Abstract
The present study investigated the effect of ferulic acid (FA; 0–1000 µM) on early growth, and rhizogenesis in mung bean (Vigna radiata) hypocotyls and associated biochemical changes. FA severely affected the radicle elongation and number of secondary roots after 72 h. The root and shoot length, number and length of secondary roots, and seedling dry weight of one-week-old seedlings of mung bean were decreased by 64%. The rooting potential (percent rooting, number and length of adventitious roots) of mung bean hypocotyls under in vitro conditions was significantly inhibited in response to 1–100 µM FA. At 1000 µM there was complete cessation of rooting. FA caused a reduction in the contents of water-soluble proteins and endogenous total phenolics, whereas the activities of proteases, peroxidases, and polyphenol peroxidases increased. The study concludes that FA inhibits root growth and development, and in vitro rooting process in mung bean by interfering with biochemical processes that are crucial for root formation.
| Abbreviations | ||
| Ferulic acid | = | FA |
| Dry weight | = | DW |
| Root length | = | RL |
| Shoot length | = | SL |
| Lower rooted hypocotylar region | = | LRHR |
| Peroxidases | = | PODs |
| Polyphenol oxidases | = | PPOs |
| Total endogenous phenolic content | = | TP |
| 2,3,5-triphenyl tetrazolium chloride | = | TTC |
Introduction
Plants synthesize an array of chemical compounds as a result of secondary metabolism and these are involved in a variety of interactions with environment (Hadacek Citation2002; Kaur et al. Citation2011). Among them, phenolic acids constitute a major group and are ubiquitously distributed in plants (Boudet Citation2007; Mutlu & Atici Citation2009). In plants, they play a variety of roles, namely, structural (as component of plant and floral pigments), protective (against harmful insects, microorganisms), and internal physiological regulators (as chemical messengers) (Li et al. Citation2010). They are an important component of the plant-soil systems. However, several studies have indicated their role in ecological interactions including vegetation patterning and allelopathy (Rice Citation1984; Singh et al. Citation2003; Li et al. Citation2010).
Ferulic acid (FA; 4-hydroxy-3-methoxycinnamic acid) is widely distributed in plants and implicated in allelopathic interactions (Rice Citation1984). It is virtually present in all plant parts, including seeds, fruits, leaves, stem, and roots. FA is a product of lignin degradation and released from the plants into soil environment through root exudation, leachation, and residue decomposition (Rice Citation1984). FA inhibits seed germination, root and shoot growth, cell division, and dry weight (DW) accumulation (Devi & Prasad Citation1996; Herrig et al. Citation2002; Esmaeili et al. Citation2012), and induces several physiological alterations in the plants. For example, FA inhibits photosynthesis (Hussain & Reigosa Citation2011), reduces leaf chlorophyll content (Esmaeili et al. Citation2012), disrupts ion and water uptake, and plant water utilization (Booker et al. Citation1992; Hussain & Reigosa Citation2011), cause stomatal closure by reducing turgor and osmotic pressure (Einhellig Citation2004) and inhibits foliar expansion (Blum Citation2005).
Though negative effects of FA on radicle growth and root division have been well documented, yet the effect of FA on physiological and biochemical processes during rhizogenesis remains unexplored. The present study investigated the effect of exogenous FA on the early root growth, rooting potential in hypocotyls of mung bean (Vigna radiata [L.] Wilczek=Phaseolus aureus Roxb.; hereafter mung bean), activity of associated enzymes–peroxidases (PODs), polyphenol oxidases (PPOs), proteases–, and total endogenous phenolic (TP) content during rhizogenesis. We selected mung bean as a target species since its hypocotyls are widely used to study adventitious root formation and associated physiological aspects in non-woody cuttings (Batish et al. Citation2008).
Materials and methods
Materials
Technical grade FA (MW = 191.18; 99% purity) was purchased from Sigma–Aldrich Pvt. Ltd., St Louis, USA. A stock solution of FA (1000 µM) was prepared by dissolving the requisite amount in ethanol and the final volume was made-up with distilled water. The final concentration of ethyl alcohol in the solution was ~0.10% and same volume was added to distilled water (control). FA stock solution was diluted to get working concentrations of 100, 10, and 1 µM. These concentrations are ecologically relevant, as natural soil concentration of phenolic acids vary between 10 and 100 µM (Kuiters Citation1990; Baleroni et al. Citation2000). Further, the pH of FA solutions was in the range of 5.7–6.5. Since the pH of FA solutions was not too low, these were used as such and not buffered.
For dose-response bioassay and assessing rooting response, mung bean (Vigna radiata (L.) Wilczek =Phaseolus aureus Roxb.) seeds were purchased locally from the market. Prior to use, these were surface sterilized with sodium hypochlorite (0.05%, w/v) for 15 min, washed under running tap water followed by distilled water.
Dose-response bioassay
The effect of FA on early growth of mung bean was studied under laboratory conditions in a Petri dish bioassay. Mung bean seeds preimbibed in FA or distilled water (control) for 8 h were placed equidistantly in 15-cm diameter Petri dishes on a Whatman no. 1 filter paper moistened with 7 ml of respective treatment solution. For each treatment, there were five Petri dishes each having 20 seeds. The experiment was laid in a completely randomized design in an environmentally controlled growth chamber at 25±2°C, ~75% RH, and under a 16 h photoperiod of 220 µmol m−2s−1 photosynthetic flux density (PFD). After 72 h, radicle length and number and length of secondary roots were measured. After a week, root length (RL), number and length of secondary roots, shoot length (SL), and seedling DW were determined.
Rooting response in mung bean hypocotyls
The effect of FA on rooting in mung bean hypocotyls was studied as per the protocol described by Batish et al. (Citation2008). Briefly, presoaked (for 8 h in distilled water) mung bean seeds were germinated for a week in polypropylene trays (32×26×7 cm) lined with moistened cotton wad layer under environmentally controlled conditions in a growth chamber set at 25±2°C, ~75% RH, and under a 24-h light photoperiod of 220 µmol m−2s−1 PFD. After five days, the uniform-sized mung bean seedlings were selected and their cotyledons removed. The cuttings were made ~3.5 cm below the cotyledonary node, keeping the above epicotylar portion intact. The resultant explants consisting of lower region of hypocotyls and epicotyls with two leaves were used for the rooting experiments. Five hypocotyls were dipped in 20 ml of respective FA solution or distilled water (as control) in glass vials. Ten replicates were maintained for each treatment in a completely randomized design in a growth chamber under controlled environmental conditions (as described above for dose-response bioassay). After a week, the percent number of hypocotyls that rooted, number of roots and primordia per hypocotyl, and the average length of adventitious roots were determined. A hypocotyl was considered rooted, if RL was ≥0.2 cm. The lower rooted hypocotylar region (LRHR; ~1.5–1.7 cm) was excised and used for the determination of total water-soluble proteins, enzymatic activities, TP, and tissue viability.
Enzyme activities
LRHRs (200 mg) were homogenized in 10 ml of 0.1 M phosphate buffer (; pH 7.0) in a prechilled pestle and mortar. The homogenates were centrifuged at 15,000 g for 20 min at 4°C rotor temperature in a refrigerated SIGMA centrifuge (Model 2K15; Sigma Laborzentrifugen GmbH, Osterade, Germany). The supernatants were stored at 4°C and used for assaying the activities of proteases (EC: 3.4.4.1), PODs (EC: 1.11.1.7) and PPOs (EC: 1.14.18.1). An aliquot of the homogenate was used for the determination of total protein content according to Lowry et al. (Citation1951) against bovine serum albumin as a calibration standard.
Proteases activity was determined using 1% (w/v) casein in 0.1 M buffer (pH 6.0) as a substrate (Basha & Beevers Citation1975). Activity of PODs was determined using 0.2 M H2O2 as a substrate, according to Malik and Singh (Citation1980). The reaction mixture comprised 3.5 ml of 0.1 M
buffer (pH 6.5), 0.5 ml of enzyme extract, 0.1 ml of dianisidine (1 mg ml−1 methanol), and 0.2 ml of 0.2 M H2O2. The activity was measured in terms of increase in absorbance at 430 nm and expressed as kat sec−1 mg−1 protein. PPOs activity was determined using 0.01 M catechol in 0.1 M
buffer (pH 6.0) as substrate, according to the method of van Lelyveld and Pretorius (Citation1973). The reaction mixture consisted of 3 ml of catechol and 0.5 ml of enzyme extract. The activity was measured in terms of increase in absorbance at 495 nm and expressed as kat sec−1 mg−1 protein.
Endogenous total phenolics
TP content in LRHRs was determined using Folin-ciocalteu reagent according to the method of Swain and Hillis (Citation1959). Briefly, LRHR (100 mg) was homogenized in 5 ml of distilled water, centrifuged at 3500 g for 5 min, and the aqueous extract was used for the estimation of TP. To 1 ml of the extract was added 1 ml of Folin-ciocalteu reagent (50%, v/v) and 1 ml of 20% Na2CO3. The contents were shaken, kept in the dark for 30 min, and the intensity of blue color was read at 700 nm on a Shimadzu spectrophotometer against a standard of FA (25 mg l−1).
Tissue viability
Viability of LRHRs was determined by TTC (2,3,5-triphenyl tetrazolium chloride) reduction assay resulting in red-colored triphenyl formazan formation. LRHR (50 mg) was treated with 5 ml of TTC solution (0.4%, w/v) and 5 ml of 66.7 mM buffer (pH 7.0). The contents were incubated for 3 h at 40°C, and 2 ml of 2 N H2SO4 was added (Shen et al. Citation1991). Then, tissue was homogenized in 10 ml of ethyl acetate to extract red triphenyl formazan. The absorbance of the extract was measured at 485 nm and tissue viability expressed as percent of control.
Statistical analyses
All the experiments were conducted in a completely randomized design and repeated. Data presented is the mean of two experiments. For each enzyme assay or other biochemical analyses, there were five replicated (independent) tissue samples. The data are presented as mean±SE and analyzed by one-way ANOVA followed by the separation of treatment means from control and among themselves at p < 0.01 and p < 0.05 applying post hoc Dunnett's or Tukey's test using SPPS/PC software (ver. 10).
Results and discussion
FA affects radicle growth of mung bean
FA severely affected the early root growth of mung bean in a dose-dependent manner. Radicle length, number and length of secondary roots declined significantly (p <0.05) after 72 h in response to >1 µM FA treatment (; ). Radicle length decreased by ~57% in response to 1000 µM FA. The number and length of secondary roots declined by 43% and 70%, respectively, at 100 µM FA treatment. However, at 1000 µM FA, there was an increase in number of roots and their length, though these were lesser than in the control ().
Table 1. Effect of ferulic acid (FA) on radicle length (primary root), number and length of secondary roots of mung bean measured 72 h after treatment.
FA inhibits mung bean seedling growth
Treatment with FA also affected RL, SL, and DW of one-week-old mung bean seedlings (). The inhibitory effect of FA was more pronounced on primary root growth than on secondary RL or SL or DW. Primary RL declined by 51% (p <0.05) in response to 100 µM FA (). Likewise, the number of secondary roots decreased in response to ≥100 µM; whereas at 1000 µM FA the number of secondary roots increased by 28% (). The length of secondary roots and SL declined (p <0.05) with FA treatment, except at 1 µM. DW of mung bean seedlings also exhibited a similar trend of changes and declined by 52% and 60% in response to 100 and 1000 µM FA, respectively ().
Table 2. Effect of ferulic acid on primary root length, number and length of secondary roots, shoot length, and seedling dry weight of mung bean measured seven days after treatment.
The results obtained in the present study demonstrate that FA at ecologically relevant concentrations significantly inhibited radicle elongation and early seedling growth of mung bean. Such an inhibitory effect of phenolic acids, including FA, has been reported earlier (Rice Citation1984; Li et al. Citation2010). FA has been reported to decrease radicle elongation and/or root growth in Lactuca sativa (Li et al. Citation1993), Zea mays (Devi & Prasad Citation1996), Brassica napus (Baleroni et al. Citation2000), Glycine max (Herrig et al. Citation2002), B. napus (Caspersen et al. Citation1999), and Cucumis sativus, Lycopersicon esculentum, and Vicia faba (Blum Citation2005). We observed an increase in the number of secondary roots at ≥100 µM FA treatment; however, it was in sharp contrast to an earlier study reporting a decline in the number of secondary roots in B. napus upon 200 µM FA treatment (Caspersen et al. Citation1999). Nevertheless, the increased number of secondary roots in response to FA probably acts as defense strategy to overcome the stress induced by FA, since phenolic acids have been reported to induce oxidative stress in the target plant tissue and thus affect normal biochemical and physiological processes (Devi & Prasad Citation1996; Baleroni et al. Citation2000; Weir et al. Citation2004; Hussain & Reigosa Citation2011). Furthermore, greater number of roots might enable the young and growing seedlings to uptake more minerals and ions. Phenolic acids, including FA, have been reported to inhibit ion uptake (Booker et al. Citation1992), negatively affect plant–water relationships (Barkosky et al. Citation2000; Hussain & Reigosa Citation2011), cause water stress in plants (Barkosky et al. Citation2000), and disrupt membrane permeability (Baziramakenga et al. Citation1995), thereby leading to a cascade of secondary effects. These vital processes are required for maintaining cell homeostasis required for normal plant growth and development.
FA inhibits rooting of mung bean hypocotyls
FA inhibited morphogenetic response of mung bean hypocotyls in a dose-dependent manner (). FA treatment significantly decreased percent rooting, roots/root primordia per cutting, and RL in mung bean hypocotyls. At 1 µM FA, there was a reduction of 28% in the number of rooted hypocotyls, whereas at 1000 µM none of the hypocotyls could root (). The number of roots/primordia decreased by ~16% (p <0.05) to 50% (p <0.01) in response to 1–100 µM FA compared to the control. RL declined by ~42–78% (p <0.01) in response to 10–100 µM FA treatment ().
Table 3. Effect of ferulic acid on the rooting potential of mung bean hypocotyls in terms of percent rooting and average root number and root length (RL) measured after seven days of treatment.
FA induces biochemical changes in mung bean hypocotyls
To explore the reason for inhibitory effect of FA on rooting in mung bean hypocotyls, biochemical studies were undertaken. In response to FA, protein content in the LRHR decreased up to 100 µM FA (p <0.05 at 10 and 100 µM). However, at 1000 µM FA, the protein content increased by ~48% (p <0.05) over the control (). The activity of the protein hydrolyzing enzyme proteases increased significantly (p <0.05) up to 100 µM FA. At 1 µM FA treatment, proteases activity was ~34% more than that in the control, and was 2.2-fold and 3.5-fold greater at 10 and 100 µM FA, respectively (). Parallel to proteases, the activity of PPOs and PODs increased significantly (p <0.05) with FA treatment compared to the control (). PPOs activity increased by ~1.3-fold and 2.8-fold at 1 µM and 100 µM FA, respectively. However, at 1000 µM FA, the increase in PPO activity was lesser than that at 10 µM FA; though, it was significantly more (~70%) than that in control (). Likewise, POD activity increased with FA treatment and showed a trend of changes similar to PPOs activity. It increased by ~1.5-fold at 1 µM FA and further enhanced by ~4.3-fold in response to 100 µM FA. However, at 1000 µM FA, POD activity, though significantly more than control, was lesser than that at 1 µM FA ().
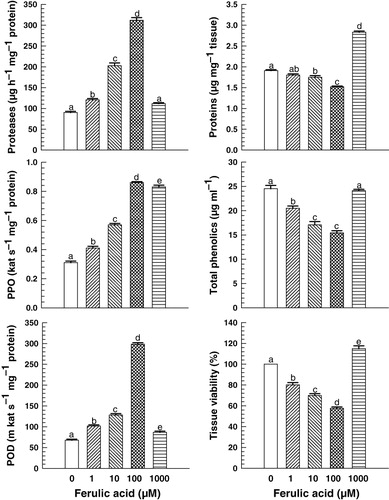
Unlike enzyme activities, the amount of endogenous TP in the mung bean hypocotyls declined significantly (p <0.05; except at 1000 µM) with FA treatment. TP content decreased by 14–37% in response to 1–100 µM FA compared to the control (). The tissue viability followed a trend of changes similar to protein and endogenous TP content in response to FA treatment. It decreased by 20–43% (p <0.05) in response to 1–100 µM FA treatment, whereas an increase was observed at 1000 µM ().
The observed impairment of morphogenetic response in mung bean hypocotyls under FA treatment is corroborated by earlier studies that allelochemicals, including phenolic acids, are potent root inhibitor (Rice Citation1984; Li et al. Citation2010), and impair the rhizogenesis (Singh et al. Citation2005; Batish et al. Citation2006, Citation2008). Adventitious root formation is an important aspect of root differentiation that involves a number of exogenous and endogenous factors and signal molecules that bring physiological and molecular changes resulting in root formation (Nag et al. Citation2001; Kollárová et al. Citation2005; Aloni et al. Citation2006). FA increased the activities of proteases, PODs, and PPOs in mung bean hypocotyls. It indicated an active role of these enzymes in ameliorating FA-induced stress. Increased activity of proteases indicates greater hydrolysis of proteins to provide precursors for root induction and other developmental processes. It, in fact, paralleled the decrease in protein content. In general, the activity of these enzymes slowed down at higher FA concentrations (1000 µM) possibly due to FA-induced stress and it manifested in lesser or no root formation.
PODs are a group of enzymes covalently and ionically bound to cell walls and involved in cell wall lignifications during root formation (Sato et al. Citation1993) and are the known inducers of rooting (Nag et al. Citation2001). PODs have been regarded as excellent markers of root formation and their activity inversely relates to the induction of rooting (Günes Citation2000). A similar observation was made in the present study where the activity of POD increased with FA treatment while the rooting decreased. These results are in agreement with earlier reports that FA-induced decline in root growth involves increased POD activity in maize (Devi & Prasad Citation1996), soybean (Baziramakenga et al. Citation1995; Herrig et al. Citation2002), and cucumber roots (Politycka et al. Citation2004). Moreover, PODs activity increases significantly in response to allelochemicals, including phenolic acids (Baziramakenga et al. Citation1995; Weir et al. Citation2004).
Singh et al. (Citation2009) demonstrated that caffeic acid inhibits root growth through generation of reactive oxygen species (ROS) in Phaseolus aureus. Baziramakenga et al. (Citation1995) demonstrated that phenolic acids undergo oxidation to form quinines that are highly reactive free radicals. To overcome the stress induced by these reactive species and protect the cells from disintegration, there is enhancement of PODs activity. At the same time, PODs (cell wall bound) are also required for lignin synthesis and thus stiffening of cell walls, thereby resulting in decreased root growth (Sánchez et al. Citation1996). The observations in the present study are similar to those of Singh et al. (Citation2005) and Batish et al. (Citation2006) who reported an inhibition of rooting with concomitant increased POD activity in mung bean hypocotyls under 2-benzoxazolinone (BOA) and L-3,4-dihydroxyphenylalanine (L-DOPA) (potent allelochemicals) treatments, respectively.
Tissue viability was inhibited up to 100 µM then increased in response to 100 µM FA. TTC reduction involves acceptance of electrons from mitochondrial electron transport chain by viable tissue, correlates directly with respiration (Comas et al. Citation2000), and thus provides an indirect measure of cellular respiration (Maness et al. Citation1999). Decreased cell viability (i.e. TTC reduction) indicates a decrease in respiration due to FA-induced stress and thus lesser availability of precursors for growth and development. However, increased value at 1000 µM FA suggests an enhanced respiratory activity that is possibly required to provide extra energy to counter severe stress induced by FA.
In the present study, the activity of PPOs increased significantly in mung bean hypocotyls in response to FA treatment; PPOs are copper-containing oxido-reductase enzymes localized in thylakoids of plastids in plants (Vaughn et al. Citation1988). PPOs are responsible for phenolic metabolism during the adventitious rooting formation (Hahlbrock & Grisebach Citation1979) and their activity increases under stress (Mohammadi & Kazemi Citation2002). Endogenous TP and PPO activities have inverse relationships during morphogenetic rooting response (Nag et al. Citation2001). Enhanced PPOs activity indicates a greater utilization of endogenous TP and root formation (Nag et al. Citation2001). In the present study, TP content decreased in mung bean hypocotyls upon FA treatment, except at 1000 µM concentration. The decreased PPO activity at 1000 µM FA was paralleled by increased TP content thus indicating their nonutilization resulting in cessation of rooting. Since PPO activity increased during stress, a decreased activity at 1000 µM FA indicates FA-induced stress. In fact, during rhizogenesis the endogenous TP content decreases due to their utilization as a cofactor to form a complex with indole-3-acetic acid (IAA) leading to root induction. In spite of a similar observation in the present study, there was a severe reduction in the rooting in mung bean hypocotyls. It indicates a possible interference of FA with the auxin activity/metabolism since auxins are essential for formation of lateral and adventitious rooting (Aloni et al. Citation2006). However, our study did not provide a direct evidence for such a negative interference of FA with auxins and it needs further exploration. Anyhow, a few studies in the past have demonstrated the negative regulation of auxins by flavonoids like apigenin and naringenin, a group of secondary metabolites related to phenolics (Brown et al. Citation2001; Imin et al. Citation2007). Earlier studies have established that basipetal auxin transport is essential for the formation of adventitious rooting (Aloni et al. Citation2006) and flavonoids inhibit rooting through the impairment of polar auxin transport (Brown et al. Citation2001). No such observation has been made with phenolic acids, in general, and with FA, in particular. Nevertheless, phenolic acids interfere with the activity of plant growth regulators like auxins and cytokinins (Einhellig Citation2004). Further, we did not explore whether the observed reduction in the rooting of mung bean hypocotyls involve any induction of oxidative stress through ROS generation, since phenolics have been reported to affect plant growth through oxidative stress induction (Weir et al. Citation2004; Oracz et al. Citation2007; Singh et al. Citation2009).
The study concludes that FA affects root growth and development, and rooting process in mung bean hypocotyls under in vitro conditions by interfering with biochemical processes that are crucial for root formation.
References
- Aloni R, Aloni E, Langhans M, Ullrich CI. 2006. Role of cytokinin and auxin in shaping root architecture: Regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann Bot. 97:888–893. 10.1093/aob/mcl027
- Baleroni CRS, Ferrarese MLL, Souza NE, Ferrarese-Filho O. 2000. Lipid accumulation during canola seed germination in response to cinnamic acid derivatives. Biol Plant. 43:313–316. 10.1023/A:1002789218415
- Barkosky RR, Einhellig FA, Butler J. 2000. Caffeic acid-induced changes in plant-water relationships and photosynthesis in leafy spurge (Euphorbia esula). J Chem Ecol. 26:2095–2109. 10.1023/A:1005564315131
- Basha SMM, Beevers L. 1975. The development of proteolytic activity and protein degradation during germination of Pisum sativum L. Planta. 124:77–87. 10.1007/BF00390070
- Batish DR, Gupta P, Singh HP, Kohli RK. 2006. L-DOPA (L-3,4-dihydroxyphenylalanine) affects rooting potential and associated biochemical changes in hypocotyl of mung bean, and inhibits mitotic activity in onion root tips. Plant Growth Regul. 49:229–235. 10.1007/s10725-006-9114-6
- Batish DR, Singh HP, Kaur S, Kohli RK, Yadav SS. 2008. Caffeic acid affects early growth, and morphogenetic response of hypocotyl cuttings of mung bean (Phaseolus aureus). J Plant Physiol. 165:297–305. 10.1016/j.jplph.2007.05.003
- Baziramakenga R, Leroux GD, Simard RR. 1995. Effects of benzoic and cinnamic acids on membrane permeability of soybean roots. J Chem Ecol. 21:1271–1285. 10.1007/BF02027561
- Blum U. 2005. Relationships between phenolic acid concentrations, transpiration, water utilization, leaf area expansion, and uptake of phenolic acids: nutrient culture studies. J Chem Ecol. 31:1907–1932. 10.1007/s10886-005-5934-5
- Booker FL, Blum U, Fiscus EL. 1992. Short-term effects of ferulic acid on ion uptake and water relations in cucumber seedlings. J Exp Bot. 43:649–655. 10.1093/jxb/43.5.649
- Boudet AM. 2007. Evolution and current status of research in phenolic compounds. Phytochemistry. 68:2722–2735. 10.1016/j.phytochem.2007.06.012
- Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, Taiz L, Muday GK. 2001. Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol. 126:524–535. 10.1104/pp.126.2.524
- Caspersen S, Sundin P, Munro M, Aðalsteinsson S, Hooker JE, Jensen P. 1999. Interactive effects of lettuce (Lactuca sativa L.), irradiance, and ferulic acid in axenic, hydroponic culture. Plant Soil. 210:115–126. 10.1023/A:1004682018888
- Comas LH, Eissenstat DM, Lasko AN. 2000. Assessing root death and root system dynamics in a study of grape canopy pruning. New Phytol. 147:171–178. 10.1046/j.1469-8137.2000.00679.x
- Devi SR, Prasad MNV. 1996. Ferulic acid mediated changes in oxidative enzymes of maize seedlings: Implications in growth. Biol Plant. 38:387–395. 10.1007/BF02896668
- Einhellig FA. 2004. Mode of allelochemical action of phenolic compounds. In: Macias FA, Galindo JCG, Molinillo JMG, Cutler HG, editors. Allelopathy – chemistry and mode of action of allelochemicals. Boca Raton, FL: CRC Press; p. 217–238.
- Esmaeili M, Heidarzade A, Pirdashti H, Esmaeili F. 2012. Inhibitory activity of pure allelochemicals on barnyardgrass (Echinochloa crus-galli L.) seed and seedling parameters. Int J Agri Crop Sci. 4:274–279.
- Günes T. 2000. Peroxidase and IAA-oxidase activities during rooting in cuttings of three poplar species. Turk J Bot. 24:97–101.
- Hadacek F. 2002. Secondary metabolites as plant traits: current assessment and future perspectives. Crit Rev Plant Sci. 21:273–322. 10.1080/0735-260291044269
- Hahlbrock K, Grisebach H. 1979. Enzymatic controls in the biosynthesis of lignin and flavonoids. Annu Rev Plant Physiol. 30:105–136. 10.1146/annurev.pp.30.060179.000541
- Herrig V, Ferrarese MLL, Suzuki LS, Rodrigues JD, Ferrarese-Filho O. 2002. Peroxidase and phenylalanine ammonia-lyase activities, phenolic acid contents, and allelochemicals-inhibited root growth of soybean. Biol Res. 35:59–66. 10.4067/S0716-97602002000100009
- Hussain MI, Reigosa MJ. 2011. Allelochemical stress inhibits growth, leaf water relations, PSII photochemistry, non-photochemical fluorescence quenching, and heat energy dissipation in three C3 perennial species. J Exp Bot. 62:4533–4545. 10.1093/jxb/err161
- Imin N, Nizamidin M, Wu T, Rolfe BG. 2007. Factors involved in root formation in Medicago truncatula. J Exp Bot. 58:439–451. 10.1093/jxb/erl224
- Kaur S, Singh HP, Batish DR, Kohli RK. 2011. Chemical characterization and allelopathic potential of volatile oil of Eucalyptus tereticornis against Amaranthus viridis. J Plant Inter. 6:297–302.
- Kollárová K, Henselová M, Liškoá D. 2005. Effect of auxins and plant oligosaccharides on root formation and elongation growth of mung bean hypocotyls. Plant Growth Regul. 46:1–9. 10.1007/s10725-005-5185-z
- Kuiters AT. 1990. Role of phenolic substances from decomposing forest litter in plant–soil interactions. Acta Bot Neerl. 39:329–348.
- Li HH, Inove M, Nishimura H, Mizutani J, Tsuzuki E. 1993. Interactions of trans-cinnamic acid, its related phenolic allelochemicals, and abscissic acid in seedling growth and seed germination of lettuce. J Chem Ecol. 19:1175–1787.
- Li Z-H, Wang Q, Ruan X, Pan C-De, De-An J. 2010. Phenolics and plant allelopathy. Molecules. 15:8933–8952. 10.3390/molecules15128933
- Lowry OH, Rosebrough NJ, Farr AL, Rendall RJ. 1951. Protein estimation with folin-phenol reagent. J Biol Chem. 193:265–275.
- Malik CP, Singh MB. 1980. Plant enzymology and histo-enzymology – a text manual. New Delhi, India: Kalyani Publishers.
- Maness P-C, Smolinski S, Blake DM, Huang Z, Wolfrum EJ, Jacoby WA. 1999. Bactericidal activity of photocatalytic TiO2 reaction: toward an understanding of its killing mechanism. Appl Environ Microbiol. 65:4094–4098.
- Mohammadi M, Kazemi H. 2002. Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. Plant Sci. 162:491–498. 10.1016/S0168-9452(01)00538-6
- Mutlu S, Atici O. 2009. Allelopathic effect of Nepeta meyeri Benth. extracts on seed germination and seedling growth of some crop plants. Acta Physiol Plant. 31:89–93. 10.1007/s11738-008-0204-0
- Nag S, Saha K, Choudhuri MA. 2001. Role of auxin and polyamines in adventitious root formation in relation to changes in compounds involved in rooting. J Plant Growth Regul. 20:182–194. 10.1007/s003440010016
- Oracz K, Bailly C, Gniazdowska A, Come D, Corbineau F, Bogatek R. 2007. Induction of oxidative stress by sunflower phytotoxins in germinating mustard seeds. J Chem Ecol. 33:251–264. 10.1007/s10886-006-9222-9
- Politycka B, Kozłowska M, Mielcarz B. 2004. Cell wall peroxidases in cucumber roots induced by phenolic allelochemicals. Allelopathy J. 13:29–36.
- Rice EL. 1984. Allelopathy. Orlando, FL: Academic Press.
- Sánchez M, Peña MJ, Revilla G, Zarra I. 1996. Changes in dehydrodiferulic acids and peroxidase activity against ferulic acid associated with cell walls during growth of Pinus pinaster hypocotyls. Plant Physiol. 111:941–946.
- Sato Y, Sugiyama M, Gorecki RJ, Fukuda H, Komamine A. 1993. Interrelationship between lignin deposition and the activities of peroxidase isozymes in differentiating tracheary elements of Zinnia. Planta. 189:584–589. 10.1007/BF00198223
- Shen HC, Zhou WJ, Xi HF, Ye QF. 1991. A preliminary study of physiological and yield effects of paclobutrazol on Brassica napus. J Zhejiang Agric Univ. 17:423–426.
- Singh HP, Batish DR, Kaur S, Setia N, Kohli RK. 2005. Effects of 2-benzoxazolinone on the germination, early growth and morphogenetic response of mung bean (Phaseolus aureus). Ann Appl Biol. 147:267–274. 10.1111/j.1744-7348.2005.00031.x
- Singh HP, Batish DR, Kohli RK. 2003. Allelopathic interactions and allelochemicals: new possibilities for sustainable weed management. Crit Rev Plant Sci. 22:239–311. 10.1080/713610858
- Singh HP, Kaur S, Batish DR, Kohli RK. 2009. Caffeic acid inhibits in vitro rooting in mung bean [Vigna radiata (L.) Wilczek] hypocotyls by inducing oxidative stress. Plant Growth Regul. 57:21–30. 10.1007/s10725-008-9314-3
- Swain T, Hillis WE. 1959. The phenolic constituents of Prunus domestica I. The quantitative analysis of constituents. J Sci Food Agric. 10:63–68. 10.1002/jsfa.2740100110
- van Lelyveld LJ, Pretorius W. 1973. Assay methods for determining enzymatic activity of α-amylase, indole-3-acetic acid oxidase, polyphenol oxidase, peroxidase and ascorbic acid oxidase in a crude extract from avocado tree bark. Agrochemophysica. 51:29–34.
- Vaughn KC, Lax AR, Duke SO. 1988. Polyphenol oxidase: the chloroplast oxidase with no established function. Physiol Plant. 72:659–665. 10.1111/j.1399-3054.1988.tb09180.x
- Weir TL, Park SW, Vivanco JM. 2004. Biochemical and physiological mechanisms mediated by allelochemicals. Curr Opin Plant Biol. 7:472–479. 10.1016/j.pbi.2004.05.007

