Abstract
Quercus liaotungensis natural secondary forest is an important vegetation formation and has a large distribution area in Lingkong Mountain Nature Reserve, Shanxi Province, China. The spatial patterns of trees at different life stages give important clues about the underlying processes driving regeneration and succession of the forest. In this paper, the trees of a population were mapped, characterized and the spatial distribution patterns and spatial associations of Q. liaotungensis among different life stages (juveniles – J, premature – P, mature – M, overmature – O) were analyzed using O-ring univariate O(r) and bivariate O(subscript 12)(r) statistics. We found that: (1) Q. liaotungensis was a discontinuously regenerating population. (2) The distribution patterns of Q. liaotungensis varied at different life stages. Q. liaotungensis (J) and Q. liaotungensis (M) showed significant aggregations at scale 0–19 m and 0–23 m, respectively. Q. liaotungensis (P) exhibited significant aggregations at the majority of scales, whereas Q. liaotungensis (O) showed a random distribution pattern at most scales. (3) Intraspecific spatial association varied with tree size and scales. Negative or independent association was a dominant pattern for Q. liaotungensis at different life stages, whereas positive associations were found at small scales for only three pairs: Q. liaotungensis (J)–Q. liaotungensis (P), Q. liaotungensis (J)–Q. liaotungensis (M), and Q. liaotungensis (P)–Q. liaotungensis (M).
Introduction
Forest stand structure is a key element in understanding forest ecosystems (Erfanifard et al. Citation2008). One of the major components of forest stand structure is the spatial pattern. It has attracted particular attention, in large part because of its significance in helping the understanding of ecological mechanisms (Duncan Citation1991; He & Duncan Citation2000; Wiegand et al. Citation2000) and dynamic processes that maintain species coexistence (Grubb Citation1977; Manabe et al. Citation2000; Nishimura et al. Citation2002). Many of these processes will hide or confound the spatial signals of many of the others, and that it is hard to identify them even using spatial statistics. Spatial patterns of plants can arise from environmental heterogeneity (Duncan Citation1993; Szwagrzyk & Czerwczak Citation1993), natural and human disturbances (Wolf Citation2005; Sánchez et al. Citation2009), intra- and interspecific competition (Stoll & Bergius Citation2005; Getzin et al. Citation2006), regeneration (Li & Zhang Citation2003; Fajardo et al. Citation2006), and mortality (Kenkel Citation1988; Barot et al. Citation1999). Diameter at breast height (DBH) and age of the individuals of the same species are significantly correlated in the same environment (Frost & Rydin Citation2000; Wang et al. Citation2003). Therefore, age classes are substituted by size classes to analyze the population structures and dynamics. Over the last decade, there has been increasing interest in the study of spatial patterns and associations at different life stages or age classes (Wang et al. Citation2003; King et al. Citation2006; Comita et al. Citation2007; Hao et al. Citation2007; Li et al. Citation2008). Ecologists study spatial patterns of species at different life stages to infer the existence of underlying processes that have generated these patterns and identify the scale at which those processes are operating. Moreover, there are many more reasons for regular tree location patterns. For example, spatial distributions of adult trees would become more regular than those of juveniles because of the different attack rates between adults and juveniles by distance/frequency-responsive pathogens or herbivores (Janzen Citation1970; Connell Citation1971). Analyzing spatial distributions of individuals within life stages and spatial associations between different life stages is essential for understanding the spatial and temporal dynamics of populations (Li et al. Citation2008). Additionally, the present spatial pattern of a particular species, especially the adult–juvenile relationship, provides useful information of regeneration process of the species (Hubbell Citation1979; Sterner et al. Citation1986; Kohyama et al. Citation1994). Thus, it is essential to analyze the stand structure and spatial associations among these age classes in forests.
Quercus liaotungensis is the dominant and widely distributed species of the climatic of the climax community in the warm temperate zone of China (Gao et al. Citation2001; Hou et al. Citation2004) because it can bear both cold-moisture climate and warm-dry climate (Wu et al. Citation2002). In Mt. Taiyue, a native woody species, Q. liaotungensis, which is the primary zonal typical vegetation, forms the community found widely in warm temperate deciduous broad leaf forests in China (Li et al. Citation2007). Human disturbance is more prevalent due to the wood of deciduous broad-leaves which has many uses in the fields of architectural, firewood, timber, edible fungi material (Gao et al. Citation2001). And these forests are now seriously deteriorated in the low hills (Chen Citation1995). Hence, the conservation, management, and restoration of Q. liaotungensis forests are urgently needed (Hou et al. Citation2004). Structural heterogeneity is an important ecosystem attribute (Luo et al. Citation2010). Ecological heterogeneity results from a variety of hierarchically structured processes from biotic interactions at fine scales to topographical shape (Holland et al. Citation2004). In order to understand the functional traits of Q. liaotungensis, it is important to examine their structural features. Many research projects have mainly focused on community characteristics (Wang et al. Citation1999; Gao et al. Citation2001), regeneration (Zhang et al. Citation2008; Mi & Hou Citation2009; Guo et al. Citation2011), genetic diversity (Li Citation2003), physiology, and ecology (Lin et al. Citation2002; Li et al. Citation2007). Spatial pattern analyses of the trees also have been performed by some researches (Wu et al. Citation2002; Hou et al. Citation2004; Yi et al. Citation2008). These studies drew different conclusions because they were done at different scales, and in different forest types, using different analyses. There are few studies linking the spatial patterns within processes in these forests, especially of the spatial patterns and spatial associations within life stages in forests. Hence, it is critical to analyze the spatial patterns for its important function in depicting the ecological processes of Q. liaotungensis populations.
To address these concerns, we studied the spatial patterns of Q. liaotungensis at different life stages (DBH classes), with a focus on intraspecific spatial associations among the life stages in 2.8 ha natural forest plot in Lingkong Mountain, northern China. The O-ring statistic was used for point pattern analysis at different scales to infer how the dominant trees compete and partition space in a forest, and how their spatial interactions change during succession. Our objectives were: (1) to detect the dynamic aspects of spatial pattern at different life stages (juveniles, premature, mature, and overmature); (2) to reveal the spatial association within different life stages among juveniles, premature, mature, and overmature; and (3) to discuss the possible mechanisms that contribute to the spatial patterns and associations of Q. liaotungensis in the plot. The study could contribute to the understanding of maintaining the long-term stability of this forest.
Materials and methods
Study site
The experiment was carried out at Lingkong Mountain Nature Reserve of Shanxi province (36°31′~36°43′N, 112°01′~112°15′E). It belongs to Taiyue Forest Bureau's Shanxi, China, with a warm temperate terrestrial monsoon climate. The mean annual temperature averages 6.2°C. In the warmest month, the average temperature is 21.5°C and monthly average temperature is −5°C in the coldest month. The annual sunshine time is about 2600 h. Precipitation amounts to 662 mm yr−1 and most of annual rainfall occurs in July, August, and September. Nonfrost period is shorter than 125 days. The stand soil is classified as mountain brown soils and the topograghy is declining from west to east with an average elevation of 1500 m. In this area, primitive Q. liaotungensis forests, however, no longer exist because of extensive harvesting and burning. The study site is a mature, secondary forest dominated by the Q. liaotungensis that have been protected since the 1990s (Yu et al. Citation2013). Its regeneration mainly relies on stem base-sprouting. The phenomenon of sprouting from stumps is very common in these forests (Yi et al. Citation2008; Yu et al. Citation2013). In addition to sprouting from stumps and bases of dead and aged individuals, new stems of seedlings and saplings also usually sprout basally.
Data collection
2.8 ha plot (200 m×140 m) were established in the study site. The plot was further subdivided into 70 contiguous 20 m×20 m quadrats. Samples were taken with the contiguous grid quadrats method, and each grid was 5 m×5 m. Within each grid, all trees at least 1 cm in DBH were mapped and identified to species, mapped to the nearest 0.1 m and tagged. Furthermore, seedlings of Q. liaotungensis were counted. The DBH and height of all adult trees were measured. Data were collected during July and August in 2010.
Data analysis
Four life stages were distinguished for Q. liaotungensis according to DBH: juveniles (J) (5 cm > DBH ≥1 cm), premature (P) (10 cm >DBH ≥5 cm), mature (M) (30 cm >DBH ≥10 cm), over mature (O) (DBH ≥ 30 cm).
Numerous statistical methods have been widely used to measure spatial patterns and associations of species (Ripley Citation1981; Kenkel Citation1988; Duncan Citation1991; He & Duncan Citation2000; Wiegand et al. Citation2000; Illian et al. Citation2008). In this paper, a technique known as the O-ring statistics were presented, which can be used to describe the local neighborhood density of the points of the pattern (Condit et al. Citation2000; Wiegand & Moloney Citation2004). The O-ring statistics include univariate and bivariate analyses (Zhang et al. Citation2010). The univariate statistic is used to analyze the spatial relationships between individuals of a particular group, while bivariate statistic is used to analyze spatial relationships between individuals of two different groups (Wiegand & Moloney Citation2004). Here, the univariate form of O-ring was used to test for the spatial structure within each developmental stage cohort (juveniles, premature, mature, and overmature). The bivariate analyses were performed to see whether there was a spatial relationship among juveniles, premature, mature, and overmature.
The O-ring statistics for uni- and bivariate point patterns were computed using Programita software (Wiegand & Moloney Citation2004). Significant distance from an underlying null model was tested by 99 Monte Carlo simulations which generates n/(n+1)×100%, hence 99% confidence limits (Bailey & Gatrell Citation1995).
Results
Stand structure and composition
The total number of individuals in the plot was 4554, consisting of 14 species, 11 genus, and 11 families. Mean stand density was 1626.4 trees ha−1. Q. Liaotungensis population is in possession of dominance of spatial occupation capacity in the plot, since 3602 individuals or 79.1% were Q. liaotungensis (3547 live and 55 dead standing) (). Armeniaca sibirica was the main associated tree species of Q. liaotungensis. The other 12 tree species together made up 15.0% of the total number. None of them was represented by more than 5%. Q. liaotungensis was the most abundant canopy species and showed the largest mean DBH (13.8 cm) and mean height (7.8 m). The largest tree diameters measured in the plot were for Q. liaotungensis (51.6 cm).
Table 1. Stand structure and composition of the forest plot.
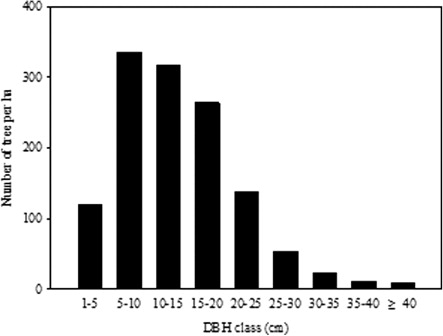
Q. liaotungensis showed a unimodal DBH distribution. The frequency increased to a peak in DBH 5–10 cm, and then gradually decreased among the larger diameters ().
Spatial patterns of Q. liaotungensis at different size classes
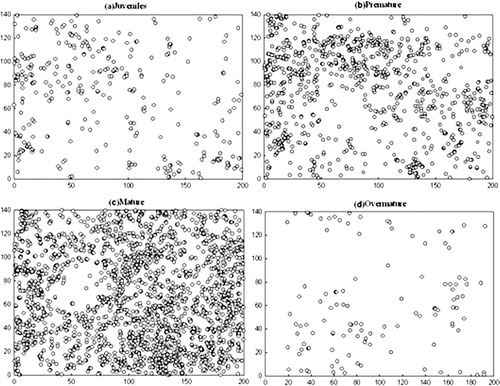
Maps of tree locations of the Q. liaotungensis across four life stages were plotted (). The maps clearly showed that Q. liaotungensis is not uniform across the plot.
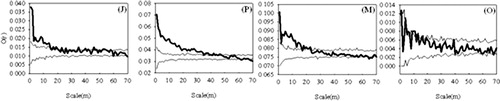
When the spatial patterns of Q. liaotungensis was analyzed at four life stages (). Q. liaotungensis (J), Q. liaotungensis (P), and Q. liaotungensis (M) showed significant aggregations at scale 0–19 m, 0–47 m, and 0–23 m, respectively, and tended to have random distributions with a fluctuation around the upper envelope line at some scales, whereas Q. liaotungensis (O) was randomly distributed at scale <70 m, note that trees beyond the interaction/correlation range simply have no statistical effect on each other.
Spatial association among life stages
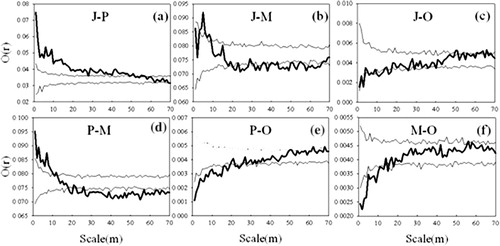
Q. liaotungensis (J) tended to have spatially positive correlation to Q. liaotungensis (P) at scales 1–57 m (). Q. liaotungensis (J) showed different patterns to Q. liaotungensis (M) at different scales: positive correlation at scales 3–8 m, spatial independence at scales 1–3 m and 8–20 m, and mainly negatively correlation at larger scales (). Q. liaotungensis (J) was dominated by spatial independence to Q. liaotungensis (O) at almost all scales (). Particularly, Q. liaotungensis (P) also showed different patterns to Q. liaotungensis (M) at different scales: positive correlation at scales 1–12 m, spatial independence at scales at 12–22 m, and mainly positive correlation at larger scales (). Q. liaotungensis (P) and Q. liaotungensis (M) were significantly and negatively correlated to Q. liaotungensis (O) at scales 1–26 m and 1–18 m, respectively, and tended to have independent associations with a fluctuation around the upper envelope line at some scales ().
Discussion
Stand structure
The size structure suggests that regeneration processes dominate the current stand development phase. Our results showed that Q. liaotungensis has temporally discontinuous regeneration, with few small juvenile individuals. The lower number of juveniles might be offset by the longer lifespan of adults, showing the lack of regeneration. One important reason for the lack of regeneration was that Q. liaotungensis was shade-intolerant species (Hou et al. Citation2004), as light is important for the establishment and survival of young individuals of Q. liaotungensis (Li & Ma Citation2003). The other reason for the present regeneration status was that although Q. liaotungensis had produced enough seeds, it had poor sapling establishment. Some studies suggested that animal predation and dispersal of acorns had been considered important factors influencing the natural regeneration of oakwoods (Kollmann & Schill Citation1996; Hammond et al. Citation1999). According to our survey, seedling regeneration in mature Q. liaotungensis forest in Lingkong Mountain is low and natural regeneration of Q. liaotungensis populations appear to rely on sprouting from stumps, bases of dead and aged individuals. The cohort of seedlings, though limited in size, has ecological significance through its role in maintaining durability and genetic diversity of oak population, while sprout has a very important significance of maintenance and stability of the oak population (Gao et al. Citation2001). Thus, the coexistence of seedlings and sprouts was the results of the adaptation to environmental stress and the co-evolution with the environment, which implied that Q. liaotungensis population had higher stability under natural conditions. Consistent with other size structure of the oak population studies (Wang et al. Citation1999; Gao et al. Citation2001), we found a clear bottleneck at the juveniles during forest regeneration. The unimodal population size structure of Q. liaotungensis in Lingkong Mountain indicated that the present environmental conditions of the stand were not favorable for the establishment and recruitment of Q. liaotungensis from seeds. Selected-cutting or canopy disturbance to permit light access to the understory can help to improve natural regeneration (Wang et al. Citation2000; Zhang Citation2001; Li & Ma Citation2003; Zhang et al. Citation2008; Duan et al. Citation2009). Therefore, suitable artificial measures should be taken to promote plant growth and enhance ecosystem stability.
Spatial patterns
Aggregated distribution in species is a widespread pattern in nature (He et al. Citation1997; Plotkin et al. Citation2002; Li et al. Citation2009). Wang et al. (Citation2010) suggested that species aggregation generally decreased with increasing size class. Unlike some previous studies (He et al. Citation1997; Condit et al. Citation2000; Seiwa et al. Citation2008), we found that mature trees to be more aggregated at small scale than juveniles and premature, perhaps because adult recruitment rates were low and dispersal was poor even in the absence of any environmental heterogeneity (Murrell Citation2009). In addition, pathogens or herbivores may also play an important role as spacing mechanisms in reducing aggregation in temperate forests (e.g. Seiwa et al. Citation2008). In contrast, we found that overmature trees were randomly distributed at almost all scales and this may be largely due to stochastic mortality or strong intra- and/or interspecific competition for resources (light, water, nutrients, etc.).
Spatial distribution of trees can exhibit different patterns at different spatial scales (Chen & Bradshaw Citation1999). Of the numerous mechanisms that contribute to spatial distribution, the major mechanisms include dispersal limitation (Thioulouse et al. Citation1997; Hubbell Citation2001), habitat heterogeneity (Harms et al. Citation2001; Queenborough et al. Citation2007), reproductive or foraging behavior (Janzen Citation1970; Connell Citation1971). Generally, the functional traits of its own population have been mainly affected spatial distribution pattern at a small scale, whereas habitat heterogeneity is considered to be the most likely explanation at a larger scale (Yuan et al. Citation2011). Poisson process complete spatial randomness (CSR) at scales r >10 m beyond direct tree–tree interactions usually interpreted as environmental heterogeneity (Getzin et al. Citation2008). In our study, juvenile, premature, and mature trees were aggregated within 10 m resulted from sprouting or limited seed dispersal, whereas juvenile, premature, and mature trees were aggregated more than 10 m resulted from heterogeneity.
Intraspecific associations
The strongly positive association of Q. liaotungensis at juvenile and premature stages may result from a facilitative relationship of larger ‘nurse plants’ providing shelter to small trees, whereas the spatial independence of Q. liaotungensis (J) to Q. liaotungensis (O) at almost all scales may be the result of strong intra- and/or interspecific competition for resources (light, water, nutrients, etc.). Negative and independent intraspecific associations were dominant in later stages. In particular, overmature had no positive associations with other life stages, indicating the suppression of early stages by later stages. Q. liaotungensis (J) facilitated the growth of Q. liaotungensis (P) at large scales, whereas Q. liaotungensis (J) was suppressed by Q. liaotungensis (M) at small scales (), and suppressed by Q. liaotungensis (O) at lager scales (). Q. liaotungensis (P) facilitated the growth of Q. liaotungensis (M) at small scales (), whereas Q. liaotungensis (P) and Q. liaotungensis (M) were inhibited by Q. liaotungensis (O) at all of the scales studied ().
The present study provides further evidence of Q. liaotungensis population dynamics and intraspecific competition at different age classes in a temperate forest. The spatial associations of Q. liaotungensis population in Lingkong Mountain not only varied with size class, but also with spatial scales. The species functional traits (regeneration, seed dispersal ability, shade-intolerance, etc.), pathogens or herbivores and environmental heterogeneity have been considered to be a primary factor controlling the distribution of species.
Acknowledgments
This project is supported by the National Bureau of Forestry 948 project (No. 2010-4-15). The authors thank all those who provided helpful suggestions and critical comments on this manuscript and anonymous reviewers. The authors also thank Dr Osbert Jianxin Sun (MOE Key laboratory for Silviculture and Conservation and Institute of Forestry and Climate Change Research, Beijing Forestry University, Beijing, China) for providing helpful information for this paper. We also thank Dr T. Wiegand for use of the Programita software, and Jinsong Wang, Qi Zhao, and Yingying Qin for their fieldwork and data collection.
References
- Bailey TC, Gatrell AC. 1995. Interactive spatial data analysis. Harlow: Longman Scientific and Technical.
- Barot S, Gignoux J, Menaut JC. 1999. Demography of a Savanna Palm tree: predictions from comprehensive spatial pattern analyses. Ecology. 80:1987–2005. 10.1890/0012-9658(1999)080[1987:DOASPT]2.0.CO;2
- Chen LZ. 1995. Deciduous broad-leaved forests in north central China. Dordrecht: Kluwer Academic Publishers.
- Chen J, Bradshaw GA. 1999. Forest structure in space: a case study of an old growth spruce-fir forest in Changbaishan Natural Reserve, PR China. For Ecol Manage. 120:219–233. 10.1016/S0378-1127(98)00543-X
- Comita LS, Condit R, Hubbell SP. 2007. Developmental changes in habitat associations of tropical trees. J Ecol. 95:482–492. 10.1111/j.1365-2745.2007.01229.x
- Condit R, Ashton PS, Baker P, Bunyavejchewin S, Gunatilleke S, Gunatilleke N, Hubbell SP, Foster RB, Itoh A, Lafrankie JV, et al. 2000. Spatial patterns in the distribution of tropical tree species. Science. 288:1414–1418. 10.1126/science.288.5470.1414
- Connell JH. 1971. On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. In: Boer, P. J. vander and Gradwell GR, editors, Dynamics of Numbers in Populations. Wageningen: Center for Agricultural Publication and Documentation; p. 298–312.
- Duan RY, Wang C, Wang XA, Zhu ZH, Guo H. 2009. Differences in plant species diversity between conifer (Pinus tabulaeformis) plantations and natural forests in middle of the Loess Plateau. Russ J Ecol. 40:501–509. 10.1134/S106741360907008X
- Duncan RP. 1991. Competition and the coexistence of species in a mixed podocarp stand. J Ecol. 79:1073–1084. 10.2307/2261099
- Duncan RP. 1993. Testing for life historical changes in spatial patterns of four tropical tree species in Westland, New Zealand. J Ecol. 81:403–416. 10.2307/2261519
- Erfanifard Y, Feghhi J, Zobeiri M, Namiranian N. 2008. Comparison of two distance methods for forest spatial pattern analysis. J Appl Sci. 8:152–157. 10.3923/jas.2008.152.157
- Fajardo A, Goodburn JM, Graham J. 2006. Spatial patterns of regeneration in managed uneven-aged ponderosa pine/douglas-fir forests of Western Montana, USA. For Ecol Manage. 223:255–266. 10.1016/j.foreco.2005.11.022
- Frost I, Rydin H. 2000. Spatial pattern and size distribution of the animal-dispersed Quercus rubur in two spruce-dominanted forests. Ecoscience. 7:38–44.
- Gao XM, Wang W, Du XJ, Ma KP. 2001. Size structure ecological significance and population origin of Quercus Wutaishanica forest in Beijing mountainous area. Acta Phyto Sin. 25:673–678.
- Getzin S, Dean C, He FL, Trofymow JA, Wiegand K, Wiegand T. 2006. Spatial patterns and competition of tree species in a Douglas-fir chronosequence on Vancouver Island. Ecography. 29:671–682. 10.1111/j.2006.0906-7590.04675.x
- Getzin S, Wiegand T, Wiegand K, He FL. 2008. Heterogeneity influences spatial patterns and demographics in forest stands. J Ecol. 96:807–820. 10.1111/j.1365-2745.2008.01377.x
- Grubb PJ. 1977. The maintenance of species richness in plant communities: the importance of the regeneration niche. Biol Rev Camb Philos Soc. 52:107–145. 10.1111/j.1469-185X.1977.tb01347.x
- Guo H, Wang XA, Zhu ZH, Wang XS, Guo JC. 2011. Seed and microsite limitation for seedling recruitment of Quercus wutaishanica on Mt. Ziwuling, Loess Plateau, China. New Forests. 41:127–137. 10.1007/s11056-010-9215-y
- Hammond DS, Brown VK, Zagt R. 1999. Spatial and temporal patterns of seed attack and germination in a large-seeded neotropical tree species. Oecologia. 119:208–218. 10.1007/s004420050778
- Hao ZQ, Zhang J, Song B, Ye J, Li BH. 2007. Vertical structure and spatial associations of dominant tree species in an old-growth temperate forest. For Ecol Manage. 252:1–11. 10.1016/j.foreco.2007.06.026
- Harms KE, Condit R, Hubbell SP, Foster RB. 2001. Habitat associations of trees and shrubs in a 50-ha neotropical forest plot. J Ecol. 89:947–959. 10.1111/j.1365-2745.2001.00615.x
- He F, Duncan RP. 2000. Density-dependent effects on tree survival in an old-growth Douglas fir forest. J Ecol. 88:676–688. 10.1046/j.1365-2745.2000.00482.x
- He FL, Legendre P, Lafrankie JV. 1997. Distribution patterns of tree species in a Malaysian tropical rain forest. J Veg Sci. 8:105–114. 10.2307/3237248
- Holland JD, Bert DG, Fahrig L. 2004. Determining the spatial scale of species' response to habitat. Bioscience. 54:227–233. 10.1641/0006-3568(2004)054[0227:DTSSOS]2.0.CO;2
- Hou JH, Mi XC, Liu CR, Ma KP. 2004. Spatial patterns and associations in a Quercus-Betula forest in northern China. J Veg Sci. 15:407–414.
- Hubbell SP. 1979. Tree dispersion, abundance, and diversity in a tropical dry forest. Science. 203:1299–1309. 10.1126/science.203.4387.1299
- Hubbell SP. 2001. The Unified neutral theory of biodiversity and biogeography. Princeton, NJ: Princeton University Press.
- Illian J, Pettinen A, Stoyan H, Stoyan D. 2008. Statistical analysis and modeling of spatial point patterns. Chichester: Wiley.
- Janzen DH. 1970. Herbivores and the number of tree species in tropical forests. Am Nat. 104:501–528. 10.1086/282687
- Kenkel NC. 1988. Pattern of self-thinning in Jack Pine: testing the random mortality hypothesis. Ecology. 69:1017–1024. 10.2307/1941257
- King DA, Wright SJ, Connell JH. 2006. The contribution of interspecific variation in maximum tree height to tropical and temperate diversity. J Trop Ecol. 22:11–24. 10.1017/S0266467405002774
- Kohyama T, Suzuki E, Hotta M. 1994. Spatial distribution pattern of representative tree species in a foothill rain forest in West Sumatra. Tropics. 4:1–15. 10.3759/tropics.4.1
- Kollmann J, Schill HP. 1996. Spatial patterns of dispersal, seed predation and germination during colonization of abandoned grassland by Quercus petrea and Corylus avellana. Vegetation. 125:193–205. 10.1007/BF00044651
- Li HJ, Zhang ZB. 2003. Effects of rodents on acorn dispersal and survival of the Liaodong oak (Quercus liaotungensis Koidz). For Ecol Manage. 176:387–396. 10.1016/S0378-1127(02)00286-4
- Li L, Huang ZL, Ye WH, Cao HL, Wei SG, Wang ZG, Lian JY, Sun IF, Ma KP, He FL. 2009. Spatial distributions of tree species in a subtropical forest of China. Oikos. 118:495–502. 10.1111/j.1600-0706.2009.16753.x
- Li L, Wei SG, Huang ZL, Ye WH, Cao HL. 2008. Spatial patterns and interspecific associations of three canopy species at different life stages in a subtropical forest, China. J Integr Plant Biol. 50 (9):1140–1150. 10.1111/j.1744-7909.2008.00690.x
- Li M, Han HR, Kang FF, Ma QY. 2007. Morphological variation of Quercus liaotungensis leaves in Lingkong Mountain, Shanxi province. Front Forest China. 2:185–191. 10.1007/s11461-007-0030-3
- Li QK, Ma KP. 2003. Factors affecting establishment of Quercus liaotungensis Koidz under mature mixed oak forest overstory and in shrubland. For Ecol Manage. 176:133–146. 10.1016/S0378-1127(02)00274-8
- Li WY. 2003. Study on genetic diversity of natural populations in Quercus mongolica [dissertation for the Doctoral Degree]. Beijing: Beijing Forestry University.
- Lin C, Ma QY, Han HR, Cao WQ, Wang ZZ, Wang ZQ, Zhang BX. 2002. Photosynthesis characteristic of Quercus liaotungensis in Taiyue Mountain Region. Acta Ecol Sin. 22:1399–1406.
- Luo ZK, Sun JX, Xu HL. 2010. A comparison of species composition and stand structure between planted and natural mangrove forests in Shenzhen Bay, South China. J Plant Ecol. 3:165–174. 10.1093/jpe/rtq004
- Manabe T, Nishimura N, Miura M, Yamamoto S. 2000. Population structure and spatial patterns for trees in a temperate old-growth evergreen broad-leaved forest in Japan. Plant Ecol. 151:181–197. 10.1023/A:1026512404110
- Mi XC, Hou JH. 2009. Regeneration pattern analysis of Quercus liaotungensis in a temperate forest using two-dimensional wavelet analysis. Front Biol. 4:491–502. 10.1007/s11515-009-0040-7
- Murrell DJ. 2009. On the emergent spatial structure of size-structured populations: when does self-thinning lead to a reduction in clustering? J Ecol. 97:256–266. 10.1111/j.1365-2745.2008.01475.x
- Nishimura N, Hara T, Miura M, Manabe T, Yamamoto S. 2002. Tree competition and species coexistence in a warm-temperate old-growth evergreen broad-leaved forest in Japan. Plant Ecol. 164:235–248. 10.1023/A:1021224429091
- Plotkin JB, Chave J, Ashton PS. 2002. Cluster analysis of spatial patterns in Malaysian tree species. Am Nat. 160:629–644. 10.1086/342823
- Queenborough SA, Burslem D, Garwood NC, Valencia R. 2007. Habitat niche partitioning by 16 species of Myristicaceae in Amazonian Ecuador. Plant Ecol. 192:193–207. 10.1007/s11258-007-9328-3
- Ripley BD. 1981. Spatial statistics. New York, NY: Wiley.
- Sánchez M, Moore MM, Bakker JD, Parysow PF. 2009. 108 years of change in spatial pattern following selective harvest of a Pinus ponderosa stand in northern Arizona, USA. J Veg Sci. 20:1–12. 10.1111/j.1654-1103.2009.01031.x
- Seiwa K, Miwa Y, Sahashi N, Kanno H, Tomita M, Ueno N, Yamazaki M. 2008. Pathogen attack and spatial patterns of juvenile mortality and growth in a temperate tree, Prunus grayana. Can J For Res. 38:2445–2454. 10.1139/X08-084
- Sterner RW, Ribic CA, Schatz GE. 1986. Testing for life historical changes in spatial patterns of four tropical tree species. J Ecol. 74:621–633. 10.2307/2260386
- Stoll P, Bergius E. 2005. Pattern and process: competition causes regular spacing of individuals within plant populations. J Ecol. 93:395–403. 10.1111/j.0022-0477.2005.00989.x
- Szwagrzyk J, Czerwczak M. 1993. Spatial patterns of trees in natural forests of East-Central Europe. J Veg Sci. 4:469–476. 10.2307/3236074
- Thioulouse J, Chessel D, Dolédec S, Doledec S, Olivier J. 1997. ADE-4: a multivariate analysis and graphical display software. Stat Comput. 7:75–83. 10.1023/A:1018513530268
- Wang W, Liu CR, Ma KP, Yu SL. 1999. Population structure and dynamics of Quercus liaotungensis in two broad-leaved deciduous forests in Dongling Mountain, Northern China. Acta Bot Sin. 41:425–432.
- Wang W, Ma KP, Liu CR. 2000. Seed shadow of Quercus liaotungensis Koidz in a broad-leaved deciduous forest. Acta Bot Sinica. 42:195–202.
- Wang XG, Ye J, Li BH, Zhang J, Lin F, Hao ZQ. 2010. Spatial distributions of species in an old-growth temperate forest, northeastern China. Can J For Res. 40:1011–1019. 10.1139/X10-056
- Wang ZF, Peng SL, Liu SZ, Li Z. 2003. Spatial pattern of Cryptocarya chinensis life stages in lower subtropical forest, China. Bot Bull Acad Sin. 44:159–166.
- Wiegand K, Jeltsch F, Ward D. 2000. Do spatial effects play a role in the spatial distribution of desert-dwelling Acacia raddiana? J Veg Sci. 11:473–484. 10.2307/3246577
- Wiegand T, Moloney KA. 2004. Rings, circles and null-models for point pattern analysis in ecology. Oikos. 104:209–229. 10.1111/j.0030-1299.2004.12497.x
- Wolf A. 2005. Fifty year record of change in tree spatial patterns within a mixed deciduous forest. For Ecol Manage. 215:212–223. 10.1016/j.foreco.2005.05.021
- Wu XP, Zheng Y, Ma KP. 2002. Population distribution and dynamics of Quercus liaotungensis, Fraxinus rhynchophylla and Acer mono in Dongling Mountain, Beijing. Acta Bot Sin. 44:212–223.
- Yi LT, Han HR, Cheng XQ, Kang FF, Zhang ZJ. 2008. Spatial distribution patterns of Quercus liaotungensis population in Lingkong Mountains. Acta Ecol Sin. 28:3244–3261.
- Yu M, Zhou ZY, Kang FF, Ouyang S, Mi XC, Sun JX. 2013. Gradient analysis and environmental interpretation of understory herb-layer communities in Xiaoshegou of Lingkong Mountain, Shanxi, China. Chinese J Plant Ecol. 37:373–383.
- Yuan ZL, Wang T, Zhu XL, Sha YY, Ye YZ. 2011. Patterns of spatial distribution of Quercus variabilis in deciduous broadleaf forests in Baotianman nature reserve. Biodivers Sci. 19:224–231. 10.3724/SP.J.1003.2011.08014
- Zhang LZ, Wang XA, Guo H, Li F. 2008. Gap characteristics and its effects on community regeneration of Quercus liaotungensis forest on Loess Plateau. Chinese J Ecol. 27:1835–1840.
- Zhang ZB. 2001. Effect of burial and environmental factors on seedling recruitment of Quercus liaotungensis Koidz. Acta Ecol Sin. 21:375–384.
- Zhang ZH, Hu G, Zhu JD, Luo DH, Ni J. 2010. Spatial patterns and interspecific associations of dominant tree species in two old-growth karst forests, SW China. Ecol Res. 25:1151–1160. 10.1007/s11284-010-0740-0