Abstract
The bacteria of PDMCd0501, PDMCd2007, and PDMZnCd2003 were isolated from a Zn/Cd contaminated soil. They were classified as salt-tolerant bacteria in this experiment. The bacteria had indole-3-acetic acids (IAA) production, nitrogen fixation, and phosphate solubilization, under 8% (w/v) NaCl condition. Biochemical test (API 20E) and 16S rDNA sequencing identified PDMCd2007 and PDMCd0501 as Serratia sp. and PDMZnCd2003 was Pseudomonas sp. The effect of Pseudomonas sp. PDMZnCd2003 on the germination and seedlings of Oryza sativa L.cv. RD6 was determined under a salinity of 0–16 dS/m. The salinity levels of 4–16 dS/m affected to decrease germination and seedlings of rice. Comparison between uninoculated and inoculated system, however, Pseudomonas sp. PDMZnCd2003 had a negative impact on the rice growth. This unexpected effect was a case that should be concerned and studied further before application as a plant growth-promoting bacteria (PGPB).
Introduction
Salinity is one of the major abiotic stresses that adversely affect modern agriculture and constitutes a problem everywhere in the world. More than 6% of the world's total land area is salt-affected; most of this salt-affected land has arisen from natural causes and the accumulation of salts over long periods of time in arid and semiarid zones (Rengasamy Citation2002; Bui Citation2013). Soils that have excessive amounts of salts (i.e., electrical conductivity (EC) > 4 dS/m) are classified as saline soils (Pierzynski et al. Citation2005). Soil salinity stresses plants in two ways: high concentrations of salts in the soil make it harder for roots to extract water and high concentrations of salts within the plant can be toxic. Salts on the outside of the roots have an immediate effect on cell growth and associated metabolism; toxic concentrations of salts take time to accumulate inside plants, before they affect plant function (Munns & Tester Citation2008).
Rice (Oryza sativa L.) is one of the most widely cultivated crops and provides food for one-half of the world's population. Rice production in the northeast of Thailand has been limited to a large extent by soil salinity. The total area salinity amounts to 28.5 thousand square kilometers of the northeastern land area (Land Development Department Citation1991). The effect of saline on rice depends on several factors: (1) the intensity of the stress, (2) the climatic conditions, and (3) the resistance level of the genotype (Asch & Wopereis Citation2001; Kanawapee et al. Citation2013). An average seasonal salinity of field water in excess of 1.9 dS/m can also reduce grain yields, and salinity affects rice yield at or above 3.0 dS/m (Grattan et al. Citation2002). Salinity has a negative impact on a number of yield components, including stand establishment; panicles, tillers, and spikelets per plant; floret sterility; individual grain size; and even delayed heading (Grattan et al. Citation2002; Rad et al. Citation2012).
One approach to solve the salt stress problem is the use of plant growth-promoting bacteria (PGPB). Many Gram-positive and -negative PGPB have been reported to colonize the plant rhizosphere and confer beneficial effects by various direct and indirect mechanisms, which can be correlated with their ability to form biofilms, chemotaxis, and the production of exopolysaccharide, indole-3-acetic acids (IAA) and aminocyclopropane-1-carboxylate (ACC) deaminase (Glick Citation1995). Investigations on the interaction of PGPB with other microbes and their effect on the physiological response of crop plants under different soil salinity regimes are still at an incipient stage (Singh et al. Citation2011). Alleviation of salt stress by PGPB inoculants has been shown in rice (Rangarajan et al. Citation2003; Sapsirisopa et al. Citation2009; Nautiyal et al. Citation2013), wheat (Egamberdiyeva Citation2009), maize (Egamberdiyeva Citation2007), cotton (Yao et al. Citation2010), lettuce (Han & Lee Citation2005), tomato (Mayak et al. Citation2004), and pepper (del-Amor & Cuadra-Crespo Citation2012; Siddikee et al. Citation2011).
Our research isolated some bacteria from the rhizosphere of Gynura pseudochina (L.) DC., growing in a zinc mining (Phatat Phadaeng sub-district, Mae Sot, Tak province, Thailand). Three bacterial isolates of PDMCd0501, PDMZnCd2003, and PDMCd2007 were uncovered that had multiple PGPR properties to promote plant growth (Meesungnoen et al. Citation2009). Therefore, this research aims to study their growth promotion abilities of N2-fixation, IAA production, phosphate solubilization, and tendency to hydrolyze starch, casein, and cellulose, under salt stress. The three isolates were identified by API-20E and 16S rDNA techniques. Finally, the effect of selected bacterial inoculation on germination and seedlings of rice (O. sativa L.cv. RD6) under salinity conditions were investigated in both plate and pot experiments.
Materials and methods
Salt tolerance assays and PBPR properties under salt stress
The three bacterial strains of PDMCd2007, PDMCd0501, and PDMZnCd2003, which were maintained in 50% (v/v) glycerol at –70°C, were re-cultivated in nutrient broth (NB) for 12–18 hours. The bacterial cells were collected by centrifugation, washed with 0.85% (w/v) sodium chloride (NaCl), and re-suspended with deionized water to obtain a suspension equal to 1.0 at optical density of 600 nm. The cell suspension was ten-fold serial diluted to 10–4 and 10–5 before being used as bacterial samples. The salt tolerance study was carried out by spreading 0.1 ml of each sample on nutrient agar (NA) (HiMedia, India) and Pseudomonas agar F (PAF medium) (Difco, USA), with each modified by 2%, 4%, 6%, 8%, or 10% (w/v) NaCl. Plates were incubated at ambient temperature (28±2°C) for 3 days. The tolerant bacteria at 8% (w/v) NaCl were selected and preserved on PAF-medium containing 8% (w/v) NaCl.
Hydrolysis of starch, cellulose, and casein
One loop of each bacterial culture was streaked on starch agar, skimmed-milk agar, and NA containing 1% (w/v) carboxymethyl cellulose (CMC). The control was the agar medium without NaCl and the treatment was the media containing 8% (w/v) NaCl. The bacterial plates were incubated for 48 hours at 28±2°C. After incubation, starch hydrolysis was tested with iodine solution. Cellulose hydrolysis was tested by flooding with 1% congo red solution for 5–10 min, then removal of the remaining congo red by washing with 1 M NaCl. Casein hydrolysis was detected from the obvious clear zone. A zone of clearing around the growth area was used to determine the solubilization index (SI), which was calculated from the diameter of the clear zone (mm) divided by the diameter of the colony (mm).
Phosphate solubilization
One loop of each bacterial culture was streaked onto NBRIP (National Botanical Research Institute's phosphate growth) agar consisting of 0.5% (w/v) tricalcium phosphate, 8% (w/v) NaCl and bromophenol blue was used as an indicator. The NBRIP agar without NaCl was used as the control. After incubation for 7 days, clear zone around each colony indicated that phosphate solubilization was being observed. The clear zone around the growth area was used to calculate the solubilization index (SI).
N2 fixation
One loop of each bacterial culture was streaked onto N-free agar containing 0.0025% (w/v) bromothymol blue and 8% (w/v) NaCl. The N-free agar without NaCl was the control. Isolates that showed growth within 7 days and intensified the blue color of the bromothymol blue around the colony were considered to be N2-fixing bacteria.
IAA production
Each bacterium was cultured in trypticase soy broth (TSB) containing 0.2% (w/v) tryptophan and 8% (w/v) NaCl, and then incubated in the dark at 30°C for 48 hours at 150 rpm in an incubated shaker (Innova 2100 Platform shaker, New Brunswick Scientific, USA). The bacteria cultures in the TSB without NaCl were the controls. The cultures were then centrifuged at 6000 rpm for 15 min at 4°C by a refrigerated centrifuge (MX-301 TOMY, USA). The supernatants were mixed with Salkowski's reagent (ratio 2:1) and left in the dark for 20 min. The optical density was measured at an absorbance of 530 nm (Bric et al. Citation1991). The IAA concentration was determined using a standard curve of authentic IAA (Sigma-Aldrich, St. Louis, MO, USA).
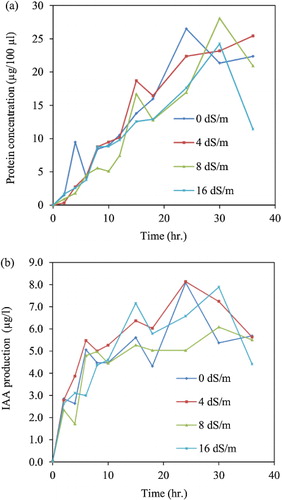
The salinity effects on IAA production and the bacterial growth-curve of Pseudomonas sp. PDMZnCd2003 were determined by cultivation in TSB containing 0.2% (w/v) tryptophan, modified with 0.21%, 0.43%, or 0.87% (w/v) NaCl to obtain the EC at 4, 8, and 16 dS/m. Total protein content was monitored for bacterial growth curve by the Bradford Protein Assay (Bradford Citation1976). The bacterial cells in each growth stages were collected by refrigerated centrifugation, and the supernatants were measured for IAA production.
Bacterial identification
Gram's staining of the bacteria was performed and investigated by a light microscopy (Olympus CH30, Japan). API-20E test kit was applied for biochemical, physiological, and nutritional tests. The API-20E test was undertaken and reported by the Thailand Institute of Scientific and Technological research (TISTR). For genetic characterization, the total genomic DNA of selected isolates was extracted by a modified phenol: chloroform procedure of Sambrook and Russel (Citation2001). Two primers of fD1 (5′-AGAGTTTGATCCTGGCTCAG-3′) and rP2 (5′-ACGGCTACCTTGTTACGACTT-3′) (Weisburg et al. Citation1991) were used for 16S rRNA amplification. Each 50 µl of polymerase chain reaction (PCR) reaction contained: 100 ng of total DNA, 0.2 mM of each dNTP, 5 unit of Taq DNA polymerase (Invitrogen, USA) in 5 µl of 10x Taq buffer, 1 mM MgCl2, 0.2 mM of each primer, and 35-µl sterile deionized water. The thermal cycling program amplification consisted of 1 cycle of 94°C for 5 min (denaturation), 57°C for 2 min (annealing), and 72°C for 2 min (extension); 29 cycles of 94°C for 2 min, 57°C for 30 sec, and 72°C for 2 min; and a final elongation cycle of 72°C for 10 min (Wood et al. Citation1998). The PCR products obtained were purified with a HiYieldTM Gel/PCR DNA Fragments extraction kit (Real Biotech Corporation, Taiwan) and cloned into the pGEM-T-Easy vector (Promega, Madison, Wis.), according to the protocols of the manufacturers. The plasmids were transformed into competent Escherichia coli JM109 by the TSS method (Chung & Miller Citation1993). Sequencing was performed on a 3730XL DNA sequencer that monitored the whole experimental process through a Laboratory Information Management System (LIMS), Macrogen Inc, Korea. The sequence data of the 16S rDNA was compared with sequences in the National Center for Biotechnology Information data bank using the BLAST program (Altschul et al. Citation1997).
The partial 16S rDNA nucleotide sequence was submitted to the NCBI database. The accession numbers for the sequences of halotolerant bacterial strains PDMCd0501, PDMCd2007, and PDMZnCd2003 are JX193587, JX193588, and JX193586, respectively.
Effect of bacterial inoculation on germination and growth of rice
O. sativa L.cv. RD6., a moderately salt tolerance variety (Kanawapee et al. Citation2013), was used in these experiments. Rice seeds were surface disinfected by immersion in 70% (v/v) ethanol plus 0.3% (v/v) tween 80 for 5 min and 3% (w/v) sodium hypochlorite containing 0.3% (v/v) Tween 80 for 15 min. They were then washed three times with sterile distilled water. To obtain germinated seeds, rice seeds were soaked in water for 12 hours and then covered with a clean cloth for 12 hours. The germinated seeds were surface sterilized by the same procedure as for seed sterilization. Pseudomonas sp. PDMZnCd2003 was cultivated in TSB for 12–18 hours to accelerate late exponential growth phase cells (Meesungnoen et al. Citation2012). The bacterial cells were collected by centrifugation and washed with 0.85% (w/v) NaCl and re-suspended with deionized water to obtain 108 CFU/ml (A600nm = 0.5).
Plate experiment
Germination testing under salt stress was carried out with 25 rice seeds per petri dish (15 cm). Each petri dish contained one sheet of paper which was moistened with 10 ml of 0% (deionized water as control), 0.21%, 0.43%, or 0.87% (w/v) NaCl, to obtain an electrical conductivity (EC) at 0, 4, 8, and 16 dS/m. Two milliliters of the bacterial suspension was applied once to the rice seeds in each treatment. The details of treatments are shown in , and three petri dishes were used for replication. The experiments were carried out under artificial light which provided a light intensity of 2100 lux for 12 hours photoperiod, a temperature of 29±3°C, and humidity of 54±8%. Rice germination was investigated for ten days by measuring germination percentage, root length, shoot length, fresh weight, and dry weight.
Table 1. The experimental details for germination and seedlings of rice under salt stress.
Pot experiment
Effect of salt stress on rice growth was performed in a modified Leonard jar. The growth unit consisted of a black plastic pot (12-cm diameter x 15-cm height) stacked into a plastic box to form a watertight seal. The vermiculite was immersed with NaCl solutions to obtain the EC at 0–16 dS/m for one day, and then autoclaved at 121°C, for 15 min. A pot was filled with 160 g of a saline vermiculite treatment. In each pot treatment (), five sterilized-germinated seeds were transplanted at the same depth (approx. 2.5 cm below the surface). Three pots were used for replication. The plants were grown under natural light, which provided a 15-hour photoperiod at temperatures of 30–35°C and 50±10% humidity. Rice seedlings were harvested on day 30 after planting. They were evaluated for root length, shoot length, fresh weight, and dry weight.
Statistical analysis
The germination and seedlings of rice was analyzed by two-way analysis of variation (Two-way ANOVA). The variance and means of separation were performed using Duncan's new multiple range test (DMRT) at P < 0.05. Statistical analysis was performed using SPSS 12.0 for Windows (SPSS Inc., USA).
Results and discussion
Salt tolerance and PGPR properties under salt stress
The salt tolerance of the three isolates of PDMCd0501, PDMCd2007, and PDMZnCd2003 was determined by total colony number on NA and PAF-medium supplemented with 0–10% (w/v) NaCl. The results showed that the three bacteria were able to grow on NA plus 0–6% (w/v) NaCl, whereas they could tolerate and grow on PAF medium plus 0–8% (w/v) NaCl. The highest percentage of NaCl to 10% (w/v) suppressed the growth of the bacteria on both the NA and PAF medium. Therefore, the population range of the three bacteria cultivated on PAF medium plus 6, 8, and 10% (w/v) NaCl was investigated to confirm their tolerance. PDMCd0501 and PDMCd2007 were able to grow well in 8% (w/v) NaCl (). Therefore, the three bacteria could be classified as halotolerant bacteria (Willey et al. Citation2009). In addition, their PGPR properties of IAA production, N2-fixation ability, phosphate solubilization under control (0% NaCl), and salt stress (8% NaCl) are shown in . The three isolates could produce IAA under the salt stress. The IAA produced by PDMZnCd2003 increased under the salinity conditions. The 8% (w/v) NaCl seemed not to affect N2-fixation by the three bacteria. Even though the salt stress decreased some properties, PDMCd2007, PDMCd0501, and PDMZnCd2003 contained P-solubility and casein hydrolysis under both control and salinity conditions. The three bacteria might not stress the plant root, because they could not hydrolyze starch and cellulose.
Table 2. Effect of salt concentrations on the isolated bacteria's growth in the PAF-medium.
The cadmium (Cd) tolerant isolates of PDMCd0501 and PDMCd2007 (Meesungnoen et al. Citation2009) tolerated higher salt stresses than PDMZnCd2003, which is a Zn-/Cd-tolerant bacterium. The reason might involve P-type ATPases, especially CPx-type ATPases, CBA transporters, and cation diffusion facilitator (CDF) family transporters in Gram-negative bacteria (Hynnienen Citation2010). P-type ATPases play critical roles in ion homeostasis in a variety of membrane types and involve a number of different cations: for example, Na+K+ ATPases, H+ ATPases, and H+K+ ATPase (Solioz & Vulpe Citation1996). In the case of IAA production, IAA is an auxin required by most plant cells for division and root initiation (Smith Citation2000). Egamberdiyeva (Citation2009) reported that IAA-producing bacteria significantly increased plant growth under salt stress. In addition, phosphate solubilization and protein hydrolysis were important characteristics due to their role in biofertilization (Vessey Citation2003; Singh et al. Citation2011). Especially in saline soil, a large portion of soluble inorganic phosphate applied to the soil as chemical fertilizer is immobilized in the soil rapidly and then becomes unavailable to the plant (Goldstein Citation1986). Therefore, the three isolates of PDMCd2007, PDMCd0501, and PDMZnCd2003, which were halotolerant bacteria containing multiple properties of PGPR, were selected for identification.
Bacterial identification
Based on the experiments of phenotypic characterization, the three isolates are gram negative and rod-shaped. The capacity to use different carbon sources of isolates PDMCd2007, PDMCd0501, and PDMZnCd2003 was evaluated using the API 20E biochemical test kit (). The results showed that isolates PDMCd2007, PDMCd0501, and PDMZnCd2003 were classified as Serratia marcescens, Serratia marcescens, and Pseudomonas aeruginosa, respectively. According to the results of the API 20E biochemical test, PDMCd2007 and PDMCd0501 were able to utilize seven out of nine carbon sources evaluated, and they were unable to utilize rhamnose and arabinose as sole carbon sources. PDMZnCd2003 was able to assimilate eight out of twelve carbon sources evaluated.
Table 3. Biochemical characterization of bacteria isolates evaluated using the API 20E biochemical test kit.
In addition, the 16S rRNA gene of the salt tolerant plant growth-promoting bacteria was sequenced using the universal primers fD1 and rP2. The PCR products of the three isolates, PDMCd2007, PDMCd0501, and PDMZnCd2003, about 1.5 kb of partial 16S rDNA gene, were purified and sequenced. The sequences obtained were analyzed using the BLAST program. The isolate PDMZnCd2003 showed a 99% identity with Pseudomonas sp., while the isolates PDMCd2007 and PDMCd0501 were assigned to Serratia sp. These finding corroborate the results obtained by the API 20E biochemical test kit. These results indicated that the bacterial isolates were a putative Pseudomonas sp. PMDZnCd2003, Serratia sp. PDMCd2007, and Serratia sp. PDMCd0501.
Effect of bacterium inoculation on rice germination and seedlings under salt stress
Bacterial selection
More specifically, the soil Pseudomonas received paramount attention because of its catabolic versatility, efficient root-colonizing ability and capacity to produce a wide range of enzymes and metabolites that help plants withstand varied biotic and abiotic stresses (Vessey Citation2003). Pseudomonas spp. have a widespread distribution in saline and non-saline soils (Rangarajan et al. Citation2002), and some salt-tolerant Pseudomonas spp. had the ability to colonize the rice rhizosphere and ability to suppress bacterial leaf blight and sheath blight pathogens of rice (Rangarajan et al. Citation2003). In addition, much research has been reported in which Pseudomonas sp. could serve as the ideal bioinoculant to promote growth for crops in saline soils (Egamberdiyeva Citation2007, Citation2009; Paul & Nair Citation2008; Yao et al. Citation2010; Singh et al. Citation2011). Especially, Pseudomonas sp. PMDZnCd 2003 can produce exopolysaccharide (EPS) (Meesungnoen et al. Citation2012). Ashraf et al. (Citation2004) suggested that inoculated selected EPS-producing bacteria could serve as a useful tool for alleviating salinity stress in salt-sensitive plants. Owing to the PGPR properties and trend to be a biofertilizer of Pseudomonas species, Pseudomonas sp. PMDZnCd 2003 was selected for inoculation to obtain an effect on rice germination and seedlings under salt stress. In addition, saline soil was descriptive of a soil having an electrical conductivity (ECe) > 4dS/m, approximately 0.2% (w/v) of NaCl or more (Richard Citation1954). A list of rice cultivated in saline paddy fields, in northeast Thailand, showed various salt-tolerant groups of sensitive (0–2 dS/m), moderately sensitive (2–4 dS/m), moderately tolerant (4–8 dS/m), and tolerant (8–16 dS/m) (Kanawapee et al. Citation2013). Therefore, the effects of salinity conditions of 0–16 dS/m was studied on the growth and IAA production of Pseudomonas sp. PMDZnCd 2003 and the germination and seedlings of O. sativa L.cv. RD6, a moderately salt tolerant variety. shows that the growth and IAA production of Pseudomonas sp. PMDZnCd 2003 were significantly unaffected by the salinity stresses of 4–16 dS/m. The results suggest that the bacterial stain should be used as a PGPR for rice grown under salt stress.
Effect of bacterial inoculation on germination and seedlings of rice
The inoculation effects of Pseudomonas sp. PDMZnCd2003 on rice germination and seedlings were investigated in plate and pot experiments, respectively. shows the salinity conditions that affected rice germination. Increasing salinity from 4 to 16 dS/m caused significant and progressive reductions in rice germination when compared with the control (0 dS/m) (P < 0.05); percentage of germination reduced from 21% to 14%, root length (cm) reduced from 3.08 to 2.09, shoot length (cm) reduced from 5.51 to 2.85, fresh weight (g) reduced from 0.39 to 0.25, and dry weight (g) reduced from 0.09 to 0.05. Interestingly, inoculation with Pseudomonas sp. PDMZnCd2003 badly affected the rice germination by significantly decreasing the rice seedlings when compared with the absence Pseudomonas sp. PDMZnCd2003 (P < 0.05). Coating rice seeds with CMC (CMC+Salt) could decrease the salt stress and the impact of inoculation of Pseudomonas sp. PDMZnCd2003 on the rice seedling.
Table 4. Effect of salinity and P. aeruginosa PDMZnCd2003 inoculation on rice germination in plate experiments.
From the pot experiment, shows that both salinity stress as well as the effect of inoculation of Pseudomonas sp. PDMZnCd2003 on the germinated rice tended to decrease their growth when the salt concentration increased from 4 to 16 dS/m. In addition, coating the germinated rice with CMC also showed a benefit of decreasing salt stress and the impact of Pseudomonas sp. PDMZnCd2003 on rice growth. The bacterial colonization on the rhizosphere of rice roots, both in plate and pot experiments, was re-checked by sampling some roots and streaking on TSA, then the bacterial colonies were obvious on the agar medium.
Table 5. Effect of salinity and P. aeruginosa PDMZnCd2003 inoculation on rice seedling in pot experiments.
Exposure to saline and inevitably salinity stress can reduce plant growth or cause plant death due to large amounts of Na+ having an impact on nutrient deficiency, nutrient imbalance within the plant's organs, and inhibition of many enzymes (del-Amor & Cuadra-Crespo Citation2012 Yao et al. Citation2010; Zhu Citation2001). Salt stress inhibits plant growth by disrupting homeostasis in water status and ionic distribution as well as causing oxidative stress and an increased production of ethylene (Shannon & Grieve Citation1999; Tester & Davenport Citation2003). In addition, many studies reported that salinity affected rice yield (Grattan et al. Citation2002). Carboxymethyl cellulose (CMC) is a cellulose-derived ester, originating from the reaction of cellulose with sodium hydroxide and with sodium monochloroacetate, which results in a long chain of anhydroglucose that in turn generates a highly hygroscopic and viscous polymer, nontoxic to humans. CMC, an amylose with many hydroxyl and carboxylic groups, can absorb water and moisture. The hydrogel made of it has many excellent properties, such as high water content, good biodegradation, and wide source, so it is low cost. The CMC gave the possibility to improve the quality of agricultural products such as paprika, petunia, and violet (Zlatković & Rašković Citation1998). Organic compost and CMC positively affect marketable maize (Zea mays L.) yield due to increasing water and fertilizer use, especially when applied as a mixture (Ali Citation2011). That might be a reason why coating rice seed with CMC resulted in higher growth.
Auxin production is widespread among PGPB. It is an important hormone that activates plant cell division. However, at high concentrations, it can suppress morphogenesis (Smith Citation2000), and causes conspicuous swelling of the root tips, this was found in pea (Pisum sativum L.) (Eliasson et al. Citation1989). Moreover, IAA has activity in the plasma membrane H+ ATPase (Cho & Hong Citation1995) that might induce Na+ loading into the root cell (Silva & Gerós Citation2009; Munns & Tester Citation2008). Therefore, as Pseudomonas sp. PDMZnCd2003 could produce high concentration of IAA under saline conditions, it might result in a negative impact on the germination and seedlings of O. sativa L.cv. RD6. In addition, Pseudomonas could be PGPB, biocontrol, or even plant pathogen (Ali et al. Citation2011; Jan et al. Citation2011; Xie et al. Citation2012; Fröhlich et al. Citation2012). Therefore, the unpleasant effect of Pseudomonas sp. PDMZnCd2003 on the rice germination and seedling should be studied further.
Conclusions
All bacteria isolated showed high salt resistance. The three isolates of PDMCd0501, PDMCd2007, and PDMZnCd 2003 had multiple plant growth properties (IAA production, N2-fixation, phosphate solubilisation and casein hydrolysis). An API 20E test and 16S rDNA gene sequencing indicated that PDMZnCd2003 was Pseudomonas sp., and PDMCd2007 and PDMCd0501 were Serratia sp. Due to the PGPR properties and trend to be a biofertilizer, Pseudomonas sp. PMDZnCd 2003 was selected for inoculation to determine the effect on rice germination and seedlings under salt stress. The growth curve and IAA production of Pseudomonas sp. PDMZnCd2003 was unaffected by salinity stress of 4–16 dS/m. However, application of salt-tolerant Pseudomonas sp. ZnCd2003 resulted in significantly unexpected results that showed a reduction in germination, root length, shoot length, fresh weight, and dry weight of O. sativa L. cv. RD6, while the CMC coating had positive effects on rice growth. The unpleasant effects possibly occur due to a high IAA production, and also through a pathogenicity of the bacterium to the rice. However, this assumption needs further study.
Acknowledgments
The authors express their thanks to Faculty of Science, Mahasarakham University for providing a grant for this research, and are grateful to Dr. Jolyon Dodgson for English proofreading. N. Panitlertumpai gratefully thanks Junior Science Talent Project, National Science and Technology Development Agency, for grant JSTP-06-54-02E.
References
- Ali LKM. 2011. Significance of applied cellulose polymer and organic manure for ameliorating hydro-physico-chemical properties of sandy soil and maize yield. Aust J Basic Appl Sci. 5:23–35.
- Ali SZ, Sandhya V, Grover M, Linga VR, Bandi V. 2011. Effect of inoculation with a thermotolerant plant growth promoting Pseudomonas putida strain AKMP7 on growth of wheat (Triticum spp.) under heat stress. J Plant Interact. 6:239–246. doi:10.1080/17429145.2010.545147
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST a new generation of protein database search programs. Nucleic acids Res. 25:3389–3402. doi:10.1093/nar/25.17.3389
- Asch F, Wopereis MCS. 2001. Responses of field-grown irrigated rice cultivars to varying levels of floodwater salinity in a semi-arid environment. Field Crop Res. 70:127–137. doi:10.1016/S0378-4290(01)00128-9
- Ashraf M, Hasnain S, Berge O, Mahmood T. 2004. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol Fertil Soils. 40:157–162.
- Bradford MM. 1976. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem. 72:248–254. doi:10.1016/0003-2697(76)90527-3
- Bric JM, Bostock RM, Silversone SE. 1991. Rapid in situ assay for indole acetic acid production by bacteria immobilization on a nitrocellulose membrane. Appl Environ Microbiol. 57:535–538.
- Bui EN. 2013. Soil salinity: a neglected factor in plant ecology and biogeography. J Arid Environ. 92:14–25. doi:10.1016/j.jaridenv.2012.12.014
- Cho HT, Hong YN. 1995. Effect of IAA on synthesis and activity of the plasma membrane H+-ATPase of sunflower hypocotyls, in relation to IAA-induced cell elongation and H+ excretion. J Plant Physiol. 145:717–725. doi:10.1016/S0176-1617(11)81286-1
- Chung CT, Miller RH. 1993. Preparation and storage of competent Escherichia coli cells. Meth Enzymol. 218:621–627.
- Del-Amor FM, Cuadra-Crespo P. 2012. Plant growth-promoting bacteria as a tool to improve salinity tolerance in sweet pepper. Funct Plant Biol. 39:82–90. doi:10.1071/FP11173
- Egamberdiyeva D. 2007. The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Appl Soil Ecol. 36:184–189. doi:10.1016/j.apsoil.2007.02.005
- Egamberdiyeva D. 2009. Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiol Plant. 31:861–864. doi:10.1007/s11738-009-0297-0
- Eliasson L, Bertell G, Bolander E. 1989. Inhibitory action of auxin on root elongation not mediated by ethylene. Plant Physiol. 91:310–314. doi:10.1104/pp.91.1.310
- Fröhlich A, Buddrus-Schiemann K, Durner J, Hartmann A, von Rad U. 2012. Response of barley to root colonization by Pseudomonas sp. DSMZ 13134 under laboratory, greenhouse, and field conditions. J Plant Interact. 7:1–9. doi:10.1080/17429145.2011.597002
- Glick BR. 1995. The enhancement of plant growth by free-living bacteria. Can J Microbiol. 41:109–117. doi:10.1139/m95-015
- Goldstein AH. 1986. Bacterial solubilization of mineral phosphates: historical perspectives and future prospects. Am J Alternative Agr. 1:57–65.
- Grattan SR, Zeng L, Shannon MC, Roberts SR. 2002. Rice is more sensitive to salinity than previously thought. Calif Agr. 56:189–195. doi:10.3733/ca.v056n06p189
- Han HS, Lee KD. 2005. Plant growth promoting rhizobacteria effect on antioxidant status, photosynthesis, mineral uptake and growth of lettuce under soil salinity. Res J Agr Biol Sci. 1:210–215.
- Hynnienen A. 2010. Zinc, cadmium and lead resistance mechanisms in bacteria and their contribution to biosensing [Academic Dissertation in Microbiolology]. Finland: University of Helsinki.
- Jan AT, Azam M, Ali A, Haq QMR. 2011. Novel approaches of beneficial Pseudomonas in mitigation of plant diseases – an appraisal. J Plant Interact. 6:195–205. doi:10.1080/17429145.2010.541944
- Kanawapee N, Sanitchon J, Srihaban P, Theerakulpisut P. 2013. Physiological changes during development of rice (Oryza sativa L.) varieties differing in salt tolerance under saline field condition. Plant Soil. 370:89–101. doi:10.1007/s11104-013-1620-5
- Land Development Department. 1991. Distribution of salt affected soils in the northeast region 1:100,000 map. Bangkok: Land Development Department.
- Mayak S, Tirosh T, Glick BR. 2004. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem. 42:565–572. doi:10.1016/j.plaphy.2004.05.009
- Meesungnoen O, Nakbanpote W, Thiwthong R, Thuamnu K. 2009. Plant growth promoting properties of heavy metal tolerant bacteria isolated from Gynura pseudochina (L.) DC.'s rhizosphere, Proceeding of International Conference on Green and Sustainable Innovation (ICGSI); 2009 Dec 2–4; Thailand: Chiang Rai. p. 618–624.
- Meesungnoen O, Nakbanpote W, Thiwthong R, Tummanu K. 2012. Zinc and cadmium resistance mechanism of Pseudomonas aeruginosa PDMZnCd2003. Res J Biol Sci. 7:4–13. doi:10.3923/rjbsci.2012.4.13
- Munns R, Tester M. 2008. Mechanisms of salinity tolerance. Ann Rev Plant Biol. 59:651–681. doi:10.1146/annurev.arplant.59.032607.092911
- Nautiyal CS, Srivastava S, Chauhan PS, Seem K, Mishra A, Sopor SK. 2013. Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol Biochem. 66:1–9. doi:10.1016/j.plaphy.2013.01.020
- Paul D, Nair S. 2008. Stress adaptations in a plant growth promoting rhizobacterium (PGPR) with increasing salinity in the coastal agricultural soils. J Basic Microbiol. 48:378–384. doi:10.1002/jobm.200700365
- Pierzynski GM, Sims JT, Vance GF. 2005. Soils and environmental quality. 3th ed. Florida: CRC Press Taylor & Francis Group.
- Rad HE, Aref F, Rezaei M. 2012. Evaluation of salinity stress affects rice growth and yield components in northern Iran. Am J Sci Res. 54:40–51.
- Rangarajan S, Saleena LM, Nair S. 2002. Diversity of Pseudomonas spp. isolated form rice rhizosphere populations grown along a salinity gradient. Microb Ecol. 43:280–289. doi:10.1007/s00248-002-2004-1
- Rangarajan S, Saleena LM, Vasudevan P, Nair S. 2003. Biological suppression of rice diseases by Pseudomonas spp. under saline soil conditions. Plant Soil. 251:73–82. doi:10.1023/A:1022950811520
- Rengasamy P. 2002. Transient salinity and subsoil constraints to dryland farming in Australian sodic soils: an overview. Aust J Exp Agr. 42:351–361. doi:10.1071/EA01111
- Richard LA. 1954. Diagnosis and improvement of saline and alkali soils. Agriculture Handbook No. 60. United States Department of Agriculture. Washington, USA: Government Printing Office.
- Sambrook J, Russel DW. 2001. Molecular cloning: a laboratory manual. 3th ed. New York: Cold Spring Harbor Laboratory Press.
- Sapsirisopa S, Chookietwattana K, Maneewan K, Khangkhan P. 2009. Effect of salt-tolerant Bacillus inoculum on rice KDML 105 cultivated in saline soil. As J Food Ag-Ind. 2:S69–S74.
- Shannon MC, Grieve CM. 1999. Tolerance of vegetable crops to salinity. Sci Hortic 78:5–38. doi:10.1016/S0304-4238(98)00189-7
- Siddikee MA, Glick BR, Chauhan PS, Yim WJ, Sa T. 2011. Enhancement of growth and salt tolerance of red peper seedlings (Capsicum annuu L.) by regulating stress ethylene synthesis with halotolerant bacteria containing 1-aminocyclopropane-1-carboxylic acid deaminase activity. Plant Physiol Biochem. 49:427–434. doi:10.1016/j.plaphy.2011.01.015
- Silva P, Gerós H. 2009. Regulation by salt of vacuolar H+-ATPase and H+-pyrophosphatase activities and Na+/H+ exchange. Plant Signal Behav. 4:718–726. doi:10.4161/psb.4.8.9236
- Singh JS, Pandey VC, Singh DP. 2011. Efficient soil microorganisms: A new dimension for sustainable agriculture and environmental development. Agr Ecosyst Environ. 140:339–353. doi:10.1016/j.agee.2011.01.017
- Smith RH. 2000. Plant tissue culture: techniques and experiments. 2nd ed. Florida: Academic press.
- Solioz M, Vulpe C. 1996. CPx-type ATPases: a class of P-type ATPases that pump heavy metals. Trends Biochem Sci. 21: 237–241.
- Tester M, Davenport R. 2003. Na tolerance and Na transport in higher plant. Ann Bot. 91:503–527. doi:10.1093/aob/mcg058
- Vessey JK. 2003. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 255:571–586. doi:10.1023/A:1026037216893
- Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 1991. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 173:697–703.
- Willey JM, Sherwood LM, Woolverton CJ. 2009. Prescott's principles of microbiology. New York: McGraw-Hill.
- Wood J, Scott KP, Avgustin G, Newbold CJ, Flint HJ. 1998. Estimation of the relative abundance of different bacteroides and Prevotella Ribotypes in gut samples by restriction enzyme profiling of PCR-amplified 16S rRNA gene sequences. Appl Environ Microbiol. 64:3683–3689.
- Xie G, Cui Z, Tao Z, Qiu H, Liu H, Ibrahim M, Zhu B, Jin G, Sun G, Almoneafy A, Li B. 2012. Genome sequence of the rice pathogen Psudomonas fuscovaginae CB98818. J Bacteriol. 194:5479–5480. doi:10.1128/JB.01273-12
- Yao L, Wu Z, Zheng Y, Kaleem I, Li C. 2010. Growth promotion and protection against salt stress by Pseudomonas putida Rs-198 on cotton. Eur J Soil Biol. 46:49–54. doi:10.1016/j.ejsobi.2009.11.002
- Zhu JK. 2001. Plant salt tolerance. Trends Plant Sci. 6:66–71. doi:10.1016/S1360-1385(00)01838-0
- Zlatković S, Rašković L. 1998. The effect of the polyacrylamide, polyvinylalcohol, and carboxymethylcellulose on the aggregation of the soil and on the growth of the plants. Facta Universitatis: Working and Living Environmental Protection. 1:17–23.