Abstract
To verify the important role of nitrogen in detoxifying plants from heavy metals in Populus, the influence of nitrogen and cadmium on growth, chlorophyll (Chl) synthesis, and the expression of the Glutamine synthetase gene (GS2) were studied in poplar plants. Experiments were carried out in potted plants treated with (NH4)2CO3, Cd(NO3)2, CdCl2 and CdCl2 plus (NH4)2CO3. After treatment, plant height, biomass, chlorophyll content, the precursors content and GS2 were investigated. Results showed that the plants treated with cadmium showed toxicity symptoms, decrease in growth and Chl content. Cd inhibited Chl synthesis seriously by blocking the site located on the steps between UrogenIII and Coprogen III. However, the plants treated with cadmium and nitrogen grew well without any toxicity symptoms. Nitrogen supplement can alleviate Cd inhibition on chlorophyll synthesis by unblocking the pathway. The results indicated that nitrogen can effectively alleviate cadmium toxicity to poplar plants.
| Abbreviations | ||
| cadmium | = | Cd |
| chlorophyll | = | Chl |
| nitrogen | = | N |
| Glutamine synthetase II gene | = | GS2 |
| glutathione | = | GSH |
| Glutamine | = | Gln |
| Phytochelatins | = | PC |
| reactive oxygen species | = | ROS |
Introduction
The development of urbanization and industrialization has increased heavy metal contamination which is produced and released by human activities. This increase of heavy metals may create oxidative stress on the generation of plants in the surrounding environment, which will in turn result in the ecology of the area to undergo unbalanced deterioration. Cadmium (Cd) is one of the most toxic pollutants found in the air, water and soil, which is non-essential for plants. It can be transferred through the food chain (Wagner Citation1993), and its accumulation in cereals represents a risk for animal and human health (Chaney et al. Citation1999; McLaughlin et al. Citation1999). Cd interacts with photosynthetic, respiratory and nitrogen (N) metabolism in plants, resulting in poor growth and low biomass accumulation (Sanitá & Gabbrielli Citation1999; Pereira et al. Citation2002). Recently, Cd pollution has been more serious, which intensifies environmental deterioration. Many farmlands have been classified as the core polluted regions in the world.
Cd harms plants through oxidative stress. It is a non-redox metal, which does not participate in redox reactions, but leads to the formation of reactive oxygen species (ROS), such as superoxide anion radicals and hydrogen peroxide (Dietz et al. Citation1999; Sandalio et al. Citation2001; Romero-Puertas et al. Citation2006). Therefore, a mechanism to interrupt such an autocatalytic process is required.
Although plant growth, Chl content, stomatal opening, transpiration and photosynthesis have been reported to be inhibited by Cd in nutrient solutions (Jiang & Li Citation1992; Baryla et al. Citation2001; Drążiewicz & Baszyński Citation2005; Sun et al. Citation2005), there are others’ reports that Cd treatments have no effect on photosynthesis or growth (Greger & Lindberg Citation1986; Haag-Kerwer et al. Citation1999; Li et al. Citation2005; Zhou & Qiu Citation2005). Our early study (in press) showed that after adding nitrogen to plants, the damage caused by Cd stress could be efficiently alleviated to better growth. Li et al.'s study (Citation2007) indicates that adding the appropriate N (NO3− or NH4+) in nutrient solution could enhance the development of root systems and increase the accumulation of Cd in Sedum alfredii Hance. Sedum alfredii Hance is a plant tolerant to cadmium and zinc (Yang et al. Citation2004)
The important physiological role of N in detoxifying plants from heavy metals has not been studied. However, the nitric oxide acting as bioactive signaling molecule in plant responses to heavy metal stress has been reported in many studies (Hassan et al. Citation2005; Floryszak-Wieczorek et al. Citation2006; Grün et al. Citation2006; Arasimowicz & Floryszak-Wieczorek Citation2007). The exogenous nitric oxide reducing the destructive action of heavy metals, ethylene and herbicides on plants was documented (Kopyra & Gwózdz Citation2003). Garcia-Mata and Lamattina (Citation2001) reported that nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress.
If nitrogen plays an important role in detoxifying plants from Cd, using tall, woody plants in combination with nitrogen fertilization to remediate contaminated sites would be an aesthetically pleasing and beneficial approach that not only promotes positive landscape effects, but reduces erosion. This study is aimed at further verifying the important physiological role of N in detoxifying plants from Cd in Populus species.
Materials and methods
Plant materials and experimental design
Two dormant, one-year-old, unrooted, 10-centimeter-long hardwood sticks of poplar clone ‘107’ (Populus deltoides×P. nigra) were set in a polyethylene pot with 5 kg of dry soil. The dimensions of the pot were 30 cm×20 cm×50 cm. The 25 pots were divided into 5 groups; each group was placed in a plant growth chamber with a 16 h photoperiod (photosynthetic active radiation about 250 µmolm−2.s−1), a 25/20 °C temperature-period, 70% air humidity, and was watered daily. A polyethylene disc was placed under each pot to avoid run-off. When the plants reached 15 cm height, approximately after a month, the following treatments were carried out: (1) a control treatment consisting of tap water without Cd or ammonium carbonate ((NH4)2CO3) amendments (CK); (2) a 1.680 g Cd(NO3)2 treatment added to each pot every 5 days (T1); (3) a 1.303 g CdCl2 treatment added to each pot every 5 days (T2); (4) a 1.303 g CdCl2 and 0.682 g (NH4)2CO3 treatment added to each pot every 5 days (T3); (5) 0.682 g (NH4)2CO3 treatment added to each pot every 5 days (T4). The amount of Cd (Cd2 +) added to each pot each time was equal to all the Cd treatments (T1, T2 and T3); similarly the amount of N added per pot each time was equal among T1, T3 and T4. The treatments were made until chlorosis was evident in T2. The duration of the experiment was 53 d. Total Cd weighing 7.988 g and N 1.989 g were added to the soil during the whole treatment.
The physicochemical properties of soil used
The experimental soil is sampled from the farmland of Sichuan agricultural university. Soil samples were collected from the surface (0–15 cm depth) of cultivated soils, and were air-dried, sieved, and analyzed in the laboratory using standard techniques. Its texture was identified by following the procedure described by Boyd (Citation1995). The pH of the soil was obtained by a soil pH meter (USA). The organic carbon content was determined by the wet oxidation method as described by Nelson and Summers (Citation1982). The total nitrogen and hydrolyzable nitrogen content were determined according to the methods described by Grimshaw et al. (Citation1989). Total phosphorus and available phosphorus content were determined colourimetrically by the molybdo-phosphoric – blue method using ascorbic acid as a reducing agent (Murphy & Riley Citation1962). The total potassium was extracted with 1 M ammonium acetate (1 M NH4OAc) solution buffered at pH 7.0 and was detected as described by Anderson and Ingram (Citation1998). The physicochemical properties of soil are as followings: pH 5.8, organic matter 10.65 g·kg−1, total nitrogen 0.21 g·kg−1, total phosphorus 0.31 g·kg−1, total potassium 2.56 g·kg−1, hydrolyzable nitrogen 43.21 mg·kg−1, available phosphorus 17.58 mg·kg−1, available potassium, 46.17 mg·kg−1.
Cadmium and nitrogen assay
Functional leaves were sampled to wash 3 times with distilled water, oven-dried for 24 h at 80 °C, and ground to a fine powder. The concentration of Cd in leaves and soil was determined with ICP-OES (OPTIMA 2000A, USA) after wet-digesting in HNO3-H2SO4-HCLO4 (Wu & Ge Citation1999). Nitrate-N and ammonia-N soil concentrations were measured with UV-spectrophotometer method and the phenol hypochlorite colorimetric method (Berthelot reaction) respectively.
Growth and biomass assay
Plant height and basal diameter were measured at the beginning and end of the Cd addition treatments. After the final treatment, biomass (dry weight) was determined. Samples were oven-dried at 90 °C for 15 min, kept at 70 °C for 24 h to obtain a constant dry weight and weighted for biomass.
Chlorophyll assay
Chl was extracted in 80% acetone. Absorbance was measured at 663 and 645 nm by a spectrophotometer (UV-2450, Shimadiu Corporation, Japan). Extinction coefficients and equations reported by Lichtenthaler (Citation1987) were used to calculate the amounts of Chl a and b. Measurements were done in triplicate.
The metabolic intermediates of chlorophyll biosynthesis extraction and quantization δ-aminolevulinic acid (ALA) assay
ALA was extracted and quantified by methods of Wang et al. (Citation1997) with some modifications. Fresh leaves (0.4 g) were ground in 4 mL of trichloroacetic acid (4%). Samples were centrifuged at 12,000 g for 10 min, and 1 mL supernatants were sampled and added with 500 µL NaAc (1 mol/L) and 50 µL Acetyl acetone. Following incubation in boiling water for 10 min, the liquid was cooled to room temperature, and then, it was centrifuged at 12,000 g for 10 min. The supernatants were sampled and added with the same volume of Ehrlich-Hg, and finally placed in a dark room for 15 min. The concentration of ALA was calculated according to the absorption at 553 nm.
Porphobilinogen (PBG) assay
PBG was measured by the methods of Bogorad (Citation1962) with some modifications. Fresh leaves (0.2 g) were ground in liquid nitrogen and tissue was homogenized in 4 mL buffer (0.6 M Tris, 0.1 M EDTA, pH = 8.2). Samples were centrifuged at 12,000 g for 10 min. The supernatants were sampled and added with the same volumes of Ehrlich-Hg, and then placed in a dark room for 15 min. The concentration of PBG was calculated in the absorption at 553 nm.
Uroporphyrinogen III (Urogen III) and Coproporphyrinogen III (Coprogen III) assay
UrogenIII and CoprogenIII were extracted and quantified as described as Bogorad (Citation1962) with some modifications. Fresh leaves (0.2 g) were ground in liquid nitrogen and tissue was homogenized in 4 mL of 0.067 M potassium phosphate buffer (pH 7.8). Samples were centrifuged at 12,000 g for 10 min. The supernatants were combined with 200 µL Na2S2O3 (1%), shaken quickly, and then placed under the light for 20 min. The combined liquid was changed to pH 3.5 with cold glacial acetic acid and was added to a final Diethyl ether. The Urogen III concentrations were determined at 405.5 nm by a spectrophotometer (UV-2450, Shimadiu Corporation, and Japan). The final supernatants were extracted three times with 0.1 mol·L−1 HCL. The concentration of Coprogen III was calculated in the absorption at 399.5 nm.
Protoporphyrin IX (ProtoIX) assay
Leaf samples (0.2 g fresh leaves) were ground in liquid nitrogen and tissue was homogenized in 6 mL of cold acetone: 0.1 M NH4OH (90/10, v/v) and transferred into a centrifuge tube. Samples were centrifuged at 12,000 g for 10 min. The supernatants were combined and washed successively with equal volume of hexane prior to spectrophotometric analysis. The concentration of Proto IX was detected based on the method of Rebeiz et al. (Citation1975).
Mg-protoporphyrin IX (Mg-Proto IX) and Protochlorophyllide (Pchlide) assay
Mg-Proto IX and Pchlide were extracted and measured by the method reported by Terry and Kendrick (Citation1999) with small modifications. Leaf samples (0.2 g fresh leaves) were ground in liquid nitrogen and tissue was homogenized in 6 mL of cold acetone: 0.1 M NH4OH (90/10, v/v) and transferred into a centrifuge tube. Samples were centrifuged at 12,000 g for 10 min. The supernatants were combined and washed successively with an equal volume and a one-third volume of hexane prior to spectrophotometric analysis. For determination of Pchlide ester, the hexane washes were pooled and analyzed directly.
Absorption spectroscopy of Mg-Proto IX samples was performed by a spectrophotometer (UV-2450, Shimadiu Corporation, and Japan). The concentration of Mg-Proto IX was determined based on a molar absorption coefficient of 16,200 M−1 cm−1 at 503 nm, and the Pchlide concentrations were determined by using a molar absorption coefficient of 31,100 M−1 cm−1 at 626 nm.
Nitrate reductase activity assay
Nitrate reductase was extracted from frozen leaf tissue stored at −80 °C. Nitrate reductase was extracted and the maximum extractable activity was measured at 4 °C (Ferrario-Mé et al., Citation1997; Chaffei et al., Citation2004).
Expression assay of Glutamine synthetase II gene
RNA isolation
Total RNA was extracted from leaves as described by Verwoerd et al. (Citation1989). RNA integrity was verified by electrophoresis using ethidium bromide staining. First, the strand cDNA was reverse transcribed based on the standard protocol of TaKaRa prime ScriptTM RT-PCR Kit. About 500 ng RNA, 1.0 µl of 10 mmol/l dNTPs, 1.0 µl of 2.5 µmol/µl OligodT primer, 1.0 µl of 20 µmol/µl random primer and 2 µl of RNase-Free H2O were mixed and heated at 65 °C for 5 min. The mixture was immediately placed on ice for 2 min and was transitorily centrifuged. Then 4.0 µl of 5×First-Strand buffer, 0.5 µl of 40 U/µl RNase inhibitor, 0.5 µl of the Primer ScriptTM RTase and 5 µl RNase-Free H2O were added and mixed. 20 µl of reaction volume was incubated for 10 min at 30 °C, 25 min at 45 °C, 5 min at 95 °C, followed by heating at 70 °C for 15 min to stop the synthesis reaction and then held at 4 °C.
Real-time RT-PCR
The first cDNA strand was reverse transcribed from 500 ng of DNase-treated RNA as described above. Based on the homologous sequences of GS2, gene-specific primers were designed by the Primer Express software, version 2.0 (Applied Biosystems, Courtaboeuf, France). The forward primer was 5′-TGCGTAGCAAGTCAAGGA-3′, and the reverse primer was 5′-TTCAGCCAGTAGCGAGGT-3′. Real-time quantitative PCR was performed by Bio-Rad iQ5 (Bio-Rad Company), and the results were analyzed using an optical system version 2.0 (Bio-Rad) software. The actin gene of the poplar used as the inner reference was amplified in parallel with the target gene allowing gene normalization and quantification. Based on the reaction procedure of the SYBR Premix Ex TaqTM II Kit (TaKaRa), the reaction system was listed as follows: 10 µl of SYBR Premix Ex TaqTM II (2×), 0.8 µl of each Primer (10 µmol/l), 1.5 µl template (cDNA solution), 6.9 µl of dH2O. The two-step PCR reaction procedure used was as follows: 95 °C pre-denaturalized for 10 s, 95°C for 5 s and 55 °C for 20 s, the PCR reaction continued for 40 cycles, and was then kept at 72 °C for 2 min. After the PCR reaction, a melting curve was obtained with the optical version 2.0 (Bio-Rad) software and the parameters were set as the reading plate for two cycles for every increase of 0.5 °C in the range from 68 °C to 95°C. Three technical replicates of each reaction were performed. The relative changes in gene expression were quantified using the 2−ΔΔCT method as described by Livak and Schmittgen (Citation2001).
Statistical analysis
Analyses of variance (ANOVA) with orthogonal contrasts and mean comparison procedures were used to detect differences among treatments. Mean separation procedures were carried out by the multiple range tests with Fisher's least significant difference (LSD) (P<0.01).
Results
Cadmium and nitrogen content in soil
Cd concentration in soil and leaves reached from 9.96 to 520 mg·kg−1 respectively in the end of the experiment. The Cd in the soil of T2 was significantly higher than that of T1 and T3, while the Cd content in the leaves of T2 was lower than that of T1 and T3 (). The Cd in the soil and in the plant increased with adding exogenous Cd(NO3)2 or CdCl2. The results indicate that the plants treated with Cd and N (NO3− or NH4+) absorbed more Cd than the plants treated only with CdCl2. N concentrations in the soil of T1 and T3 were substantially higher than that of CK and T2 (). Together, the results suggest that the addition of N significantly promotes one clone of populus deltoides×populus nigra's absorbtion of Cd.
Table 1. Addition of nitrogen may significantly increase cadmium absorption by poplar plants from soil.
Plant morphology and growth
No measurable differences were recorded in the treated plants at the beginning of the study. However, various phenotypes diverged in the experimental treatments. Typical treatment differences were exhibited by growth rate and leaf color. The T2 plants grew slower than the control or other treatments. The leaves of T1 and T3 gradually turned dark-green with the treatment, while leaf color in T2 slowly changed from a normal shade of green to yellow-green. At the end of the experiment, leaf chlorosis was evident in T2. CdCl2 severely inhibited growth; a significant decrease in plant height, plant basal diameter and biomass was observed in comparison with CK, and its average plant height, basal diameter and dry weight decreased 18.6%, 16.0% and 31.0% respectively in comparison to the control. Conversely, both the T1 and T3 treatments induced a significant increase in plant growth (). Plant height, basal diameter and biomass increased 40.7%, 14.0% and 20.1% respectively under Cd(NO3)2 treatment in comparison to the control, and Plant height, basal diameter increased 15.8%, 3.6% and biomass decreased 3.2% compared to (NH4)2CO3 only treatment respectively. Similarly, plant height, basal diameter and dry weight increased 38.3%, 24% and 32.6% under CdCl2+(NH4)2CO3 treatment compared to the control, and increased 13.8%, 12.7% and 6.9% compared to sole (NH4)2CO3 treatment respectively.
Table 2. Effects of different Cd resources on plant height (cm), basal diameter (mm) and dry weight (mg plant−1).
Chlorophyll content
Chl content varied widely in treatments (). Chl a, Chl b, and total Chl content in T2 were significantly lower than CK in respective measured reductions of 17.0%, 95.9% and 34.5%. The Chl a:b ratio (70.6:1) of T2 was substantially higher than the ratio of CK (3.49:1), which is indicative of the severe inhibition that Cd had on Chl b synthesis. On the other hand, Chl a and Chl b contents were increased in T1 and T3. T1 induced an increase of 77.0% and 74.1% from CK for Chl a and b. The Chl a and b contents of T3 respectively increased by 1.8 and 33.8 times CK. Interestingly, Chl a, Chl b, and total Chl content in T1 or T3 were significantly higher than those in T4. The results suggest that the addition of N can significantly increase Chl content in leaves under Cd stress.
Table 3. Effects of different Cd resources on the chlorophyll content in leaves of poplar.
Nitrate reductase (NR) activity
T2 showed an obvious increase in NR leaf activity compared to CK (). Thus, NR activity of plants is accentuated under Cd stress. When N was supplied under Cd stress (T1 and T3), NR activity was also significantly increased. The result is similar to Hecht et al.'s study. Hecht et al. (Citation1988) reported that the addition of NO3− can up-regulate the activity of NR. NR is a key enzyme for nitrate assimilation in plants. Neill et al. (Citation2003) showed that NR can induce the increment of nitric oxide (NO). However, nitric oxide (NO) as a bioactive signaling molecule plays an important role in plant responses to heavy metals stress (Arasimowicz & Floryszak-Wieczorek Citation2007). So the increase of NR activity is a help to promote plant to be tolerant to heavy metals.
The precursors of chlorophy II synthesis
The levels of seven precursors (ALA, PBG, Urogen III, Coprogen III, Proto IX, Mg-proto and Pchlide) in poplar leaves were determined by absorption spectroscope. showed that ALA, PBG and Urogen III levels in T2 were significantly higher than CK with respective increments of 24.1%, 45.3% and 30.7%. While, Coprogen III, Proto IX, Mg-proto and Pchlide contents in T2 were obviously lower than CK with respective reduction of 44.9%, 32.9%, 52.9% and 61.3%. On the other hand, in T1 and T3, there was no remarkable difference in the seven precursors’ levels compared with CK. T2 treatment plants accumulated 1.3 fold more Urogen III and one half less Coprogen III compared to CK. These data suggested that the increased Urogen III and the reduced Coprogen III was a direct consequence of the inhibition of CoprogenIII synthesis induced by sole Cadmium stress. So, Cd seriously inhibited Chl synthesis by blocking the site located on the steps between Urogen III and Coprogen III. However, the plants treated with cadmium and nitrogen grew well without any toxicity symptoms. Nitrogen supplement can alleviate Cd inhibition on chlorophyll synthesis by unblocking the pathway.
Table 4. Analysis of seven precursors of chlorophy II synthesis in poplar leaves. They were quantified by absorption spectroscopy.
Expression of GS2 gene
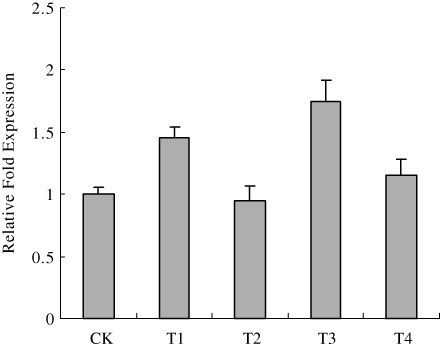
Total expressed RNA was subjected to electrophoresis on 1% formaldehyde denaturation agarose gel. The total RNA bands of leaves were all trim and clear, which indicates that the extracted RNA did not degrade and could be used for quantitative PCR assay. The actin gene of the poplar used as the inner reference was amplified in parallel with the target gene allowing gene normalization and quantification. The transcript level of GS2 varied with treatment. The expression of GS2 showed a significant decrease in T2, but an appreciable increase in T1 and T3 (). The transcript level of GS2 in T1 and T3 was respectively 1.22 and 1.72 times higher than those in T2 and 1.26 and 1.52 times higher than those in T4. Therefore, Cd alone inhibited the expression of GS2, while N significantly promoted gene expression in poplar plants under Cd stress.
Note: CK indicates control. T1, T2, T3 and T4 indicate Cd(NO3)2, only CdCl2, CdCl2 plus (NH4)2CO3, only (NH4)2CO3 treatments respectively.
Discussion
In general terms, Cd is inhibitory to Chl synthesis (Hammami et al. Citation2004; Amani Citation2008) and plant growth (Vassilev et al. Citation1995; Hammami et al. Citation2004; John et al. Citation2008). In the study, plant height, dry weight and Chl content of the plants exposed to Cd, all significantly decreased when compared to the controls. Cd seriously inhibited the Chl synthesis. The blocking site of Cd inhibition in Chl synthesis was determined to be on the steps from Urogen III to Coprogen III. Nitrogen addition could promote the growth, increase content of Chl of plants under stress of Cd, and effectively alleviate the inhibition of Cd to Chl synthesis by unblocking the pathway. The above results demonstrated that nitrogen played an important role in detoxifying heavy metals from plants. According to related research, the detoxification strategies may include: (1) Regulation of metal uptake by the root system (Hart et al. Citation1998; Benavides et al. Citation2005; Hassan et al. Citation2005); (2) Constraint by the cell wall (Hart et al. Citation1998); (3) The presence of some chelate complexes, such as phytochelatin-Cd2 + or GSH-Cd2 + (Mendoza-Cózatl & Moreno-Sánchez Citation2006; Quan et al. Citation2006; Gustavo et al. Citation2007); (4) Regulation of related gene expression (Benavides et al. Citation2005; Xu et al. Citation2006; Wu et al. Citation2012). Especially, sequestration with glutathione (GSH) and phytochelation (PC) is an important pathway to detoxifying Cd, which has earlier been confirmed (Singhal et al. Citation1987). Both GSH and PC could bind Cd2 + to form a stable chelate (GSH-Cd2 + or PC-Cd2 +) and then transfer chelates into vacuoles. In addition, the synthesis of glutathione occurs in two ATP-dependent steps (Meister Citation1995). First, glutamate-cysteine ligase (GCL) catalyzes formation of γ-glutamylcysteine from Cys and Glu. This step is thought to be the rate-limiting reaction of the pathway. In the second step, glutathione synthetase (GS) adds Gly to γ-glutamylcysteine to yield glutathione. GSH also can be used for PC synthesis. So the synthesis of GSH needs the participation of glutamate (Glu). At the same time, the synthesis of Glu requires the participation of nitrogen. The above researches revealed that the important role of GSH in detoxifying Cd from plants was closely related to nitrogen. The results also demonstrated that nitrogen can effectively alleviate cadmium toxicity to poplar plants. Based on the study and other related researches on Cd toxicity, a hypothesis is proposed to explain the mechanism of detoxification of Cd in higher plants. The hypothesis includes the following main points:
Sequestration with GSH and PC is an important pathway to detoxify heavy metals. GSH binds Cd directly or will be used for PC synthesis and transport of sequestered Cd into the vacuole. Although GSH and PC contents were not detected in our study, others have confirmed that low molecular weight thiols, such as cysteine, GSH and PC, will significantly increase in plants under Cd stress (Cataldo et al. Citation1983; Strohm et al. Citation1995; Zhu et al. Citation1999; Miflin & Habash Citation2002; Li et al. Citation2006).
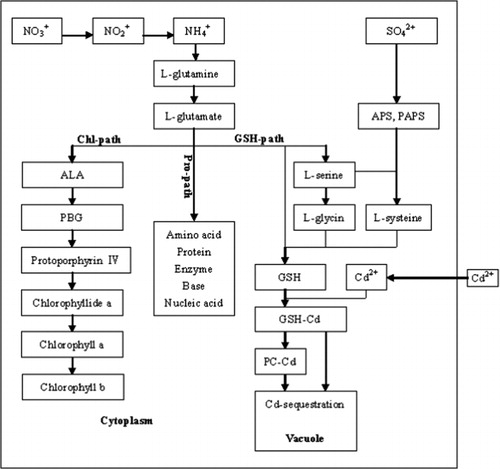
There are three metabolic pathways that utilize Gln as a resource (): Chl synthesis pathway (Chl path); GSH synthesis pathway (GSH path); and amino acid, protein, nucleic acid synthesis pathway (Pro path). The pathways are mutually competitive; namely, when the total amount of glutamate is limited; if the consumption of glutamate increases in one of the three pathways, it will decrease in the other two. The three pathways are in balance in the absence of Cd.
The response of plants to Cd stress includes two phases, detoxification and toxification. Detoxification is the first response. As soon as exogenous Cd invades mesophyll cells, corroding protection is activated to preclude intercellular Cd toxicity. Typically, the genes responsible for Gln, GSH and PC synthesis would be up-regulated so that, subsequently, more GSH and PC are available for Cd chelation. No toxic symptoms will be expressed no matter how much Cd enters the cell, as long as the free Cd concentration is controlled at low levels and the amount of glutamate for biosynthesis occurs at normal levels. In the presence of exogenous N, the plant not only has enough glutamate to synthesis GSH for free Cd chelation, but also an excess amount for manifold biosyntheses, increased metabolism and plant growth.
The toxification phase occurs when glutamate is limited by an inadequate N supply with increasing amounts of Cd2 + entering the cell. In these conditions, enhanced GSH synthesis would consume more glutamate, which would lead to glutamate deficiency and decreases in Chl, protein and nucleic acid, resulting in chlorosis and growth inhibition. Therefore, primary toxic symptoms of plants under Cd treatment are not directly caused by Cd reaction, but are induced by insufficient glutamate for the Chl-pathway and Pro-pathway in the cells. If GSH and PC are insufficient to bind the invading Cd, the ions will directly react with some proteins and enzymes, or be replaced with other metal ions, such as Mg2 + and Fe2 +, leading to more serious toxicity. Thus Cd toxicity includes both indirect and direct effects.
Because GSH synthesis needs cysteine in addition to glutamate, sulfur also plays an important role in the N-based detoxification of Cd. Namely, the detoxification depends on sufficient quantities of sulfur.
The detoxification and toxification of poplar to Cd may be a common mechanism of all plants and other heavy metals such as Pb, Cu, As, Hg, and so on. Our hypothesis needs verification in follow-up studies.
In the study, hybrid poplar ‘107’ (Populus deltoides×P. nigra) plants were selected as the materials and the potential species for phytoremediation because Populus ‘107’ has many excellent features, such as fast growth rates, profuse vegetative propagation, adaptability to various ecological conditions, aesthetic values and wood values. ‘107 poplar’ was widely cultivated in China and it has become one of the most economically important groups of tress. In addition, populus species, as the only woody model plants, provide the physiology and molecular basis for study of other woody plants. Using tall, woody plants in combination with nitrogen fertilization to remediate contaminated sites would be an aesthetically pleasing and beneficial approach that, not only promotes positive landscape effects, but reduces erosion and provides the valuable timber. For example, Komárek et al. (Citation2007) used poplar plants to remediate the lead-contaminated agricultural soil. Giachetti and Sebastiani (Citation2006) reported that poplar plants grown in industrial waste developed well, and showed high Cd accumulation. In addition, eucalyptus and populus, the short rotation woody crops, were also used to remediate phosphate mined lands in Florida USA (2006).
Conclusions
Although the toxic effects of Cd have been reported by many authors, the mechanisms are not completely understood yet. In our experiments, the poplar plants treated with very high concentrations of Cd grew well repeatedly. It is well-established that the low-molecular-weight thiol compounds, such as cysteine, GSH and PC, have an important function on Cd sequestration. Based on our results and related research, we draw the conclusion that Cd exposure does not always lead to toxic symptoms in plants. Ample N mitigates high Cd concentrations, leading to poplar plants that grew better than the controls in our experiments. Cd toxicity may be divided into indirect action and direct action; the former refers to the toxic action caused by glutamate deficiency as a result of GSH and PC's detoxification, while the direct action is caused by the interaction between Cd and protein. Both N and sulfur play an important role on the detoxification of Cd in plants. There are three biosynthesis pathways (Chl, GSH and protein), which compete for L-glutamate. N plays a key role in the detoxification or toxification process via glutamate and GSH. The detoxification process is realized by GSH- or PC-binding Cd and transporting into vacuoles. When glutamate is limited, competitive inhibition results with reductions in the biosynthesis of Chl, protein and nucleic acid, because the GSH synthesis consumes more glutamate under Cd stress. With more and more Cd2 + entering the cell, the plant would transfer into the toxification phase, leading to chlorosis and growth loss. The detoxification and toxification of poplar to Cd may be a common mechanism of all plants and other heavy metals such as Pb, Cu, As, and Hg. If the reasoning is confirmed in the future, phytoremediation of contaminated soil may be enhanced with N fertilization.
Acknowledgments
The authors thank Dr Brian Stanton, Dr Chris Boswell and Dr Zhang Li for their helpful comments that improved the manuscript. This work was supported by the 12th Five Years Key Programs for forest breeding of Sichuan Province (No. 2011YZGG).
References
- Amani AL. 2008. Cadmium induced changes in pigment content, ion uptake, proline content and phosphoenolpyruvate carboxylase activity in Triticum aestivum seedlings. Austra J Basic Applied Sci. 2:57–62.
- Anderson JS, Ingram JIS. 1998. Tropical soil biology and fertlity. A handbook of methods. 2nd ed. USA: Oxford University Press; p. 221.
- Arasimowicz M, Floryszak-Wieczorek J. 2007. Nitric oxide as a bioactive signaling molecule in plant stress responses. Plant Sci. 172:876–887. 10.1016/j.plantsci.2007.02.005
- Baryla A, Carrier P, Franck F, Coulomb C, Sahut C, Havaux M. 2001. Leaf chlorosis in oilseed rape plants (Brassica napus) grown on cadmium-polluted soil: cause and consequences for photosynthesis and growth. Planta 212:696–709. 10.1007/s004250000439
- Benavides M, Gallego S, Tomaro M. 2005. Cadmium toxicity in plants. Braz J Plant Physiol. 17: 21–34. 10.1590/S1677-04202005000100003
- Bogorad L. 1962. Porphydn synthesis. In: Colowick SP, Kapha NO, editors. Methods in enzymology [C]. New York: Academic Proc; p. 885–891.
- Boyd CE. 1995. Bottom soils, sediment and pond aquaculture. New York: Chapman and Hall.
- Cataldo DA, Garland TR, Wildung RE. 1983. Cadmium uptake kinetics in intact soybean plants. Plant Physiol. 73:844–848. 10.1104/pp.73.3.844
- Chaffei C, Pageau K, Suzuki A, Gouia H, Ghorbel MH, Céline MD. 2004. Cadmium toxicity induced changes in nitrogen management in Lycopersicon esculentum leading to a metabolic safeguard through an amino acid storage strategy. Plant cell physiol. 45:1681–1693. 10.1093/pcp/pch192
- Chaney RL, Ryan JA, Li YM, Brown SL. 1999. Soil cadmium as a threat to human health. In: McLaughlin MJ, Singh BR, editors. Cadmium in soils and plants. Dordrecht: Kluwer Academic Publishers; p. 219–256.
- Dietz KJ, Baier M, Krämer U. 1999. Free radicals and reactive oxygen species as mediators of heavy metals toxicity in plants. In: Prasad MNV, Hagemeyer J, editors. Heavy metal stress in plants. Berlin and Heidelberg: Springer-Verlag; p. 73–97.
- Drążiewicz M, Baszyński T. 2005. Growth parameters and photosynthetic pigments in leaf segments of Zea mays exposed to cadmium, as related to protection mechanisms. J Plant Physiol. 162:1013–1021. 10.1016/j.jplph.2004.10.010
- Ferrario-Mé S, Cthibaud M, Bettsche T, Valadier MH, Foyer C. 1997. Modulation of carbon and nitrogen metabolism, and of nitrate reductase, in untransformed and transformed Nicotiana plumbaginifolia during CO2 enrichment of plants grown in pots and in hydroponic culture. Planta. 202:510–521. 10.1007/s004250050156
- Floryszak-Wieczorek J, Milczarek G, Arasimowicz M, Ciszewski A. 2006. Do nitric oxide donors mimic an endogenous NO-related response in plants. Planta. 224:1363–1372. 10.1007/s00425-006-0321-1
- Garcia-Mata C, Lamattina L. 2001. Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol. 126:1196–1204. 10.1104/pp.126.3.1196
- Giachetti G, Sebastiani L. 2006. Metal accumulation in poplar plant grown with industrial wastes. Chemosphere. 64:446–454 10.1016/j.chemosphere.2005.11.021
- Greger M, Lindberg S. 1986. Effects of Cd2 + and EDTA on young sugar beets (Beta vulgaris) I. Cd2 + uptake and sugar accumulation. Plant Physiol. 66:69–74. 10.1111/j.1399-3054.1986.tb01235.x
- Grimshaw HM, Allen SE, Parkinson JA. 1989. Nutrient elements. In: Allen SE, editor, Chemical analysis of ecological material. Oxford: Blackwell Scientific; p. 81–159.
- Grün S, Lindermayr C, Sell S, Durner J. 2006. Nitric oxide and gene regulation in plants. J Exp Bot. 57:507–516. 10.1093/jxb/erj053
- Gustavo G, Ana JF, Diego MS, María LT. 2007. Glutathione reductase activity and isoforms in leaves and roots of wheat plants subjected to cadmium stress. Phytochem. 68:505–512. 10.1016/j.phytochem.2006.11.016
- Haag-Kerwer A, Heiss S, Walter C, Rausch T, Schafer HJ. 1999. Cadmium exposure in Brassica juncea causes a decline in transpiration rate and leaf expansion without effect on photosynthesis. J Exp Bot. 341:1827–1835.
- Hammami SS, Chaffai R, Ferjani EE. 2004. Effect of cadmium on sunflower growth, leaf pigment and photosynthetic enzymes. Pak J Biol Sci. 7:1419–1426. 10.3923/pjbs.2004.1419.1426
- Hart JJ, Welch RM, Norvell WA, Sullivan LA, Kochian LV. 1998. Characterization of cadmium binding, uptake, and translocation in intact seedlings of bread and durum wheat cultivars. Plant Physiol. 116:1413–1420. 10.1104/pp.116.4.1413
- Hassan MJ, Wang F, Ali S, Zhang G. 2005. Toxic effect of Cd on rice as affected by nitrogen fertilizer form. Plant Soil. 277:359–365. 10.1007/s11104-005-8160-6
- Hecht U, Oelmüller R, Schmidt S. 1988. Action of light, nitrate and ammonium on the levels of NADH-and ferredoxin-dependent glutamate synthase in the cotelydons of mus mustard seedlings. Planta. 175:130–138. 10.1007/BF00402890
- Jiang WZ, Li JL. 1992. Effect of cadmium on the construction of photosynthetic membrane system. J Yunnan Univ. 14:318–323.
- John R, Ahmad P, Gadgil K, Sharma S. 2008. Effect of cadmium and lead on growth, biochemical parameters and uptake in Lemna polyrhiza L. Plant Soil Environ. 54:262–270.
- Komárek M, Tlustos P, Száková J, Chrastný V, Ettler V. 2007. The use of maize and poplar in chelant-enhanced phytoextraction of lead from contaminated agricultural soils. Chemosphere. 67:640–651. 10.1016/j.chemosphere.2006.11.010
- Kopyra M, Gwózdz EA. 2003. Nitric oxide stimulates seed germination and counteracts the inhibitory effect of heavy metals and salinity on root growth of Lupinus luteus. Plant Physiol Biochem. 41:1011–1017. 10.1016/j.plaphy.2003.09.003
- Li JC, Guo JB, Xu WZ, Ma M. 2006. Enhanced cadmium accumulation in transgenic tobacco expressing the phytochelatin synthase gene of Cynodon dactylon L. J of Integr Plant Biol. 48:928–937. 10.1111/j.1744-7909.2006.00314.x
- Li JG, Jin SL, Chen YQ, Lin GL, Han XR, Li TQ, Yang XE, Zhu E. 2007. Effects of nitrogen fertilizer on the root morphology and cadmium accumulation in low cadmium treatment Sedum alfredii Hance. Chin Agric Sci Bull. 23:260–265. ( In Chinese).
- Li DM, Zhu ZJ, Liu YH. 2005. Influence of cadmium on photosynthesis of Brassica campestris ssp. Chinensis L. J Zhejiang University (Agric & Life Sci.). 31:459–464. ( In Chinese).
- Lichtenthaler HK. 1987. Chls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 148:350–382.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 25:402–408. 10.1006/meth.2001.1262
- McLaughlin MJ, Parker DR, Clarke JM. 1999. Metals and micronutrients-food safety issues. Field Crop Res. 60:143–163. 10.1016/S0378-4290(98)00137-3
- Meister A. 1995. Glutathione metabolism. Methods Enzymol. 252:26–30.
- Mendoza-Cózatl DG, Moreno-Sánchez R. 2006. Control of glutathione and phytochelatin synthesis under cadmium stress. Pathway modeling for plants. J Theor Biol. 238:919–936. 10.1016/j.jtbi.2005.07.003
- Miflin BJ, Habash DZ. 2002. The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J Exp Bot. 53:979–987. 10.1093/jexbot/53.370.979
- Murphy J, Riley JP. 1962. A modified single solution method for the determination of phosphate in natural water. Anal Chem Acta. 27:31–36. 10.1016/S0003-2670(00)88444-5
- Neill SJ, Desikan R, Hancock JT. 2003. Nitric oxide signaling in plants. New Phytol. 159:11–35. 10.1046/j.1469-8137.2003.00804.x
- Nelson DW, Sommers LE. 1982. Organic carbon. In: Page AL, editor. Methods of soil analysis, part 2. Agron. Monograph. 9. Madison, WI: ASA; p. 570–571.
- Pereira GJG, Molina SMG, Lea PJ, Azevedo RA. 2002. Activity of antioxidant enzymes in response to cadmium in Crotalaria juncea. Plant Soil. 239:123–132. 10.1023/A:1014951524286
- Quan XQ, Zhang HT, Shan L, Bi YP. 2006. Advances in plant metallothionein and its heavy metal detoxification mechanisms. Hereditas. 28:375–382.
- Rebeiz CA, Mattheis JL, Smith BB, Rebeiz CC, Dayton DF. 1975. Chloroplast biosynthesis and accumulation of protochlorophyll by isolated etioplasts and developing chloroplasts. Arch Biochem Biophys. 171:549–567. 10.1016/0003-9861(75)90065-X
- Romero-Puertas MC, Corpas FJ, Sandalio LM, Leterrier M, Rodríguez-Serrano M, del Río LA, Palma JM. 2006. Glutathione reductase from pea leaves: response to abiotic stress and characterization of the peroxisomal isozyme. New Phytol. 170:43–52. 10.1111/j.1469-8137.2006.01643.x
- Sandalio LM, Dalurzo HC, Gomez M, Romero-Puertas MC, del Rio LA. 2001. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot. 52:2115–2126.
- Sanità di Toppi L, Gabbrielli R. 1999. Response to cadmium in higher plants. Environ Exp Bot. 41:105–130. 10.1016/S0098-8472(98)00058-6
- Singhal RK, Anderson ME, Meister A. 1987. Glutathione, a first line of defense against cadmium toxicity. Faseb J. 1:220–223.
- Strohm M, Jouanin L, Kunert KJ, Pruvost C, Polle A, Foyer CH, Rennenberg H. 1995. Regulation of glutathione synthetase in leaves of transgenic poplar (Populus tremula×P. alba) over-expressing glutathione synthase. Plant J. 7:141–145. 10.1046/j.1365-313X.1995.07010141.x
- Sun GW, Chen RY, Liu HC. 2005. Advances on investigation of effect of cadmium on photosynthesis and nitrogen metabolism of plant. Chin Agric Sci Bull. 9:234–236, 251. (In Chinese).
- Terry MJ, Kendrick RE. 1999. Feedback inhibition of chlorophyll synthesis in the phytochrome chromophore-deficient aurea and yellow-green-2 mutants of tomato. Plant Physiol. 119:143–152. 10.1104/pp.119.1.143
- Vassilev A, Iordanov I, Chakalova E, Kerin V. 1995. Effect of cadmium stress on growth and photosynthesis of young barley (H vulgare L.) plants. 2. Structural and functional changes in the photosynthetic apparatus. Bulg J Plant Physiol. 21:12–21.
- Verwoerd TC, Dekker BMM, Hoekema AA. 1989. Small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 17:2362–2362. 10.1093/nar/17.6.2362
- Wagner GJ. 1993. Accumulation of cadmium in crop plants and its consequences to human health. Adv Agron. 51:173–212.
- Wang LJ, Ni DA, Ye XF, Xia ZA. 1997. Determination of d-aminolevulinic acid in plant leaves. Plant Physiol Commun. 33:439–441. ( In Chinese).
- Wu HL, Chen CL, Du J, Liu HF, Cui Y, Zhang Y, He YJ, Li JM, Feng ZY, Wang YQ, Chu CC, Ling HQ. 2012. Co-overexpression FIT with AtbHLH38 or AtbHLH39 in Arabidopsis enhanced cadmium tolerance via increased cadmium sequestration in roots and improved iron homeostasis of shoots. Plant Physiol. 158:790–800. 10.1104/pp.111.190983
- Wu JZ, Ge Y. 1999. Comparative studies on five pretreatment methods in the determination of elements in plant standard sample by ICP-AES. SpectroSc Spectral Anal. 19:369–372. ( In Chinese).
- Xu ZH, Shen GJ, Zhu CQ, Xu LJ, He Y, Yu GS. 2006. Molecular mechanisms of plant resistance to cadmium toxicity. Chin J Appl. 17:1112–1116.
- Yang XE, Long XX, Ye HB, He ZL, Calvert DV, Stoffella PJ. 2004.Cadmium tolerance and hyperaccumulation in a new Zn-hyperaccumulating plant species (Sedum alfredii Hance). Plant Soil. 259:181–189. 10.1023/B:PLSO.0000020956.24027.f2
- Zhou WB, Qiu BS. 2005. Effects of cadmium hyperaccumulation on physiological characteristics of Sedum alfredii Hance (Crassulaceae). Plant Sci. 169:737–745. ( In Chinese).
- Zhu YL, Pilon-Smits E, Jouanin L, Terry N. 1999. Over-expression of glutathione gynthetase in India mustard enhances cadmium accumulation and tolerance. Plant Physiol. 1:73–80.
