Abstract
Two-dimensional electrophoresis (2-DE) showed the variation expression of Arabidopsis thaliana root proteins between wild type and its salt-tolerant mutant obtained from cobalt-60 γ ray radiation. Forty-six differential root protein spots were reproducibly presented on 2-DE maps, and 29 spots were identified by matrix assisted laser desorption ionization-time of flight/time of flight mass spectrometry (MS). Fifteen protein spots corresponding to 10 proteins, and 14 protein spots corresponding to 9 proteins were constitutively up-regulated and down-regulated in the salt-tolerant mutant root. Bioinformatic analysis indicated that those differential proteins might be involved in the regulation of redox homeostasis, nucleotide metabolism, signal transduction, stress response and defense, carbohydrate metabolism, and cell wall metabolism. Peroxidase 22 might be a versatile enzyme and might play dual roles in both cell wall metabolism and regulation of redox homeostasis. Our work provides not only new insights into salt-responsive proteins in root, but also the potential salt-tolerant targets for further dissection of molecular mechanism adapted by plants during salt stress.
| Abbreviations | ||
| MS | = | mass spectrometry |
| GO | = | gene ontology |
| 2-DE | = | two-dimensional electrophoresis |
| ROS | = | reactive oxygen species. |
1. Introduction
Soil salinity is a major abiotic stress that inhibits plant productivity worldwide. A total land area of over 280 million square kilometers has been severely salinized. Soil salinization has seriously affected development, growth, yield, and quality of crops (Shaheen et al. Citation2013). Plant roots are in contact with Na+ in the soil, which are both the main organ of Na+ absorption and the important barrier to prevent excessive Na+ from entering the plant. Therefore, plant roots research is becoming a target to disclose the mechanism of plant salt tolerance (Zhao et al. Citation2013). Understanding the root proteins involved in salt response played a key for the interpretation of plant salt-tolerance mechanisms. Generally, Na+ has two ways to enter the plant roots through a symplastic or apoplastic way. The former included many Na+ transport transmembrane proteins such as Na+/H+ antiporters (NHXs; Tester & Davenport Citation2013) and a high affinity K+ transporter (HKT; Rus et al. Citation2001).
In recent years, microarray technology has been applied to the roots of chickpea (Jiang & Deyholos Citation2006) and Arabidopsis (Misra et al. Citation2010) under salt stress. The altered genes were involved in active oxygen scavenging, signal transduction, transcription factors, hormones, and other functions. Since transcripts may undergo a lot of posttranscriptional and posttranslational modifications, changes at the protein level can provide direct understanding of salt-adaptive mechanisms (Guo et al. Citation2012).
In this paper, salt-tolerant Arabidopsis mutant was used as the material. High-resolution Two-dimensional (2D) gel electrophoresis followed by mass spectrometry (MS) revealed the important, constitutively differential Arabidopsis root proteins between wild type (WT) and mutant. These analyses provide an in-depth understanding for the improvement of crop salt tolerance and productivity.
2. Materials and methods
2.1. Mutant isolation, plant materials, and growth conditions
For the salt-tolerant Arabidopsis mutant isolation, 2000Gy Cobalt-60-mutagenized M2 seeds were germinated on an MS medium containing 0.8% (w/v) agar and 3% (w/v) sucrose at 22°C under constant illumination at 60 µmol m−2 s−1 for four days. Then, M2 seedlings were transferred to a high-salt medium to screen for the putative salt-tolerant mutants, whose cotyledons showed no chlorotic phenotype after 7–10 days. The Na+ for the high-salt medium was determined by testing phenotypes of WT Arabidopsis seedlings in a medium containing different Na+, and the final Na+ in the high-salt medium was 200 mM throughout this study. The putative salt-tolerant mutants were grown under normal conditions to harvest M3 seeds, and the salt-tolerant phenotype was further confirmed with M3 seedlings in the high-salt medium (Figure S1Footnote1). The selected M3 mutants were backcrossed with the WT plants, and phenotypes of their F1 and F2 generations were tested in the high-salt medium for genetic analyses. For seed harvest and hybridization, Arabidopsis plants were grown in potting soil mixture (rich soil: vermiculite =2:1, v/v) and kept in growth chambers at 22°C with illumination at 120 µmol m−2 s−1 for a 14-hr daily light period. The relative humidity was approximately 70% (±5%).
Arabidopsis WT and mutant seeds were sown in the normal MS medium. After vernalization in the dark at 4°C for three days, they were transferred into the incubator at 22°C and 60 µmol m−2 s−1 for 7–8 days. Till the first pair of true leaves was unfolded, WT and mutant seedling root tissues were clipped and quickly stored in liquid nitrogen. Finally, the roots were stored at −80°C refrigerator roots for the use.
2.2. Extraction of Arabidopsis WT and mutant root proteins
Roots were ground to powder in liquid nitrogen, dissolved for 2 hr in lysis solution (7 M urea, 2 M thiourea, 4% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, 65 mM dithiothreitol [DTT]) and centrifuged at 40,000×g for 1 hr at 4°C. The supernatant was precipitated with 4 volumes of ice-cold acetone, stored for 1 hr at −20°C, thawed and centrifuged at 20,000×g for 1 hr at 4°C. Precipitates were washed with 90% ice-cold acetone, dissolved in 5 mL lysis solution and stored at −80°C. Sample protein concentrations were determined with the Bradford assay (Bio-Rad; Gotham et al. Citation1988).
2.3. Gel electrophoresis, mass spectrometric analyses, and protein identification
Two-dimensional gel electrophoresis was performed as previously described (Gorg et al. Citation2004). Before the second dimensional electrophoresis, nonlinear pH 3–10 immobilized pH gradient strips (13 cm) used for one-dimensional isoelectric focusing were equilibrated in two steps: reduction with DTT and carboxymethylation with iodoacetamide. The equilibrated strips were run on 12% (w/v) SDS-PAGE at 25 mA per gel and stained with Coomassie Brilliant Blue R-350 (Amersham Biosciences). Gels were made in triplicate to confirm the spot patterns and were scanned with a Z320 scanner (Founder, Beijing, China). Gel images were processed with an ImageMaster (GE Healthcare).
Protein spots excised from 2D gels were destained with 25 mM NH4HCO3/50% (v/v) acrylonitrile and dried, followed by in-gel digestion with 0.01 µg trypsin in 0.01 mL 25 mM NH4HCO3 for 12 hr at 37°C. Digestion buffer was removed to a new 1.5 mL, clear microtube (Axygen Scientific, Union City, CA), 50 µl of 1% trifluoroacetic acid (v/v) in 50% (v/v) acetonitrile was added to the gel plugs, which were sonicated for 30 min. This extract was removed and combined with the digestion buffer and freeze-dried (Labconco, Kansas City, MO; Kumarathasan et al. Citation2005).
Peptides were then resuspended in 15 µL of 0.5% (v/v) trifluoroacetic acid in Milli-Q water. Peptides were analyzed by an ultraflex III TOF/TOF (Bruker Daltonik GmbH, Germany) in the positive reflection mode under the control of Compass 1.2 and WarpLC 1.1 software (Bruker Daltonik, GmbH, Germany). Detected peptide compounds with a signal-to-noise ratio higher than 10 were subjected to matrix assisted laser desorption ionization-time of flight/time of flight (MALDI-TOF) MS/MS. External calibration was performed with the ProteoMass peptide and protein MALDI/MS calibration kit (Sigma). Each mass spectrum represents the sum of 150–200 laser shots collected from ≥30 different positions within each spot. Masses frequently detected that arose from the matrix, trypsin, or known contaminants (e.g. keratins) were not analyzed.
2.4. Data mining and bioinformatic analysis
MS and MS/MS spectra were subjected to database search employing MASCOT (www.matriscience.com) and National Center for Biotechnology Information (NCBI) sequence database (NCBInr 20130101) restricted to Arabidopsis (35375 sequences; 14477754 residues). The algorithm was set to use trypsin as the enzyme, allowing for one missed cleavage site and assuming carbamidomethyl as a fixed modification of cysteine and oxidized methionine as a variable modification. For protein mass fragment (PMF) data, peptide mass tolerance was set to±0.3 Da. For MS/MS database searches, mass tolerance of precursor ions and fragment ions was set to 150 ppm and±0.4 Da. Protein hits were considered identified if the Mascot score was greater than 60 and matched at least four peptides for peptide mass fingerprinting and 37 for MS/MS analysis (significance level, p<0.05). If more than one protein was identified in a spot, the single protein member with the highest score (top rank) was chosen from the multiprotein family.
Proteins were distinguished functionally by a step-by-step classification and each protein was placed in only one category. The proteins were first scored according to their function reported in the literature and the Kyoto Encyclopedia of Genes and Genomes database (Release 52.0): proteins that could not be defined were sought in the Protein Information Resource (PIR) database (Release 15.9). Proteins were also scanned for protein domains by InterPro database (Release 23.0).
3. Results
3.1. 2-DE analysis of WT and salt-tolerant mutant proteins in Arabidopsis roots
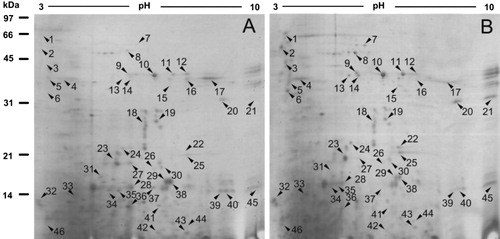
To investigate the constitutively differential protein profiles between WT and salt-tolerant mutant proteins in Arabidopsis roots, 2-DE analysis of the total proteins from three biologically independent replicate experiments was carried out. A representative gel is shown in . Approximately 600 protein spots were detected on Coomassie brilliant blue-stained gels and about 400 protein spots were matched between WT and mutant. Quantitative image analysis revealed a total of 46 protein spots that changed their abundance (vol%) significantly (p<0.05) by more than 2.0-fold.
3.2. MALDI-TOF/TOF-MS analysis of constitutively differential protein profiles between WT and salt-tolerant mutant proteins in Arabidopsis roots
Forty-six constitutively differentially expressed spots described above were selected and excised for tryptic digestion and analysis by MALDI-TOF-MS. From the 46 gel plugs excised, 29 protein spots were successfully identified (). Among them, 15 constitutively differentially expressed spots corresponding to 10 proteins were significantly up-regulated in the salt-tolerant mutant root, and 14 constitutively differentially expressed spots corresponding to 9 proteins were significantly up-regulated. showed the PMF and MS/MS spectra of differential protein spot 9 (peroxidase 22).
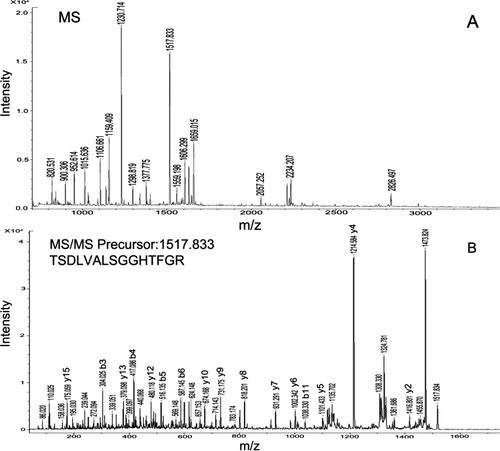
Table 1. Identification of 29 differential root proteins of Arabidopsis thaliana WT and salt-resistant mutant with MALDI-TOF/TOF-MS.
3.3. Heterogeneity of constitutively differential root protein between WT and salt-tolerant mutant
Based on MALDI-TOF/TOF-MS identification, it was found that some differential proteins were identified in more than one spot, although they were excised from the same gel (). For example, glycosyl hydrolase family protein such as beta-glucosidase 23 was identified in more than two spots. As for beta-glucosidase 23, two spots were up-regulated, and three spots were down-regulated. Its electrophoresis patterns showed the inferred mass or isoelectric point values of these spots differed, due to various posttranslational modifications such as glycosylation and phosphorylation or degradation, which caused the change of the molecular weight and/or change of proteins.
3.4. Classification of biological function of constitutively differential root proteins between Arabidopsis WT and salt-tolerant mutant
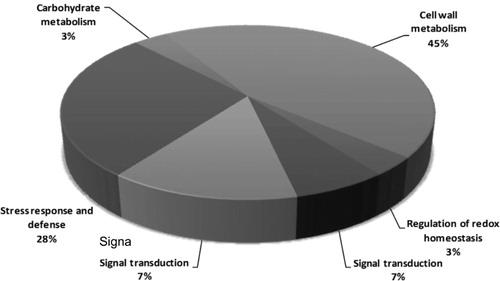
Functional classification analysis according to gene ontology (GO) annotations and PubMed references revealed that the proteins were clustered into several categories. Those 29 differential proteins were involved in regulation of redox homeostasis (4%), nucleotide metabolism (14%), signal transduction (7%), stress response and defense (4%), carbohydrate metabolism (3%), and cell wall metabolism (45%; , ).
4. Discussion
Proteomic technologies have improved the researches of global root protein profiling, and especially 2-DE technology has been widely used for discovering salt-responsive proteins in plant roots. 2-DE is a powerful technology with high resolution in protein separation, especially for protein isoforms. MALDI-TOF/TOF-MS has been applied for protein identification in roots (Lee et al. Citation2004; Jiang et al. Citation2007). This study aimed to show the constitutively differential root protein between WT and salt-tolerant mutant. Those proteins may be involved in the effective salt response of plant roots, which could demonstrate the advantages of the proteomic approach in studies of plant biology and identify candidate proteins in salt stress signaling network, contribute to the elucidation of the salt-tolerance mechanisms in roots, and provide an in-depth understanding for effective engineering strategies to improve crop salt tolerance.
4.1. Cell wall metabolism-related root proteins
Under salt stress plants will usually increase the level of cell wall lignification to prevent turgor and cell wall-induced collapse. In this study, 3 differential points corresponding to peroxidase 22 were significantly highly expressed in salt-tolerant mutant root. Peroxidase 22 belongs to class III plant peroxidases, which are plant-specific oxidoreductases that are implicated in many physiological processes such as auxin catabolism, liginfication, and suberization (Degenhardt & Gimmler Citation2000). They participated in the cross-linking of several compounds to form a physical barrier to prevent the water loss. At the transcript level, under salt stress, the majority of class III peroxidases are responsive in Arabidopsis roots (Jiang & Deyholos Citation2006). Our proteomic result showed its member-peroxidase 22 was constitutively highly expressed in salt-tolerant root, which may improve its salt resistance. The study also found that glycosyl hydrolase family members were significantly differentially expressed in both WT and salt-tolerant mutant roots. Glycosyl hydrolase played a key role in cell wall remodeling (Hiraga et al. Citation2001; Bray Citation2004). In addition, reversibly glycosylated polypeptide (RGP1) has been reported to be involved in plant cell wall synthesis (Dhugga et al. Citation1997). Comparing with WT root, those cell wall-related proteins were constitutively highly expressed in salt-tolerant mutant root, which would increase its potential of salt resistance.
4.2. Stress response and defense-related proteins
During salt condition, stress response and defense-related proteins played an important role. In this study, jacalin-like lectin domain-containing protein was highly expressed in the salt-tolerant mutant. It has been reported that under 150 mM NaCl stress, lectin family proteins were up-regulated in Arabidopsis roots (Jiang et al. Citation2007). Therefore, those proteins were constitutively highly expressed in salt-tolerant mutant which was helpful to increase the salt tolerance. Additionally, three differential spots were identified as isoforms of START/RHO_alpha_C/PITP/Bet_v1/CoxG/ CalC (SRPBCC) ligand-binding domain-containing protein. The protein has a function of plant choline monooxygenase catalyzed biosynthesis of betaine. Betaine could protect membrane integrity and biological activity of biological macromolecules such as enzymes, which improve the plant adaptability under drought, salt, low cold, and isostatic stress conditions (Xing & Rajashekar Citation2001). Compared to WT, major latex protein (MLP)-like protein 328 and two homologous protein of pyruvate kinase 1 (PYK1)-binding protein 1 in the roots of salt-tolerant mutants were down-regulated. The former facilitates correct aggregation of PYK10 protein when the plant suffered pathogen infection or mechanical damage (Nagano et al. Citation2005), and the latter has a similar function to pathogenesis-related protein 10 involved in the defense of pathogen infections (Lytle et al. Citation2009). Those two constitutively differential proteins of the salt-tolerant mutants were down-regulated, which may indicate the existence of complex defense function system.
4.3. Nucleic acid metabolism-related proteins
Our work found that DNA topoisomerase-like protein was highly expressed in tolerant mutant roots, which was the enzyme participating in catalyzing DNA strand breaks and binding, and regulating template supercoiling in the process of RNA transcripts. But there were no similar reports in Arabidopsis. Under salinity stress, salt-tolerant wheat increased the expression of DNA/RNA helicase (Wang et al. Citation2008). In this study, salt-tolerant mutants constitutively highly expressed DNA topoisomerase-like protein whether it had a similar role or needed further study. Salt stress induced the increase of glycine-rich RNA-binding protein abundance in Arabidopsis roots (Jiang et al. Citation2007). This protein can promote gene expression and typically accumulate in the vascular tissue, which played an important role in plant defense processes (Mousavi & Hotta Citation2005). But in the present study, glycine-rich RNA-binding protein 8 was of low expression in mutant roots, which may be different from other family members and play a versatile role in salinity tolerance.
4.4. Signal transduction-related proteins
This study found two signal transduction molecules: TNF receptor associated factor (TRAF)-like family protein and subtilase family protein. The former was constitutively of low expression in salt-tolerant mutant roots, and the latter was of high expression in the salt-tolerant mutant roots. TRAF family proteins had a MATH domain, which mediated protein interactions between TRAFs members and receptors, among TRAFs members, and between TRAFs members and several intracellular signaling molecules (Zapata Citation2003). Now, 59 MATH domain-containing genes have been found in Arabidopsis (Oelmüller et al. Citation2005), but its corresponding protein function needs further study. The researches of subtilase family protein early focused on its functions of protease and/amino reuse, but recently, it was used as protease to shear membrane-associated signaling molecules AtbZIP17, and then the modified AtbZIP17 could enter into the nucleus to activate the expression salt-responsive genes (Liu et al. Citation2007). In this study, a member of subtilase family enzymes was found constitutively highly expressed in salt-tolerant mutant, which might improve its early salt response to increase its salt tolerance.
4.5. Regulation of redox homeostasis-related proteins
Under salt stress, plants could increase antioxidant enzyme activity to remove excess reactive oxygen species and protect the membrane system from attack and damage. The studies showed that halophytes under salt stress exhibited higher antioxidant enzyme activity than glycophyte. In order to maintain reactive oxygen species (ROS) homeostasis under salt stress, plants developed a variety of antioxidant enzymes system for the elimination of reactive oxygen species including glutathione S-transferase. That enzyme can be used as a substrate to reduce H2O2 to H2O (Edwards et al. Citation2000). In the present study, glutathione S-transferase in the salt-tolerant mutants was constitutively of low expression, which indicated there may be complex salt-tolerant mechanism. In this study, peroxidase 22 was a versatile enzyme. In addition to playing a role in cell wall synthesis and metabolism, it was also involved in the reduction of H2O2. Peroxidase 22 was constitutively of high expression in mutant root, and it is likely associated with enhanced salt tolerance.
4.6. Carbohydrate metabolism-related proteins
Dealing with salinity stress, plants required energy to synthetize and degrade salt-responsive protein. Thereby, in plant carbohydrate energy metabolism (glycolysis pathway, etc.) related enzymes were responsive to salt stress (Zhao et al. Citation2013). Glyceraldehyde 3-phosphate dehydrogenase was the backbone of the glycolytic pathway involved in the synthesis of ATP. After 200 mM NaCl-treated wheat roots, expression abundance of glyceraldehyde 3-phosphate dehydrogenase increased (Wang et al. Citation2008). In our work, constitutively high expression of glyceraldehyde 3-phosphate dehydrogenase in mutant root may be associated with the increasing of salt adaptation.
5. Conclusion
Comparing with WT roots through differential proteome, some important proteins were successfully found in salt-mutant root. Those proteins were involved in stress response and defense, cell wall metabolism, nucleic acid metabolism, signal transduction, redox reactions balance adjustment, and carbohydrate metabolism. The discovery of these proteins contributed to a better understanding of plant root salinity adaptation, and to the in-depth interpretation of the salt-tolerance mechanisms in roots.
Supplemental Material.pdf
Download PDF (238.6 KB)Acknowledgments
Supported by grants from Shandong Provincial Natural Science Foundation, China (ZR2010CQ024) and A Project of Shandong Province Higher Educational Science and Technology Program (J10LC72). The authors declare no competing interests.
Notes
1. Supplemental Content may be viewed online at http://dx.doi.org/10.1080/17429145.2013.833653
References
- Bray EA. 2004. Genes commonly regulated by water-deficit stress in Arabidopsis thaliana. J Exp Bot. 55: 2331–2341.10.1093/jxb/erh270
- Degenhardt B, Gimmler H. 2000. Cell wall adaptations to multiple environmental stresses in maize roots. J Exp Bot. 51:595–603.10.1093/jexbot/51.344.595
- Dhugga KS, Tiwari SC, Ray PM. 1997. A reversibly glycosylated polypeptide (RGP1) possibly involved in plant cell wall synthesis: purification, gene cloning, and trans-Golgi localization. Proc Natl Acad Sci USA. 94:7679–7684.10.1073/pnas.94.14.7679
- Edwards R, Dixon DP, Walbot V. 2000. Plant glutathione S-transferases: enzymes with multiple functions in sickness and in health. Trends Plant Sci. 5:193–198.10.1016/S1360-1385(00)01601-0
- Gorg A, Weiss W, Dunn MJ. 2004. Current two-dimensional electrophoresis technology for proteomics. Proteomics. 4:3665–3685.10.1002/pmic.200401031
- Gotham SM, Fryer PJ, Paterson WR. 1988. The measurement of insoluble proteins using a modified Bradford assay. Anal Biochem. 173:353–358.10.1016/0003-2697(88)90199-6
- Guo ML, Yang AH, Zhou CX, Liu X. 2012. The new understanding of Arabidopsis thaliana proteins associated with salinity. J Plant Interact. 7:348–355.10.1080/17429145.2011.640438
- Hiraga S, Sasaki K, Ito H, Ohashi Y, Matsui H. 2001. A large family of class III plant peroxidases. Plant Cell Physiol. 42:462–468.10.1093/pcp/pce061
- Jiang YQ, Deyholos MK. 2006. Comprehensive transcriptional profiling of NaCl-stressed Arabidopsis roots reveals novel classes of responsive genes. BMC Plant Biol. 6:25.10.1186/1471-2229-6-25
- Jiang YQ, Yang B, Harris NS, Deyholos MK. 2007. Comparative proteomic analysis of NaCl stress-responsive proteins in Arabidopsis roots. J Exp Bot. 58:3591–3607.10.1093/jxb/erm207
- Kumarathasan P, Mohottalage S, Goegan P, Vincent R. 2005. An optimized protein in-gel digest method for reliable proteome characterization by MALDI-TOF-MS analysis. Anal Biochem. 346:85–89.10.1016/j.ab.2005.06.004
- Lee S, Lee EJ, Yang EJ, Lee JE, Park AR, Song WH, Park OK. 2004. Proteomic identification of annexins, calcium-dependent membrane binding proteins that mediate osmotic stress and abscisic acid signal transduction in Arabidopsis. Plant Cell. 16:1378–1391.10.1105/tpc.021683
- Liu JX, Srivastava R, Che P, Howell SH. 2007. Salt stress responses in Arabidopsis utilize a signal transduction pathway related to endoplasmic reticulum stress signaling. Plant J. 51:897–909.10.1111/j.1365-313X.2007.03195.x
- Lytle BL, Song J, de la Cruz NB, Peterson FC, Johnson KA, Bingman CA, Phillips GN, Jr Volkman BF. 2009. Structures of two Arabidopsis thaliana major latex proteins represent novel helix-grip folds. Proteins. 76:237–243.10.1002/prot.22396
- Misra P, Pandey A, Tiwari M, Chandrashekar K, Sidhu OP, Asif MH, Chakrabarty D, Singh PK, Trivedi PK, Nath P, Tuli R. 2010. Modulation of transcriptome and metabolome of tobacco by Arabidopsis transcription factor, AtMyb12, leads to insect resistance. Plant Physiol. 152:2258–2268.10.1104/pp.109.150979
- Mousavi A, Hotta Y. 2005. Glycine-rich proteins: a class of novel proteins. Appl Biochem Biotech. 120:169–174.10.1385/ABAB:120:3:169
- Nagano AJ, Matsushima R, Hara-Nishimura I. 2005. Activation of an ER-body-localized beta-glucosidase via a cytosolic binding partner in damaged tissues of Arabidopsis thaliana. Plant Cell Physiol. 46:1140–1148.10.1093/pcp/pci126
- Oelmüller R, Peškan-Berghöfer T, Shahollari B, Trebicka A, Sherameti I, Varma A. 2005. MATH domain proteins represent a novel protein family in Arabidopsis thaliana, and at least one member is modified in roots during the course of a plant–microbe interaction. Physiol Plantarum. 124:152–166.10.1111/j.1399-3054.2005.00505.x
- Rus A, Yokoi S, Sharkhuu A, Reddy M, Lee BH, Matsumoto TK, Koiwa H, Zhu JK, Bressan RA, Hasegawa PM. 2001. AtHKT1 is a salt tolerance determinant that controls Na+ entry into plant roots. Proc Natl Acad Sci USA. 98:14150–14155.10.1073/pnas.241501798
- Shaheena S, Naseera S, Ashrafa M, Akramb NA. 2013. Salt stress affects water relations, photosynthesis, and oxidative defense mechanisms in Solanum melongena L. J Plant Interact. 8:85–96.10.1080/17429145.2012.718376
- Tester M, Davenport R. 2013. Na+ tolerance and Na+ transport in higher plants. Ann Bot. 91:503–527.10.1093/aob/mcg058
- Wang MC, Peng ZY, Li CL, Li F, Liu C, Xia GM. 2008. Proteomic analysis on a high salt tolerance introgression strain of Triticum aestivum/Thinopyrum ponticum. Proteomics. 8:1470–1489.10.1002/pmic.200700569
- Xing WB, Rajashekar CB. 2001. Glycine betaine involvement it freezing tolerance and water stress in Arabidosis thaliana. Environ Exp Bot. 46:21–28.10.1016/S0098-8472(01)00078-8
- Zapata JM. 2003. TNF-receptor-associated factors as targets for drug development. Expert Opin Ther Tar. 7:411–425.10.1517/14728222.7.3.411
- Zhao Q, Zhang H, Wang T, Chen SX, Dai SJ. 2013. Proteomics-based investigation of salt-responsive mechanisms in plant roots. J Proteomics. 82:230–253.10.1016/j.jprot.2013.01.024