values <0.05 unless otherwise stated.
Results
The result in the field showed that the leaf, stem, and root biomass of A. philoxeroides in CE were all significantly lower than in CKA habitat (; n = 3, P < 0.001), and the ratio of root to shoot of A. philoxeroides in CE was also significantly lower than in CKA habitat (n=3, P < 0.05). The leaf, stem, and root biomass of H. scandens in CE habitat was lower than in CKH habitat, but only leaf biomass was significant (n = 3, P < 0.001), and the ratio of root to shoot of H. scandens in CE was lower than that of CKH habitat (n=3, P < 0.05). The total biomass of A. philoxeroides in CKA was significantly higher than that of H. scandens in CKH (n=3, P < 0.05); however, when the two species co-occurred in CE habitat, the biomass did not significantly differ between the two species. The TRY and the CR of H. scandens to A. philoxeroides were 0.444 and 9.834, respectively.
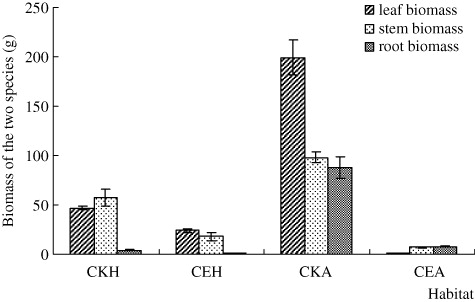
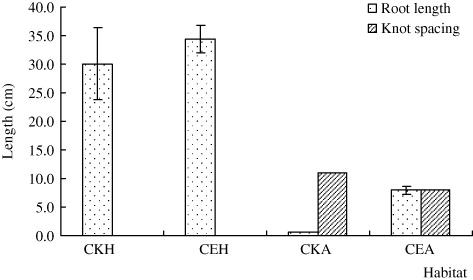
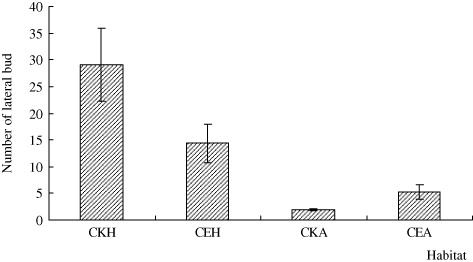
The whole stem length of H. scandens and A. philoxeroides in CE was significantly shorter than in CKH and CKA habitats, respectively (n=11, p < 0.05), and the stem length of H. scandens is always greater than that of A. philoxeroides, regardless of habitat (; F=5.249, p < 0.05). On the other hand, the knot spacing and root length of A. philoxeroides in CE were significantly longer than in CKA (; n=6 for knot spacing and 5 for root length, p < 0.05). The lateral bud number and root length of H. scandens in CE were significantly lower than in CKH (; n=3, P < 0.05), but the lateral bud number of A. philoxeroides did not differ with habitat.
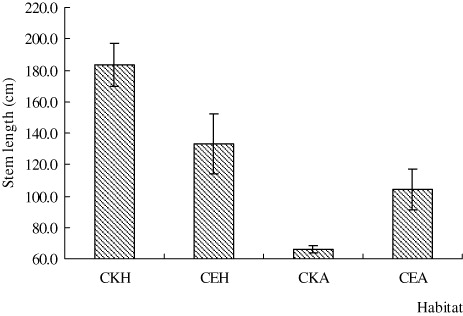
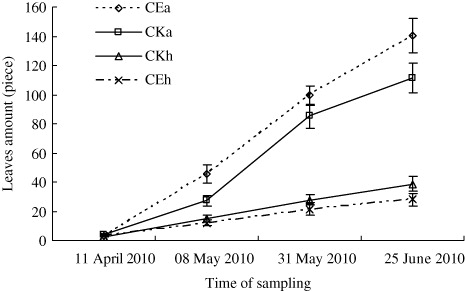
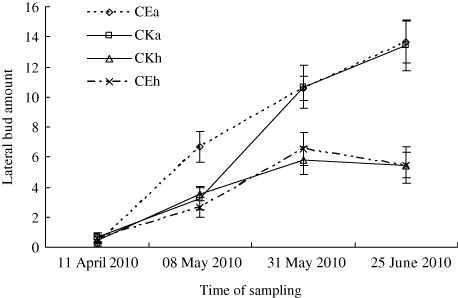
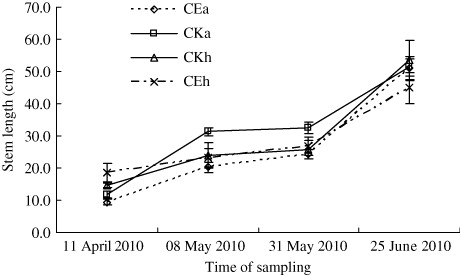
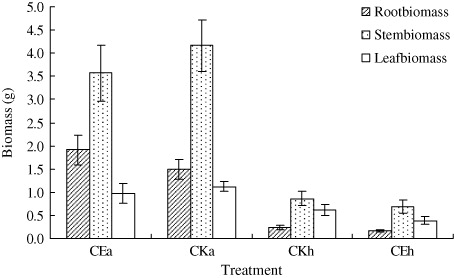
The results of the greenhouse experiment showed that the number of leaves and lateral buds on A. philoxeroides in CEA was significantly higher than in CKA treatment, except for during the first sampling on 11 April (; P < 0.05, n=9). On H. scandens, the number of leaves in CEH was higher than in CKH treatment, and the number of lateral buds in CE was very close to counts in CKH over the four-month duration of the experiment (; P < 0.05). The stem length of A. philoxeroides in CE was marginally lower than in CKA, and the difference increased throughout the treatment period (; P < 0.05). However, when the two species were mix-planted (CE), stem length of H. scandens was always lower than that of A. philoxeroides except for the first sampling, and the difference increased throughout the treatment period. The root, stem, and leaf biomass of both plants in CE were all significantly lower than in CKA. However, the root, stem, and leaf biomass of A. philoxeroides were significantly higher than those of H. scandens when they were mix-planted (CE; ; P < 0.05).
Discussion
Competition is a situation where two individuals strive for the same resource, which is present in limited quantities. Whatever one species acquires out of the environment is deducted from what is available for the second one, and vice versa. Thus, both species of the interacting pair must be mutually affected (Agami & Waisel Citation1985). Competitive relationships between two sympatric species can be identified by the level of growth inhibition that is inflicted on one species by the other (De Wit Citation1960). The result of our field investigation showed that the CR of H. Scandens to A. philoxeroides was 9.834 which means that H. scandens is more competitive than A. philoxeroides. Moreover, the leaf, stem, and root biomass and the ratio of root to shoot of A. philoxeroides in CE were significantly lower than those in CKA, which means that the growth of A. philoxeroides was strongly inhabited when co-occurring with H. scandens. Thus, we conclude that the invasive ability of these two plants differs, and according to the competitive exclusion principle, which predicts that closely related species with overlapping niches are unlikely to occupy the same habitat (Gause Citation1932; Hutchinson Citation1959; Schluter Citation2000), H. scandens would competitively exclude A. philoxeroides.
The greenhouse treatment results showed that the number of lateral bud on H. scandens in CE was very close counts in the CKH treatment over the four-month duration of the experiment, but the number of leaves on H. scandens in CE was higher than that in CKH, which means that H. scandens try to enhance their competitiveness by increasing the number of leaves. On the other hand, the number of leaves and lateral buds on A. philoxeroides in CE was significantly higher than in the CKA treatment. The root, stem, and leaf biomass of A. philoxeroides were significantly higher than those of H. scandens when they were mix-planted (). The reason for this finding may be that the seedlings of H. scandens in this experiment, which were transplanted from other habitats, were not well adapt to the greenhouse habitats. Moreover, we observed in the field that germination of H. scandens seeds occurred earlier than budding of A. philoxeroides in the spring. In the greenhouse, when the lateral buds of A. philoxeroides emerged from the subterranean stems, they shaded the seedlings of H. scandens (personal observation). Nevai and Vance (Citation2007) examined the influence of canopy partitioning on the outcome of competition between two plant species that interact only by mutually shading each other. And the principal conclusion is that two clonal plant species which compete for sunlight and place their leaves at different heights above the ground but differ in no other way can, under suitable parameter values, experience stable coexistence even though they occupy an environment which varies neither over horizontal space nor through time. Thus, we suspect that the growth of A. philoxeroides would have been restrained by H. scandens if we had sowed seeds instead of transplanting the seedlings of H. scandens in this experiment.
Researches on biological invasions have largely focused on the impacts of introduced species and on methods of their control (Devin & Beisel Citation2007). Classical biological control of exotic weeds, which involves the deliberate release of specialist natural enemies from the target weed's native range to its invasive range, can be a highly cost-efficient approach to mitigate the negative impact of invasive weeds on biodiversity, human welfare, and economy (Müller-Schärer & Schaffner Citation2008). However, as an amphibious perennial species with perennial roots (Pan et al. Citation2006), A. philoxeroides is resistant to classical biological control and physical methods including mechanical or artificial removal (Wilson et al. Citation2007). Biological control of A. philoxeroides has been attempted through the introduction of natural enemies including Arcola malloi and Amynothrips andersoni (Vogt et al. Citation1992), which have had little control effect on A. philoxeroides, especially in terrestrial conditions, where the stems of A. philoxeroides lignify (Vogt et al. Citation1992; Spencer & Coulson Citation1976; Julien & Chan Citation1992; Ma & Wang Citation2004; Pan et al. Citation2007). Therefore, it is urgently necessary to find an effective environment-friendly way of controlling the invasion of A. philoxeroides.
Invasive species have been repeatedly found to be more competitive than native species (Delph Citation1986; Wardle et al. Citation1994; Weihe & Neely Citation1997; Chittka & Schurkens Citation2001; Brown et al. Citation2002; Kandori et al. Citation2009). Therefore, it is impossible to control invasive species using native plant species. In the present study, we found that the invasive abilities of A. philoxeroides and H. scandens differed and intense competition existed between the two plant species when they cohabitated the same field conditions. H. scandens was more competitive, and could inhibit the invasion of A. philoxeroides through interspecific competition. Although H. scandens is also an invasive species, as an annual herb, it can be easily eliminated by harvesting before the seeds mature in autumn. Therefore, sowing H. scandens seeds in habitats invaded by A. philoxeroides would be an innovative and efficient biological control method.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (31060069/C030401) and Dr. Startup Fund of Jinggangshan University (JZ10041) awards. The authors acknowledge members of the key laboratory of the School of Life Science, Jinggangshan University, for things they shared with us. We would also like to thank Christine Verhille at the University of British Columbia for her assistance with English language and grammatical editing of the manuscript.
References
- Agami M, Waisel Y. 1985. Inter-relationships between Najas marina and three other species of aquatic macrophytes. Hydrobiologia. 126:169–173. 10.1007/BF00008684
- Bohn T, Amundsen PA, Sparrow A. 2007. Competitive exclusion after invasion? Biol Invasions. 10:359–368. 10.1007/s10530-007-9135-8
- Brown BJ, Mitchell RJ, Graham SA. 2002. Competition for pollination between an invasive species (purple loosestrife) and a native congener. Ecology. 83:2328–2336. 10.1890/0012-9658(2002)083[2328:CFPBAI]2.0.CO;2
- Braks MAH, Honório NA, Lounibos LP, Lourenço-De-Oliveira R, Juliano SA. 2004. Interspecific competition between two invasive species of container mosquitoes, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Brazil. Ann Entomol Soc Am. 97:130–139. 10.1603/0013-8746(2004)097[0130:ICBTIS]2.0.CO;2
- Callaway RM, Aschehoug ET. 2000. Invasive plants versus their new and old neighbors: a mechanism for exotic invasion. Science. 290:521–523. 10.1126/science.290.5491.521
- Chittka L, Schurkens S. 2001. Successful invasion of a floral market-an exotic Asian plant has moved in on Europe's river-banks by bribing pollinators. Nature. 411:653. 10.1038/35079676
- Cheng XY, Xie PZ, Cheng FX, Xu RM, Xie BY. 2009. Competitive displacement of the native species Bursaphelenchus mucronatus by an alien species Bursaphelenchus xylophilus (Nematoda: Aphelenchida: Aphelenchoididae): a case of successful invasion. Biol Invasions. 11:205–213. 10.1007/s10530-008-9225-2
- De Wit CT. 1960. On competition. Versl. Landbouwk. Onderz. 66:1–82.
- Delph LF. 1986. Factors regulating fruit and seed production in the desert annual Lesquerella gordonii. Oecologia. 69:471–476. 10.1007/BF00377071
- Devin S, Beisel JN. 2007. Biological and ecological characteristics of invasive species: a gammarid study. Biol Invasions. 9:13–24. 10.1007/s10530-006-9001-0
- Edgerly JS, Willey MS, Livdahl TP. 1993. The community ecology of Aedes egg hatching: implications for a mosquito invasion. Ecol Entomol. 18:123–128. 10.1111/j.1365-2311.1993.tb01193.x
- Fan XH, Li WM. 2001. Research on quarantine strategy for biosafety protection in China. Biodiversity Sci. 9(4):439–445 (in Chinese).
- Gause GF. 1932. Experimental studies on the struggle for existence. J Exp Biol. 9:389–402.
- Gurevitch J, Padilla DK. 2004. Are invasive species a major cause of extinctions? Trends in Ecol Evol. 19:470–474. 10.1016/j.tree.2004.07.005
- Holway DA. 1999. Competitive mechanisms underlying the displacement of native ants by the invasive Argentine ant. Ecology. 80:238–251. 10.1890/0012-9658(1999)080[0238:CMUTDO]2.0.CO;2
- Holway DA, Suarez AV. 1999. Animal behavior: an essential component of invasion biology. Trends in Ecol Evol. 14:328–330. 10.1016/S0169-5347(99)01636-5
- Hutchinson GE. 1959. Homage to Santa Rosalia, or why are there so many kinds of animals? Am Nat. 93:145–159. 10.1086/282070
- Juliano SA. 1998. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition. Ecology. 79:255–268. 10.1890/0012-9658(1998)079[0255:SIARAM]2.0.CO;2
- Julien MH, Chan RR. 1992. Biological control of alligator weed: unsuccessful attempts to control terrestrial growth using the flea beetle Disonycha argentinensis (Chrysomelidae). Entomophaga. 37:215–221. 10.1007/BF02372420
- Kandori I, Hirao T, Matsunaga S, Kurosaki T. 2009. An invasive dandelion unilaterally reduces the reproduction of a native congener through competition for pollination. Oecologia. 159:559–569. 10.1007/s00442-008-1250-4
- Lin W, Zhou G, Cheng X, Xu R. 2007. Fast economic development accelerates biological invasions in China. PloS ONE. 2:1–6.
- Ma RY, Wang R. 2004. Effect of morphological and physiological variations in the ecotypes of alligator weed, Alternanthera philoxeroides on the population rate of its biocontrol agent Agasicles hygrophila. Acta Phytoecologica Sin. 28:24–30 (in Chinese).
- Mooney HA, Hobbs RJ. 2000. Invasive species in a changing world. Washington, DC: Island Press.
- Mooney HA, Cleland EE. 2001. The evolutionary impact of invasive species. Proc Natl Acad Sci. 98:5446–5451. 10.1073/pnas.091093398
- Müller-Schärer H, Schaffner U. 2008. Classical biological control: exploiting enemy escape to manage plant invasions. Biol Invasions. 10:859–874. 10.1007/s10530-008-9238-x
- Nevai AL, Vance RR. 2007. Plant interspecies competition for sunlight: a mathematical model of canopy partitioning. J Math Biol. 55:105–145. 10.1007/s00285-007-0073-y
- Pan XY, Geng YP, Sosa A, Zhang WJ, Li B, Chen JK. 2007. Invasive Alternanthera philoxeroides: biology, ecology and management. Acta Phytotaxonomica Sin. 45(6):884–900 (in Chinese). 10.1360/aps06134
- Pan XY, Liang HZ, Sosa A, Geng YP, Li B, Chen JK. 2006. Patterns morpological variation of alligator weed (Alternanthera philoxeroides): from native to introduced regions. Biodiversity Sci. 14:232–240 (in Chinese). 10.1360/biodiv.050223
- Petren K, Case TJ. 1996. An experimental demonstration of exploitation competition in an ongoing invasion. Ecology. 77:118–132. 10.2307/2265661
- Petren K, Bolger DT, Case TJ. 1993. Mechanisms in the competitive success of an invading sexual gecko over an asexual native. Science. 259:354–358. 10.1126/science.259.5093.354
- Schluter D. 2000. Ecological character displacement in adaptive radiation. Am Nat. 156:S4–S16. 10.1086/303412
- Spencer NR, Coulson JR. 1976. The biological control of alligator weed, Alternanthera philoxeroides, in the United States of America. Aquat Bot. 2:177–190. 10.1016/0304-3770(76)90019-X
- Taylor DR, Aarssen. 1989. On the density dependence of replacement-series competition experiments. J Ecol. 77:975–988. 10.2307/2260817
- Vitousek PM, D'Antonio CM, Loope LL, Rejmánek M, Westbrooks R. 1997. Introduced species: a significant component of human-caused global change. New Zeal J Ecol. 21:1–16.
- Vogt GB, Quimby JPC, Kay SH. 1992. Effects of weather on the biological control of alligator weed in the lower Mississippi Valley region. Tech Bulletin. 1766:1973–1983.
- Wauters LA, Tosi G, Gurnell J. 2002. Interspecific competition in tree squirrels: do introduced grey squirrels (Sciurus carolinensis) deplete tree seeds hoarded by red squirrels (S. vulgaris)? Behav Ecol Sociobiology. 51:360–367. 10.1007/s00265-001-0446-y
- Wardle DA, Nicholson KS, Ahmed M, Rahman A. 1994. Interference effects of the invasive plant Carduus nutans L. against the nitrogen fixation ability of Trifolium repens L. Plant Soil. 163:287–297.
- Weigelt A, Jolliffe P. 2003. Indices of plant competition. J Ecol. 91:707–720.
- Weihe PE, Neely RK. 1997. The effects of shading on competition between purple loosestrife and broad-leaved cattail. Aquat Bot. 59:127–138.
- Wilson KA, Magnuson JJ, Lodge DM, Hill AM, Kratz TK, Perry WL, Willis TV. 2004. A long-term rusty crayfish (Orconectes rusticus) invasion: dispersal patterns and community change in a north temperate lake. Can J Fish Aquat Sci. 61:2255–2266.
- Wilson JRU, Yeates A, Schooler S, Julien MH. 2007. Rapid response to shoot removal by the invasive wetland plant, alligator weed (Alternanthera philoxeroides). Environ Exp Bot. 60:20–25.
- Xie Y, Li ZY, William PG, Li D. 2001. Invasive species in China: an overview. Biodivers Conserv. 10:1317–1341 (in Chinese).
- Zang LS, Liu SS, Liu YQ, Ruan YM, Wan FH. 2005. Competition between the B biotype and a non-B biotype of the whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) in Zhejiang, China. Biodiversity Sci. 13:181–187.
