Abstract
A pot experiment was conducted to study the effects of sodium nitroprusside (SNP, a nitric oxide [NO] donor) on cadmium (Cd) toxicity in peanut plants. SNP solution was poured into Cd-contaminated soil at sowing, seedling, flowering, and pod setting stages, respectively. The results showed that exogenous NO increased the biomass and yield of peanuts and improved chlorophyll content, photosynthesis (Pn), and transpiration (Tr). Cd-induced oxidative damages were eliminated by exogenous NO, reflected by decreased accumulation of superoxide anion (), hydrogen peroxide (H2O2), and malondialdehyde (MDA). Moreover, exogenous NO increased Cd-decreased activities of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and soluble protein and proline contents also reached a normal level. Furthermore, exogenous NO improved the uptake of nutrient elements and reduced Cd accumulation in the roots, shoots, and kernels under Cd stress. These data suggest that SNP application at sowing and seedling stages had better alleviation effects on Cd toxicity in peanut plants.
| Abbreviations | ||
| CAT | = | catalase |
| Cd | = | cadmium |
| H2O2 | = | hydrogen peroxide |
| MDA | = | malondialdehyde |
| NO | = | nitric oxide |
|
| = | superoxide anion radical |
| POD | = | peroxidase |
| ROS | = | reactive oxygen species |
| SNP | = | sodium nitroprusside |
| SOD | = | superoxide dismutase |
1. Introduction
Cadmium (Cd) is a heavy metal widely recognized as a very dangerous environmental pollutant due to its high toxicity to both plants and animals (Xiong et al. Citation2010; Magdalena et al. Citation2011). It can be readily absorbed by plant roots and concentrated by many cereals, potatoes, vegetables, and fruits (Wagner Citation1993). Daily consumption of crop products containing high Cd level results in increased toxic accumulation in organs, such as the kidneys and liver, and may impair their functions (Zhang et al. Citation2011). Numerous experimental studies have demonstrated the highly toxic effects of Cd on many plant metabolic processes such as photosynthesis (Pn), respiration, nitrogen metabolism, transpiration (Tr), and nutrient uptake (Sanita di Toppi & Gabbrielli Citation1999; Clemens Citation2006). At the cellular level, Cd has numerous negative effects, such as membrane distortion, production of reactive oxygen species (ROS), inhibition of enzyme activities, and ion leakage (Romero-Puertas et al. Citation2004; Hsu & Kao Citation2007). To survive against Cd stress, plants have evolved protective mechanisms of mitigating and repairing its damages, including increased antioxidant capacity, metal exclusion, active excretion, restricted distribution of toxic metal in sensitive tissue, metal binding to the cell wall, chelation by organic molecules, compartmentalization in vacuoles, and the regulation of root cell wall composition (Wang et al. Citation2008; Xiong et al. Citation2009).
Efforts have been made to develop practical techniques for alleviating the Cd stress on plants. One of the strategies is to develop or select the species or genotypes with high Cd tolerance. Moreover, the application of some plant growth regulators also plays important roles in activating the defense system of the plants against Cd stress, such as supply with salicylic acid (SA), jasmonic acid (JA), abscisic acid (ABA), gibberellins (GA), silicon (Si), and nitric oxide (NO). As a gaseous signaling molecule in plants, NO plays significant roles in regulating many crucial physiological processes (Besson-Bard et al. Citation2008). Sodium nitroprusside (SNP) was often used as a source of NO in many studies. The effects of exogenous NO alleviating Cd toxicity were reported in different plant species. For instance, Cd-induced oxidative stress on rice and sunflower leaves was alleviated by the addition of exogenous NO (Hsu & Kao Citation2004; Laspina et al. Citation2005). Similarly, Cd-induced oxidative stress on wheat roots was alleviated by supplementation with exogenous NO (Singh et al. Citation2008). Xiong et al. (Citation2009) demonstrated that exogenous NO enhanced Cd tolerance of rice by increasing the pectin and hemicellulose content in the cell wall of roots. In addition, our previous study also indicated that NO supply can alleviate Cd toxicity by increasing antioxidant capacity, improving uptake of nutrition elements, and restricting Cd translocation from roots to shoots in perennial ryegrass (Wang et al. Citation2013).
In China, peanut is a kind of edible food, oilseed crop, and raw material used as food additives, with a high nutritional and economic value. In the shortage of protein and serious cases of oil supply security, peanut is expected to be of more use in the future (Wan Citation2003). It is known that peanut is one of four major oil crops with high capacity to tolerate Cd stress and can accumulate high Cd level in vegetative parts and seeds (McLaughlin et al. Citation2000; Shi & Cai Citation2009). Some phosphorus and trace element fertilizers may contain elevated amounts of Cd and repeated uses of the fertilizers at high rates over time may increase Cd uptake by plants (Huang et al. Citation2003). A wide use of phosphorous fertilizers could accumulate Cd in the peanut field. Therefore, it is necessary to find an approach to alleviate Cd toxicity in peanut plants and decrease Cd accumulation in peanut seeds. To our knowledge, most of the studies on exogenous NO alleviating Cd toxicity were carried out in a hydroponic experiment. However, little knowledge is reported regarding the effects of application of exogenous NO in Cd-contaminated soil on plant growth and development in the whole growth period, and further, or how the growing plants cope with Cd toxicity through physiological and biochemical adaptations.
In this study, we poured SNP solution in Cd-contaminated soil to supply NO for peanut plants. SNP solution was supplied at sowing, seedling, flowering, and pod setting stages, respectively. This paper reported the toxic effects of Cd on the growth and development in peanut plants, the responses of plant to Cd stress, and the effects of application of exogenous NO at different growth stages on Cd toxicity in peanut plants. The aim of this study is to understand the mechanism of Cd detoxification in peanut plants, and explore the proper growth stages of supplying NO to promote plant growth in the fields with Cd contamination.
2. Materials and methods
2.1. Plant materials, growth, and treatment conditions
A pot experiment was conducted on brown clay loam soils (a soil group in Chinese genetic soil classification; named Hapli-Udic Argosols in Chinese soil taxonomy), at the College of Resources and Environment, Shandong Agricultural University, China. Soil in pots was taken from uncontaminated topsoil (0–20 cm) in Shandong Agricultural University's experimental field. The soil contains 14.1 g kg−1 organic matter, 0.622 g kg−1 total nitrogen, 46.5 mg kg−1 available nitrogen, 22.5 mg kg−1 available phosphorus, and 106.1 mg kg−1 available potassium. Soil subsamples were mixed with appropriate amounts of analytical grade solid CdCl2 in order to add 50 mg kg−1 Cd of soil (oven-dry basis). Basal fertilizers applied were compound fertilizer, which were provided by the Shandong Kingenta Ecological Engineering Co., Ltd. The nutrient content of compound fertilizer was 15% N, 15% P2O5, and 15% K2O. The mass of the fertilizer applied in each pot was 2.0 g, and the fertilizer was thoroughly mixed with the soil. Every pot (diameter: 30 cm and height: 40 cm) was full with 7.5 kg of soil.
Peanut seeds (Arachis hypogaea L.) were surface sterilized with 0.5% (v/v) sodium hypochlorite and washed with distilled water, then sowed on 28 April 2012. Seeds were sown in plastic pots (eight plants per pot). The pots were arranged in randomized block designs with three replicates. After the seedlings emerged, the pots were lessened to five seedlings per pot. During the growing season, plants were managed under commonly used agronomic and irrigation practices.
The experimental design is given as follows: CK, no Cd contaminated soil; Cd, 50 mg kg−1 Cd-contaminated soil; S1, SNP solutions (200 µM, 50 mL) were poured into 50 mg kg−1 Cd-contaminated soil at sowing stage; S2, SNP solutions (200 µM, 50 mL) were poured into 50 mg kg−1 Cd-contaminated soil at seedling stage; S3, SNP solutions (200 µM, 50 mL) were poured into 50 mg kg−1 Cd-contaminated soil at flowering stage; S4, SNP solutions (200 µM, 50 mL) were poured into 50 mg kg−1 Cd-contaminated soil at pod setting stage. SNP was used as a donor of NO. At every growth stage, SNP was made into 200 µM of SNP solution in the lab. Before the SNP solution (200 µM, 50 mL) was poured into soil, it was stored in the dark, and the time interval of twice pour actions was five days.
2.2. Determination of chlorophyll content
The chlorophyll content was determined according to the method of Knudson et al. (Citation1977). Young leaves were taken at the pod setting stage. Fresh leaves (0.5 g of fresh weight) were extracted in 2 mL 95% ethanol for 24 h in the dark, and the extracted solution was analyzed. The amounts of chlorophyll a, chlorophyll b, and carotenoids were determined spectrophotometrically (SHIMADZU UV-2450, Kyoto, Japan), by reading the absorbance at 665, 649, and 470 nm, respectively. The chlorophyll content results are expressed as milligram per gram fresh weight (mg g−1 FW).
2.3. Determinations of Pn and Tr
Net Pn and Tr were monitored in vivo with a portable Pn system (LI-6400, LICOR, Lincoln, NB, USA). Measurements were made on three plants, in three replications per plant. The leaves were fully formed and located in the upper third part of the crown, oriented toward the south. Measurements were done in sunny and clear weather, in the period between 09:00 and 11:00 h. All the measurements were taken at a constant airflow rate of 500 µmol s−1 and at saturation irradiance with incident photosynthetic photon flux density of 1200 µmol m−2 s−1. The ambient CO2 concentration was approximately 350 cm3 m−3 and the temperature was approximately 25°C.
2.4. Determination of the  generation rate
generation rate
For the measurement of the generation rate, fresh leaf samples (0.3 g) were ground in liquid N2 and extracted in 3 mL of ice-cold 50 mM sodium phosphate buffer (PBS) (pH 7.0). The
generation rate was determined by monitoring the A530 of the production of the hydroxylamine reaction following the modified method as described by He et al. (Citation2005). One milliliter supernatant of fresh leaf extraction was added to 0.9 mL 65 mM phosphate buffer solution (pH 7.8) and 0.1 mL 10 mM hydroxyl ammonium chloride. The reaction was incubated at 25°C for 35 min. A 0.5 mL solution from the above reaction mixture was then added in 0.5 mL 17 mM sulfanic acid and 0.5 mL 7.8 mM α-naphthylamine solution. After 20 min of reaction, 2 mL of ether was added into the above solution, and then mixed well. The solution was centrifuged at 1500×g at 4°C for 5 min. The absorbance of the pink supernatant was measured at 530 nm with the spectrophotometer. Absorbance values were calibrated to a standard curve generated with known concentrations of HNO2.
2.5. Determination of H2O2 concentration
Fresh samples (1.0 g) were homogenized in 2 mL ice-cold acetone. Titanium reagent (2% TiCl2 in conc. HCl) was added to a known volume of extract supernatant to give a Ti (IV) concentration of 2%. The Ti–H2O2 complex, together with unreacted Ti, was then precipitated by adding 0.2 mL 17 M ammonia solution for each 1 mL of extract. The precipitate was washed five times with ice acetone by resuspension, drained, and dissolved in 1 M H2SO4 (3 mL). The absorbance of the solution was measured at 410 nm against blanks, which had been prepared similarly but without plant tissue (Patterson et al. Citation1984).
2.6. Assay of antioxidant enzyme activities
For extraction of antioxidant enzymes, leaves and roots were homogenized with 50 mM Na2HPO4–NaH2PO4 buffer (pH 7.8) containing 0.2 mM EDTA and 2% insoluble polyvinylpyrrolidone (PVP) in a chilled pestle and mortar. The homogenate was centrifuged at 12,000×g for 20 min and the resulted supernatant was used for the determination of enzyme activities. The whole extraction procedure was carried out at 4°C. Protein content was estimated according to the method of Bradford (Citation1976). Spectrophotometric analysis was conducted on a SHIMADZU UV-2450 spectrophotometer (Kyoto, Japan).
Superoxide dismutase (SOD) (EC 1.15.1.1) activity was measured in terms of its capacity to inhibit photochemical reduction of nitroblue tetrazolium (NBT), as described by Giannopolitis and Ries (Citation1977). The reaction mixture (3 mL) contained 50 mM phosphate buffer (pH 7.8), 0.1 mM EDTA, 130 mM methionine, 0.75 mM NBT, 0.02 mM riboflavin, and 0.1 mL enzyme extract. Riboflavin was added as the last component and the reaction was initiated by placing the tubes under two 20 W fluorescent lamps. The reaction was terminated after 10 min by removing the reaction tubes from the light source. Nonilluminated and illuminated reactions without supernatant served as calibration standards. Absorbance of the reaction mixture was read at 560 nm. One unit of SOD activity (U) was defined as the amount of enzyme required to cause 50% inhibition of the NBT photoreduction rate.
Analysis of guaiacol peroxidase (POD, EC 1.11.1.7) capacity was based on the oxidation of guaiacol using hydrogen peroxide (Zhang & Kirham Citation1994). The enzyme extract (0.02 mL) was added to the reaction mixture containing 0.02 mL guaiacol solution and 0.01 mL hydrogen peroxide solution in 3 mL of phosphate buffer solution (pH 7.0). The addition of enzyme extract started the reaction and the increase in absorbance was recorded at 470 nm for 5 min. Enzyme activity was quantified by the amount of tetraguaiacol formed using its molar extinction coefficient (26.6 mM−1 cm−1).
Catalase (CAT, EC 1.11.1.6) activity was determined using the method of Cakmak and Marschner (Citation1992), with minor modifications. The reaction solution (3 mL) consisted of 100 Mm phosphate buffer (pH 7.0), 0.1 µM EDTA, 0.1% H2O2, and 0.1 mL enzyme extract. The reaction was initiated by adding the enzyme extract. The decrease of H2O2 was monitored at 240 nm for at least 3 min and quantified by its molar extinction coefficient (36 M−1 cm−1).
2.7. Determination of lipid peroxidation
Lipid peroxidation was determined by measuring malondialdehyde (MDA), a major thiobarbituric acid reactive species (TBARS) and product of lipid peroxidation (Heath & Packer Citation1968). Samples (0.2 g) were ground in 3 ml of trichloroacetic acid (TCA) (0.1%, w/v). The homogenate was centrifuged at 10,000×g for 10 min and 1 mL of the supernatant fraction was mixed with 4 mL of 0.5% (w/v) thiobarbituric acid (TBA) in 20% (w/v) TCA. The mixture was heated at 95°C for 30 min, chilled on ice, and then centrifuged at 10,000×g for 5 min. The absorbance of the supernatant was measured at 532 nm. The value for nonspecific absorption at 600 nm was subtracted. The amount of MDA was calculated using the extinction coefficient of 155 mM−1 cm−1 and expressed as nmol g−1 FW.
2.8. Soluble protein and proline assay
Proteins were estimated by Bradford's (Citation1976) method. Fresh leaves (0.5 g) were homogenized in 1 mL phosphate buffer (pH 7.0). The crude homogenate was centrifuged at 5000×g for 10 min. Half milliliter of freshly prepared TCA was added and centrifuged at 8000×g for 15 min. The debris was dissolved in 1 mL of 0.1 N NaOH and 5 mL of Bradford's reagent was added. Absorbance was recorded photometrically at 595 nm (SHIMADZU UV-2450, Japan) using bovine serum albumin as a standard.
Proline concentration was determined using the method of Bates et al. (Citation1973). Fresh leaves (0.3 g) were homogenized in 10 mL of 30% aqueous sulphosalicylic acid. The homogenate was centrifuged at 9000×g for 15 min. A 2 mL aliquot of the supernatant was mixed with an equal volume of acetic acid and acid ninhydrin (1.25 g ninhydrin in 30 mL acetic acid and 20 mL of 6N H3PO4) and incubated for 1 h at 100°C. The reaction was terminated in an ice bath and extracted with 4 mL of toluene. The extract was vortexed for 20 s and the chromatophore-containing toluene was aspirated from the aqueous phase and absorbance determined photometrically at 520 nm (SHIMADZU UV-2450, Japan) using toluene for a blank.
2.9. Determination of Cd and mineral element concentrations
At harvest, the roots, shoots, and kernels were separated and oven-dried for 30 min at 105°C, then at 70°C till the materials reach their constant weights. The dried tissues were weighed and ground into powder for the determination of Cd and mineral element concentrations, which was measured by the flame atomic absorbance spectrometry (SHIMADZU AA-6300, Kyoto, Japan) after digestion with mixed acid [HNO3+HClO4 (3:1, v/v)] (Wang et al. Citation2013).
2.10. Statistical analysis
Statistical analyses were carried out by analysis of variance (ANOVA) using SAS software (SAS Institute, Cary, NC). Differences between treatments were separated by the least significant difference (LSD) test at a 0.05 probability level.
3. Results
3.1. Biomass and yield
shows the biomass, yield, and water content of peanut at harvest time. Compared with CK, the reduction of root and shoot biomass grown in 50 mg kg−1 Cd soil was 38.5% and 15.6%, respectively. Yield, kernel yield, and pods of Cd-treated plants were decreased by 29.6%, 31.7%, and 30.0%, respectively. In addition, Cd stress decreased the water content in roots and shoots significantly. Addition of exogenous NO at sowing, seedling, and flowering stages improved Cd-induced decrease of the biomass, yield, and water content, especially supplying NO at sowing and seedling stages. However, the application of NO at pod setting stage did not have an obvious effect on plant biomass, yield, and water content.
Table 1. Effects of the application of exogenous NO at different growth stages (S1, sowing stage; S2, seedling stage; S3, flowering stage; S4, pod setting stage) on biomass, yield, and water content of peanut plants grown in 50 mg kg−1 Cd-contaminated soil at harvested time.
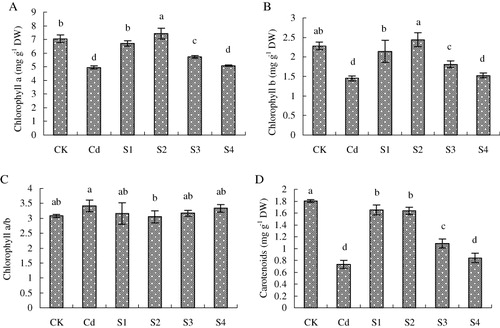
3.2. Chlorophyll content
At pod setting stage, Cd-treated plants showed a significant decrease in contents of chlorophyll a, chlorophyll b, and carotenoids, while chlorophyll a/b was not affected significantly (). SNP solutions added in soil at sowing, seedling, and flowering stages increased Cd-decrease in chlorophyll a, chlorophyll b, and carotenoids contents, especially the application of exogenous NO at seedling stage. NO supplementation at seedling stage increased chlorophyll a, chlorophyll b, and carotenoids by 50.1%, 68.3%, and 124.7%, respectively, compared with Cd treatment. However, SNP addition at pod setting stage had no effect on the chlorophyll contents of plants grown in Cd-contaminated soil. In addition, compared with CK, SNP supplementation at the four growth stages did not obviously change chlorophyll a/b.
3.3. Photosynthesis and Transpiration
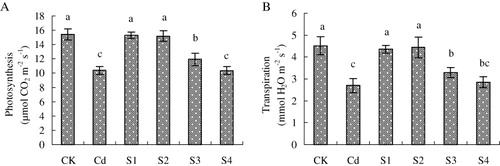
Nutrient uptake came to a climax at pod setting stage, when the pegs interposed into the soil to have pod, and the Pn was most vigorous (Zhang et al. Citation2012). shows Pn and Tr of peanut at pod setting stage. Cd stress induced an obvious decrease of Pn and Tr as relative to the normal plants. The decrease in Pn and Tr was alleviated by the addition of NO at sowing, seedling, and flowering stages significantly, especially NO was supplied at sowing and seedling stages. However, the addition of NO at pod setting stage had no obvious change on Pn and Tr compared with Cd treatment.
3.4.  production rate, H2O2accumulation, and MDA content
production rate, H2O2accumulation, and MDA content
shows production rate, H2O2 accumulation, and MDA content of peanut leaves at pod setting and maturity stages. At pod setting stage, Cd stress enhanced the
production rate compared with CK (). Addition of exogenous NO at sowing and seedling stages decreased Cd-induced production rate of
significantly. However, NO supplied at pod setting stage did not reduce Cd-increased
production rate. Similar trends of
production rate were detected at maturity stage.
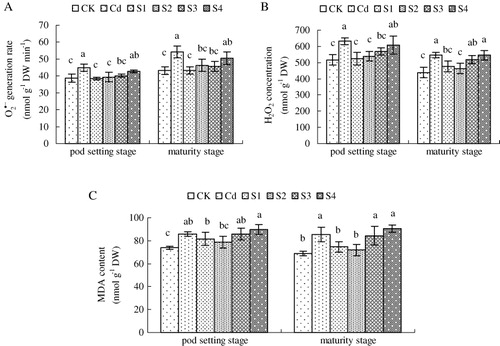
Compared with the control, Cd stress caused a significant increase in H2O2 accumulation at pod setting and maturity stages (). Addition of exogenous NO at sowing and seedling stages decreased Cd-induced accumulation of H2O2 at pod setting and maturity stages. Compared with Cd treatment, NO supplementation at flowering stage reduced H2O2 accumulation at pod setting stage but had no change at maturity stage. In addition, NO supplied at pod setting stage did not inhibit Cd-caused increase in H2O2 accumulation at pod setting and maturity stages.
MDA, a product of lipid peroxidation, has been considered an indicator of oxidative damage. MDA content was investigated at pod setting and maturity stages (). Cd stress induced MDA accumulation at pod setting and maturity stages compared with CK. At pod setting stage, the application of exogenous NO at sowing, seedling, flowering, and pod setting stages did not decrease Cd-induced MDA accumulation. At maturity stage, compared with Cd-stressed plants, SNP supplementation at sowing and seedling stages inhibited MDA content. However, the addition of exogenous NO at flowering and pod setting stages had no effect on MDA accumulation.
3.5. Antioxidant enzymes
Activities of several representative antioxidant enzymes, including SOD, POD, and CAT in peanut seedlings were determined to assess the physiological mechanism of exogenous NO in regulation of these antioxidant enzymes upon Cd stress. As shown in , compared with CK, Cd stress decreased SOD, POD, and CAT activities dramatically when investigating SOD activity at pod setting and maturity stages. However, the application of exogenous NO at sowing and seedling stages increased Cd-decreased SOD activity of peanut plants (). In addition, SNP solution supplied at flowering and pod setting stages had no obvious effect on increasing Cd-decreased activity of SOD at pod setting and maturity stages.
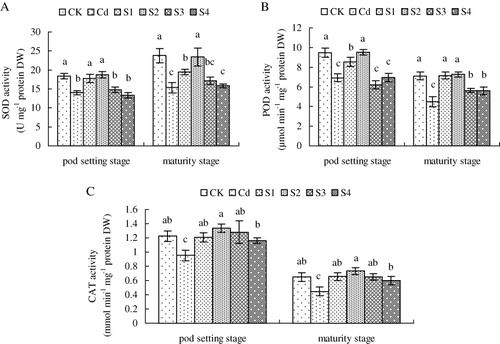
At pod setting stage, SNP supplementation at sowing and seedling stages enhanced Cd-decreased activity of POD activity, while the addition of exogenous NO at flowering and pod setting stages did not improve Cd-inhibited POD activity (). When investigating POD activity at maturity stage, the application of exogenous NO at sowing, seedling, flowering, and pod setting stages increased POD activity compared with Cd treatment, especially at seedling stage.
The application of exogenous NO at sowing, seedling, flowering, and pod setting stages increased CAT activity dramatically compared with Cd-treated plants, especially at seeding stage ().
3.6. Soluble protein and proline
The Cd treatment declined the soluble protein content of peanut plants to 54.0% and 56.5% at pod setting and maturity stages compare with CK, respectively (). SNP solutions added in soil at sowing, seedling, and flowering stages increased the content of soluble protein at pod setting stage significantly. However, the application of exogenous NO at pod setting stage did not increase Cd-decreased content of soluble protein. At maturity stage, NO application at sowing and seedling stages had obvious effects on increasing soluble protein content compared with Cd treatment. However, the addition of NO at flowering and pod setting stages did not improve the content of soluble protein.
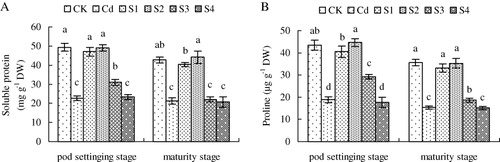
As shown in , at pod setting and maturity stages, Cd stress decreased proline content significantly, compared with CK. The application of exogenous NO at sowing, seedling, and flowering stages increased Cd-decreased content of proline dramatically, especially at seedling stage. However, SNP supplementation at pod setting did not improve proline content compared with Cd treatment.
3.7. P, K, Ca, and Fe absorption
At maturity stage, P, K, Ca, and Fe concentrations in the leaves and roots were measured (). Cd stress decreased P, K, Ca, and Fe concentrations in the leaves and roots significantly compared with CK. NO addition at sowing, seedling, and flowering stages had effects on improving P and K contents in leaves and roots under Cd stress. However, the application of NO at the pod setting stage did not obviously change absorption of P and K. The addition of NO at sowing, seedling, flowering, and pod setting stages had obvious positive effects on increasing Ca concentration in leaves and roots under Cd stress. Fe concentration in leaves and roots were significantly increased by the addition of NO at sowing and seedling stages. In addition, NO supply at flowering stage increased Fe content in leaves. However, the addition of NO at pod setting stage did not increase Fe absorption.
Table 2. Effects of the application of exogenous NO at different growth stages (S1, sowing stage; S2, seedling stage; S3, flowering stage; S4, pod setting stage) on the contents of mineral nutrients (P, K, Ca, and Fe) in roots and leaves of peanut grown in 50 mg kg−1 Cd-contaminated soil. The contents were investigated at maturity stages.
3.8. Cd accumulation in the roots, shoots, and seeds
Cd accumulation in the roots, shoots, and seeds were measured at harvest time of plants (). Cd treatment dramatically increased Cd concentration in the roots, shoots, and seeds compared with CK. In root tissue, NO addition at sowing and seedling stages reduced Cd absorption by the roots. However, NO supplementation at flowering and pod setting stages had no effect on Cd content. In the shoots, the addition of NO at the four stages decreased Cd concentration effectively. In seeds, SNP supplementation at sowing, seedling, and flowering stages had an obvious effect on inhibiting Cd accumulation. However, the application of NO at pod setting stage did not diminish Cd accumulation in the seeds.
Table 3. Effects of the application of exogenous NO at different growth stages (S1, sowing stage; S2, seedling stage; S3, flowering stage; S4, pod setting stage) on Cd concentration in the roots, shoots and kernels of peanut grown in 50 mg kg−1 Cd-contaminated soil. The contents were investigated at harvest time.
4. Discussion
Increasing evidence indicates that exogenous NO plays important roles in plant tolerance to Cd stress (Hsu & Kao Citation2004; Laspina et al. Citation2005; Xiong et al. Citation2009; Wang et al. Citation2013). In our lab, Zhang et al. (Citation2012) supplied SNP (a donor of NO) for peanut plants growing on calcareous soil and their study found that the SNP added into the soil effectively increased peanut tolerance to Fe deficiency stress. In addition, Gao et al. (Citation2011) demonstrated that SNP solutions poured into Cd-contaminated soil alleviated Cd toxicity in seedlings of Malus hupehensis Rehd. under continuous cropping. In this study, we also supplied NO for peanut grown in Cd-contaminated soil by adding SNP solution in soil, and exogenous NO was applied at different growth stages of peanut. By investigating the physiological characteristics of peanut, we detected that the effect of the application of NO at different growth stages varied. The addition of NO at sowing and seedlings stages had better alleviation effects.
Cd stress induced detrimental effects on plant growth and development, just as reported by Wang et al. (Citation2008) on Bechmeria nivea (L.) Guad, Qiu et al. (Citation2013) about wheat seedlings, and Wang et al. (Citation2013) on perennial ryegrass. However, this work presented strong evidence supporting a role for exogenous NO in increasing Cd tolerance of peanut plants. Addition of exogenous NO to 50 mg kg−1 Cd-contaminated soil at sowing and seedling stages effectively alleviated Cd-induced growth inhibition in peanut plants, described as its ability to prevent inhibition of biomass and yield, increase chlorophyll content, and improve Pn, achieving a similar growth condition as control (, and ). Many studies suggested that decreasing leaf chlorophyll content was one of the most general toxicity effects of Cd on plants (Chen et al. Citation2008, Citation2010), which was confirmed in this study. However, more and more researches reported that exogenous NO could alleviate Cd-induced chlorophyll synthesis inhibition (Chen et al. Citation2010; Wang et al. Citation2013). In this study, Cd-decreased chlorophyll content was recovered to the level of normal plants when peanut plants were treated with exogenous NO at sowing and seedling stages (), accompanied by the recovery of Pn and Tr, which was severely affected by 50 mg kg−1 Cd (). This indicated that NO-mediated improvement in Pn and Tr, partly due to increasing chlorophyll synthesis, is important for increasing Cd tolerance. Carotenoids are kinds of photosynthetic pigments, which are necessary for Pn. They can transfer the absorbed light energy to the chlorophyll a and capture light energy for the chlorophyll pigment effectively. The application of exogenous NO increased Cd-decreased content of carotenoids (), which was responsible for the improvement of Pn. Furthermore, carotenoids are regarded as molecules providing nonenzymatic protection against the oxidative damage (Tiryakioglu et al. Citation2006). The NO-mediated increase in carotenoids might play an important role in alleviating ROS damage, which might account for the enhancement of Cd tolerance in peanut plants.
Numerous data from different plant species have suggested that Cd is a potent pro-oxidant, evoking oxidized stress by promoting the generation of ROS (Laspina et al. Citation2005; Hsu & Kao Citation2007; Singh et al. Citation2008). MDA, a product of lipid peroxidation, has been considered an indicator of oxidative damage (Wang et al. Citation2008). In this experiment, we investigated the three molecules to evaluate Cd-induced oxidative damage. , H2O2, and MDA are generated excessively in peanut plants grown in Cd-polluted soil (). The excess formation of ROS can oxidize various cellular components (for example, proteins, lipids, and nucleic acids) and result in lipid peroxidation, membrane leakage, and enzyme inactivation, which can finally lead to oxidative damages and alteration in cell structure (Romero-Puertas et al. Citation2007). The growth inhibition might be partly due to enhanced production and accumulation of ROS. However, NO-treated plants counteracted oxidative damages and had protective effects against stressful condition, which was concomitant with the decreased production rate of
, and the concentrations of H2O2 and MDA (). In particular, the application of exogenous NO at sowing and seedling stages eliminated the ROS damages effectively. This suggested that NO functions as a signaling molecule involving in a signal transduction pathway that could activate protective effects on lipid peroxidative to Cd stress. Many studies also demonstrated that the addition of exogenous NO alleviated Cd-caused oxidative damages (Hsu & Kao Citation2004; Xu et al. Citation2010; Wang et al. Citation2013). By investigating activities of antioxidant enzymes, NO-mediated improvement of SOD, POD, and CAT activities played an important role in ameliorating the ROS damages, which was consistent with the report by Wang et al. (Citation2013).
For surviving against Cd stress, plants have evolved protective mechanisms of mitigating and repairing the ROS damages caused by environmental stresses. In many studies, it was found that the function of NO alleviation in oxidative stress was attributed to induction of various ROS-scavenging enzyme activities (Laspina et al. Citation2005; Hsu & Kao Citation2007; Singh et al. Citation2008; Wang et al. Citation2013). The addition of NO to plant tissues was demonstrated to act as a signaling molecule in the induction of gene expression of the enzymes, such as CAT, APX, POD, and GR (Chen et al. Citation2010; Xiong et al. Citation2010). Our present results clearly indicated that the application of exogenous NO raised SOD, POD, and CAT activities in peanut plants under Cd stress, especially the application of NO at sowing and seedling stages. These results are in accordance with our previous study in which we demonstrated that NO counteracted Cd-induced decrease in the activities of antioxidant enzymes. Qiu et al. (Citation2013) have also reported that NO upregulated the components of the antioxidant defense machinery activities of SOD, POD, and CAT. Improved activities of SOD, POD, and CAT in Cd-stressed peanut plants prevented lipid peroxidation and MDA production, and thus the plant cells were protected from Cd stress damage. However, NO-mediated improvement in antioxidant enzymes needed proper NO concentrations. Many studies reported that higher concentrations of exogenous NO inhibited the activities of antioxidant enzymes in Dendrobium huoshanense (Fan et al. Citation2012) and perennial ryegrass (Wang et al. Citation2013). Therefore, different NO concentrations, crop types, disposal methods, and levels of stress can bring about different changes of antioxidant enzymes.
Abiotic stress may inhibit a synthesis of some proteins and promote others (Ericson & Alfinito Citation1984) with a general trend of decline in the overall content. Our studies coincide with John et al. (Citation2008) who also reported a decrease in the soluble protein content under Cd stress in Lemna polyrrhiza L. It is likely that heavy metals have induced lipid peroxidation and fragmentation of proteins due to toxic effects of ROS, which led to the reduction of protein content (Davies et al. Citation1987). However, NO supplementation improved Cd-decreased soluble protein content. The probable reason was that exogenous NO enhanced the antioxidant ability to eliminate Cd toxicity on peanut growth, accompanied by the improvement of protein content. In addition, the NO-induced increase in soluble protein of peanut plants grown in Cd-contaminated soil was more obvious by the addition of NO at sowing and seedling stages. Proline, an amino acid, is regarded as a kind of antioxidant. Cd-caused decrease of proline was reversed by the addition of NO. This suggested that NO mediated the enhancement of antioxidant ability in peanut plants. In addition, proline also acts as a major reservoir of energy and nitrogen, which can be used in resuming the growth (Chandrashekhar & Sandhyarani Citation1996) after the stress removal, resulting in that plant growth under Cd stress was recovered.
Cd toxicity has been related to the interactions between uptake and translocation of mineral nutrients in plants (Jiang et al. Citation2004). It has been reported that Cd toxicity in plants is affected by other ions in the nutrient solution (Sanita di Toppi & Gabbrielli Citation1999; Xu et al. Citation2010; Wang et al. Citation2013). Cd toxicity inhibited the uptake of P, K, Ca, and Fe by plants, disturbing intracellular ion homeostasis and exerting a toxic effect on plants (). P is an essential element in the plasma membrane, and the uptake of mineral elements by plant root is subjected to selective properties of the plasma membrane (Gussarsson Citation1994). Heavy metals including Cd may interfere with nutrient uptake by altering the plasma membrane permeability, and by affecting element transport processes across the membrane (Gussarsson Citation1994), as a result that P, K, Ca, and Fe concentrations were reduced in Cd-treated plants. Our study indicated that NO supplementation increased the absorption of P element and reduced the ROS damages, indicating that the plasma membrane permeability was enhanced, which might improve the absorption of K, Ca, and Fe. K is an essential element that plays vital roles in various aspects of plant cell growth and metabolism and is needed in large quantities (Zhu et al. Citation1998). Appropriate concentration of K is advantageous for the accumulation of divalent cations (Kahn & Hanson Citation1957). NO-increased uptake of K was important for improving the amount of divalent cations and ameliorating the effects of Cd toxicity on intracellular ion equilibrium. NO stimulates Ca release from intracellular stores (Sokolovski & Blatt Citation2007) but can also act as a strong stimulator of Ca influx across the plasma membrane (Lamotte et al. Citation2006), as a result that Ca concentrations were increased by the application of exogenous NO. The stimulative effect of NO on Fe uptake might be the consequence of a close relation between NO and Fe metabolism. Furthermore, NO has high affinity toward the Fe-containing active sites of many proteins and thus participates in regulation of Fe transport in plants (Ramirez et al. Citation2010). In addition, it is well known that H+-ATPase in the plasma membrane plays an important role in the transport of multiple ions (Palmgren & Harper Citation1999), and there is the investigation indicating that NO could induce H+-ATPase activity (Cui et al. Citation2010; Wang et al. Citation2013), which might be responsible for NO increasing absorption of P, K, Ca, and Fe in peanut plants under Cd stress. These results indicated that NO could ameliorate ion equilibrium in peanut cells under Cd stress.
Plant root is the main part that is in direct contact with Cd in the soil, and the cell walls in roots plays a significant role in heavy metal tolerance in plants (Xiong et al. Citation2009). Our study demonstrated that Cd concentration in the roots was more than shoots, and Cd accumulation in the kernels was the least (). Cataldo et al. (Citation1981) had also reported that most Cd was accumulated in the roots of soybean plants. Interestingly, addition of NO at sowing and seedling stages decreased Cd concentration in the roots, shoots, and kernels of peanut grown in Cd-contaminated soil. Rodriguez-Serrano et al. (Citation2009) found that the addition of Ca inhibited Cd accumulation in peanut plant, suggesting that the competition between Ca and Cd was for the same transporters. In this study, the application of NO increased the uptake of Ca, which might be responsible for the decreased accumulation of Cd in peanut plants. The result indicated that NO-induced increase in Ca level was important for improving Cd tolerance in peanut plants. Like essential micronutrients, Cd with similar physicochemical properties, competes with and enters the plant via transport systems operating for micronutrient acquisition (Papoyan et al. Citation2007). NO-mediated increase in P, K, Ca, and Fe might also account for the decrease of Cd content in the roots, shoots, and kernels of peanut plants.
5. Conclusion
In this study, SNP solution was poured into Cd-contaminated soil at different growth stages, aimed at supplying NO for peanut. The results provided evidence for NO-mediated alleviated effects on Cd toxicity in peanut plants. Application of exogenous NO applied in soil at sowing and seedling stages had obvious effects on alleviating Cd-induced inhibition on peanut growth and development. The alleviation mechanism contains: (1) increased chlorophyll content and Pn; (2) improved antioxidant ability and reduced ROS damages; (3) increased uptake of P, K, Ca, and Fe and decreased Cd accumulation. This finding may provide a new approach to protecting crops against heavy metal toxicity with supplying NO for plant at the proper time.
Acknowledgments
Great thanks were given to Pingping Yang, College of Animal Science Technology, Shandong Agricultural University, China, for her supplying instruments and patient guidance. Special acknowledgments are given to the editors and reviewers.
References
- Bates LS, Waldren RP, Teare ID. 1973. Rapid determination of free proline for water-stress studies. Plant Soil. 39:205–207. 10.1007/BF00018060
- Besson-Bard A, Pugin A, Wendehenne D. 2008. New insights into nitric oxide signaling in plants. Annu Rev Plant Biol. 59:21–39. 10.1146/annurev.arplant.59.032607.092830
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizating the principle of protein dyes binding. Anal Biochem. 72:248–254. 10.1016/0003-2697(76)90527-3
- Cakmak I, Marschner H. 1992. Magnesium deficiency and high light intensity on enhance activities of superoxide dismutase, peroxidase and glutathione reductase in bean leaves. Plant Physiol. 98:1222–1227. 10.1104/pp.98.4.1222
- Cataldo DA, Garland TR, Wildung RE. 1981. Cadmium distribution and chemical fate in soybean plants. Plant Physiol. 68:835–839. 10.1104/pp.68.4.835
- Chandrashekhar KR, Sandhyarani S. 1996. Salinity induced chemical changes in Crotalaria striata DC. Ind J Plant Physiol. 1:44–48.
- Chen F, Wang F, Sun HY, Cai Y, Mao WH, Zhang GP, Vincze E, Wu FB. 2010. Genotype-dependent effect of exogenous nitric oxide on Cd-induced changes in antioxidative metabolism, ultrastructure, and photosynthetic performance in barley seedlings (Hordeum vulgare). J Plant Growth Regul. 29:394–408. 10.1007/s00344-010-9151-2
- Chen F, Wang F, Zhang GP, Wu FB. 2008. Identification of barley varieties tolerant to cadmium toxicity. Biol Trace Elem Res. 121:171–179. 10.1007/s12011-007-8042-2
- Clemens S. 2006. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie. 88:1707–1719. 10.1016/j.biochi.2006.07.003
- Cui XM, Zhang YK, Wu XB, Liu CS. 2010. The investigation of the alleviated effect of copper toxicity by exogenous nitric oxide in tomato plants. Plant Soil Environ. 56:274–281.
- Davies CS, Nielsen SS, Nielsen NC. 1987. Flavor improvement of soybean preparations by genetic removal of lipoxygenase-2. J Am Oil Chem Soc. 64:1428–1433. 10.1007/BF02636994
- Ericson MC, Alfinito AE. 1984. Proteins produced during salt stress in tobacco cell cultures. Plant Physiol. 74:506–509. 10.1104/pp.74.3.506
- Fan HH, Li TC, Guan L, Li ZP, Guo N, Cai YP, Lin Y. 2012. Effects of exogenous nitric oxide on antioxidation and DNA methylation of Dendrobium huoshanense grown under drought stress. Plant Cell Tiss Organ Cult. 109:307–314. 10.1007/s11240-011-0096-3
- Gao AN, Tian CP, Hu YL, Chen Q, Mao ZQ. 2011. Effects of exogenous nitric oxide on physiological characteristics of seedlings of Malus hupehensis Rehd. under continuous cropping. Scientia Agricultura Sinica. 44:2184–2192. Chinese.
- Giannopolitis CN, Ries SK. 1977. Superoxide dismutase occurrence in higher plants. Plant Physiol. 59:309–314. 10.1104/pp.59.2.309
- Gussarsson M. 1994. Cadmium-induced alterations in nutrient composition and growth of Betula pendula seedlings: the significance of fine roots as a primary target for cadmium toxicity. J Plant Nutri. 17:2151–2163. 10.1080/01904169409364871
- Heath RL, Packer L. 1968. Photoperoxidation in isolated chloroplasts. I: kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 125:189–198. 10.1016/0003-9861(68)90654-1
- He Y, Liu Y, Cao W, Hua M, Xu B, Huang B. 2005. Effects of salicylic acid on heat tolerance associated with antioxidant metabolism in Kentucky bluegrass. Crop Sci. 45:988–995. 10.2135/cropsci2003.0678
- Hsu YT, Kao CH. 2004. Cadmium toxicity is reduced by nitric oxide in rice leaves. Plant Growth Regul. 42:227–238. 10.1023/B:GROW.0000026514.98385.5c
- Hsu YT, Kao CH. 2007. Toxicity in leaves of rice exposed to cadmium is due to hydrogen peroxide accumulation. Plant Soil. 298:232–241. 10.1007/s11104-007-9357-7
- Huang B, Kuo S, Bembenek R. 2003. Cadmium uptake by lettuce from soil amended with phosphorous and trace element fertilizers. Water Air Soil Pollut. 147:109–127. 10.1023/A:1024558228180
- Jiang XJ, Luo YM, Liu Q, Liu SL, Zhao QG. 2004. Effects of cadmium on nutrient uptake and translocation by Indian Mustard. Environ Geochem Health. 26:319–324. 10.1023/B:EGAH.0000039596.15586.b3
- John R, Ahmad P, Gadgil K, Sharma S. 2008. Effects of cadmium and lead on growth, biochemical parameters and uptake in Lemna polyrrhiza L. Plant Soil Environ. 54:262–270.
- Kahn JS, Hanson JB. 1957. The effect of calcium on potassium accumulation in corn and soybean roots. Plant Physiol. 32:312–316. 10.1104/pp.32.4.312
- Knudson LL, Tibbitts TW, Edwards GE. 1977. Measurement of ozone injury by determination of leaf chlorophyll concentration. Plant Physiol. 60:606–608. 10.1104/pp.60.4.606
- Lamotte O, Courtois C, Dobrowolska G, Besson A, Pugin A, Wendehenne D. 2006. Mechanisms of nitric oxide-induced increase of free cytosolic Ca2 +-concentration in Nicotiana plumbaginifolia cells. Free Radical Bio Med. 40:1369–1376. 10.1016/j.freeradbiomed.2005.12.006
- Laspina NV, Groppa MD, Tomaro ML, Benavides MP. 2005. Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci. 169:323–330. 10.1016/j.plantsci.2005.02.007
- Magdalena AJ, Jolanta FW, Edward AG. 2011. The message of nitric oxide in cadmium challenged plants. Plant Sci. 181:612–620. 10.1016/j.plantsci.2011.03.019
- McLaughlin MJ, Bell MJ, Wright GC, Cozens GD. 2000. Uptake and partitioning of cadmium by cultivars of peanut (Arachis hypogaea L.). Plant Soil. 222:51–58. 10.1023/A:1004771712840
- Palmgren MG, Harper JF. 1999. Pumping with plant P-type ATPases. J Exp Bot. 50:883–893.
- Papoyan A, Pineros M, Kochian LV. 2007. Plant Cd2 + And Zn2 + status effects on root and shoot heavy metal accumulation in Thlaspi caerulescens. New Phytol. 175:51–58. 10.1111/j.1469-8137.2007.02073.x
- Patterson BD, MacRae EA, Ferguson IB. 1984. Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal Biochem. 139:487–492. 10.1016/0003-2697(84)90039-3
- Qiu ZB, Guo JL, Zhang MM, Lei MY, Li ZL. 2013. Nitric oxide acts as a signal molecule in microwave pretreatment induced cadmium tolerance in wheat seedlings. Acta Physiol Plant. 35:65–73. 10.1007/s11738-012-1048-1
- Ramirez L, Zabaleta EJ, Lamattina L. 2010. Nitric oxide and frataxin: two players contributing to maintain cellular iron homeostasis. Annal Botany. 105:801–810. 10.1093/aob/mcp147
- Rodriguez-Serrano M, Romero-Puertas MC, Pazmino DM, Testillano PS, Risueno MC, del Rio LA, Sandalio LM. 2009. Cellular reponse of pea plants to cadmium toxicity: cross-talk between reactive oxygen species, nitric oxide and calcium. Plant Physiol. 150:229–243. 10.1104/pp.108.131524
- Romero-Puertas MC, Laxa M, Matte A, Zaninotto F, Finkemeier I, Jones AM, Perazzolli M, Vandelle E, Dietz KJ, Delledonne M. 2007. S-Nitrosylation of peroxiredoxin II E promotes peroxynitrite- mediated tyrosine nitration. Plant Cell. 19:4120–4130. 10.1105/tpc.107.055061
- Romero-Puertas MC, Rodríguez-Serrano M, Corpas FJ, Gómez M, del Río LA, Sandalio LM. 2004. Cadmium induced subcellular accumulation of O−2 and H2O2 in pea leaves. Plant Cell Environ. 27:1122–1134. 10.1111/j.1365-3040.2004.01217.x
- Sanita di Toppi L, Gabbrielli R. 1999. Response to cadmium in higher plants. Environ Exp Bot. 41:105–130. 10.1016/S0098-8472(98)00058-6
- Shi G, Cai Q. 2009. Cadmium tolerance and accumulation in eight potential energy crops. Biotechnol Adv. 27:555–561. 10.1016/j.biotechadv.2009.04.006
- Singh HP, Batish DR, Kaur G, Arora K, Kohli RK. 2008. Nitric oxide (as sodium nitroprusside) supplementation ameliorates Cd toxicity in hydroponically grown wheat roots. Environ Exp Bot. 63:158–167. 10.1016/j.envexpbot.2007.12.005
- Sokolovski SG, Blatt MR. 2007. Nitric oxide and plant ion channel control. In: Lamattina L, Polacco JC, editors. Nitric oxide in plant growth, development and stress physiology. Berlin/Heidelberg: Springer; p. 153–171.
- Tiryakioglu M, Eker S, Ozkutlu F, Husted S, Cakmak I. 2006. Antioxidant defense system and cadmium uptake in barley genotypes differing in cadmium tolerance. J Trace Elem Med Biol. 20:181–189. 10.1016/j.jtemb.2005.12.004
- Wagner GJ. 1993. Accumulation of cadmium in crop plants and its consequences to human health. Adv Agron. 51:173–212.
- Wan SB. 2003. Peanut cultivation of China. Shanghai: Shanghai Science and Technology Press; p. 1–2. Chinese.
- Wang QH, Liang X, Dong YJ, Xu LL, Zhang XW, Hou J, Fan ZY. 2013. Effects of exogenous nitric oxide on cadmium toxicity, element contents and antioxidative system in perennial ryegrass. J Plant Growth Regul. 69:11–20. 10.1007/s10725-012-9742-y
- Wang X, Liu YG, Zeng GM, Chai LY, Song XC, Min ZY, Xiao X. 2008. Subcellular distribution and chemical forms of cadmium in Bechmeria nivea (L.) Guad Environ Exp Bot. 63:389–395. 10.1016/j.envexpbot.2007.10.014
- Xiong J, An LY, Lu H, Zhu C. 2009. Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta. 230:755–765. 10.1007/s00425-009-0984-5
- Xiong J, Fu GF, Tao LX, Zhu C. 2010. Roles of nitric oxide in alleviating heavy metal toxicity in plants. Arch Biochem Biophys. 497:13–20. 10.1016/j.abb.2010.02.014
- Xu J, Wang WY, Yin HX, Liu XJ, Sun H, Mi Q. 2010. Exogenous nitric oxide improves antioxidative capacity and reduces auxin degradation in roots of Medicago truncatula seedling under cadmium stress. Plant Soil. 326:321–330. 10.1007/s11104-009-0011-4
- Zhang XW, Dong YJ, Qiu XK, Hu GQ, Wang YH, Wang QH. 2012. Exogenous nitric oxide alleviates iron-deficiency chlorosis in peanut growing on calcareous soil. Plant Soil Environ. 58:111–120.
- Zhang JX, Kirham MB. 1994. Drought stress-induced changes in activities of superoxide dismutase, catalase and peroxidase in wheat species. Plant Cell Physiol. 35:785–791.
- Zhang FQ, Zhang HX, Xia Y, Wang GP, Xu LL, Shen ZG. 2011. Exogenous application of salicylic acid alleviates cadmium toxicity and reduces hydrogen peroxide accumulation in root apoplasts of Phaseolus aureus and Vicia sativa. Plant Cell Rep. 30:1475–1483. 10.1007/s00299-011-1056-4
- Zhu JK, Liu JP, Xiong LM. 1998. Genetic analysis of salt tolerance in Arabidopsis: evidence for a critical role of potassium nutrition. Plant Cell. 10:1181–1191.
