Abstract
Salt is one of the major abiotic stresses limiting the productivity and the geographical distribution of crops. To gain a better understanding of NaCl stress responses in model plant Arabidopsis roots, the protein changes in the abundance (Coomassie Brilliant Blue R-350 stain) and phosphorylation (Pro-Q Diamond stain) were examined using two-dimensional electrophoresis coupled with mass spectrometry (MS). Seventeen unique proteins differentially changed in abundance, phosphorylation, or both in response to NaCl. Nonsynchronous differences were found between total proteins and phosphorylated proteins. Protein synthesis, proteolysis, post-translational modifications, and isoforms might cause the differential protein redundancies. The identified proteins are involved in binding, catalysis, signal transduction, transport, metabolisms of cell wall and energy, and reactive oxygen species (ROS) scavenging and defense. These protein changes provide new avenues of investigation into the underlying salt stress response in Arabidopsis roots and demonstrate the advantages of proteomic approach in plant biology studies.
1. Introduction
Salinity is a common abiotic stress, and seriously limits the growth and productivity of glycophytes, wherein falls major crops. It is predicted that in the next 50 years, increased salinization of arable land will result in up to 50% land loss. How to improve the salt tolerance of crop plants should be given priority in the botanic researches (Wang et al. Citation2003).
Salinity stress in plants is multifactorial and causes osmotic stress and cellular Na+ toxicity. As a result, vital enzymes and metabolic processes are inhibited. Therefore, plant life style is sensible and have to undergo a series of responses including signal perception, transduction, cellular and morphological changes to survive in the salinity environment (Yadav et al. Citation2011).
Plant roots are the primary site of salinity perception, and transmit the salt signal to the shoot for salt response in the entire plant. The better understanding of salt response in the root will disclose the plant salt-tolerant mechanisms to increase the crop productivity. A lot of salt-responsive genes have been reported in the previous reports (Shavrukov Citation2013), but mRNA level is not correlated well with protein level. Because proteins are major executors of life processes, post-transcriptional (e.g. proteomic), and post-translational (e.g. phosphoproteomic) researches are crucial for deeper understanding of mechanisms of crop adaption to salinity stress. However, the salt-responsive mechanisms of phosphoproteome or proteome have not been well characterized (Rampitsch et al. Citation2012). To show the abundance and distribution of phosphoproteins, a Pro-Q Diamond dye was widely used to specially stain the phosphoproteins for the high-throughput identification and quantification of phosphoproteins (Schulenberg et al. Citation2004; Agrawal & Thelen Citation2006).
Here, we used two-dimensional gel electrophoresis coupled with mass spectrometry to investigate salt-responsive changes in the abundance (Coomassie Brilliant Blue R-350 stain) and phosphorylation (Pro-Q Diamond stain) of proteins in the seedling roots of model plant Arabidopsis. Our study provides new insights into salt stress responses in Arabidopsis and demonstrates the advantages of proteomic analysis. These protein changes under salt stress may suggest new avenues of investigation into the molecular mechanisms of salt adaption in plants.
2. Materials and methods
2.1. Plant materials and growth conditions
Arabidopsis (wild-type, ecotype Col-0) seeds were sown in normal Murashige and Skoog (MS) medium. After vernalization in the dark at 4°C for three days, they were transferred into incubator at 22°C and 60 µmol m−2 s−1 illumination for 7–8 days. Then they were transferred to an inverted orientation on MS medium with or without 200 mM NaCl and maintained for 96 h. The control or NaCl-treated seedling root tissues were harvested and quickly stored in liquid nitrogen. Finally, the roots were stored at −80°C refrigerator roots for the use (Figure S1Footnote1).
2.2. Extraction of Arabidopsis root proteins
Roots were ground to powder in liquid nitrogen, dissolved for 2 h in lysis solution (7 M urea, 2 M thiourea, 4% CHAPS, 65 mM DTT) and centrifuged at 40,000×g for 1 h at 4°C. The supernatant was precipitated with 4 volumes of ice-cold acetone, stored for 1 h at −20°C, thawed and centrifuged at 20,000×g for 1 h at 4°C. Precipitates were washed with 90% ice-cold acetone, dissolved in 5 mL lysis solution, and stored at −80°C. Sample protein concentrations were determined with the Bradford assay (Bio-Rad; Gotham et al. Citation1988).
2.3. Gel electrophoresis and gel staining
Two-dimensional gel electrophoresis was performed as previously described (Gorg et al. Citation2004). Before the two-dimensional electrophoresis, nonlinear pH 3–10 immobilized pH gradient strips (13 cm) used for one-dimensional isoelectric focusing were equilibrated in two steps: reduction with dithiothreitol (DTT) and carboxymethylation with iodoacetamide. The equilibrated strips were run on 12% (w/v) sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) at 25 mA per gel.
Two kinds of stains were applied to each gel. First for phosphorylated protein staining, the gel was immersed in 250 mL of fixation solution (50% methanol, 10% acetic acid) with gentle agitation at least twice for 30 min each time. The gel was then washed with 250 mL distilled water (three changes, 10 min per wash). Then, the gel was incubated with 200 mL Pro-Q Diamond phosphoprotein stain for 4 h in the dark, and destained with 250 mL of destaining solution in the dark (20% ACN, 50 mM sodium acetate pH 4.0, two changes, 1 h per wash). The image was acquired on Typhoon 9410 (Amersham Biosciences) with a 532-nm laser excitation and a 580-nm band-pass emission filter. For total protein staining, following the image acquisition, the gel was stained with Coomassie Brilliant Blue R-350 (Amersham Biosciences). Gels were run in triplicate to confirm the spot patterns and were scanned with a Z320 scanner (Founder, Beijing, China). Gel images were processed and analyzed with an Imagemaster 6.0 software (GE Healthcare).
2.4. Mass spectrometric analyses and protein identification
Protein spots excised from two-dimensional gels were destained with 25 mM NH4HCO3/50% (v/v) ACN and dried, followed by in-gel digestion with 0.01 µg trypsin in 0.01 ml 25 mM NH4HCO3 for 12 h at 37°C. Digestion buffer was removed to a new 1.5 mL, clear microtube (Axygen Scientific, Union City, CA, USA), 50 µl of 1% trifluoroacetic acid (v/v) in 50% (v/v) ACN was added to the gel plugs, which were sonicated for 30 min. This extract was removed and combined with the digestion buffer and freeze-dried (Labconco, Kansas City, MO, USA; Kumarathasan et al. Citation2005). Peptides were then resuspended in 15 µL of 0.5% (v/v) trifluoroacetic acid in Milli-Q water. Peptides were analyzed by an ultraflex III TOF/TOF (Bruker Daltonik GmbH, Germany) in the positive reflection mode under the control of Compass 1.2 and WarpLC 1.1 software (Bruker Daltonik, GmbH, Germany). Detected peptide compounds with a signal-to-noise ratio higher than 10 were subjected to matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry/mass spectrometry (MS/MS). External calibration was performed with the ProteomMass peptide and protein MALDI/MS calibration kit (Sigma). Each mass spectrum represents the sum of 150–200 laser shots collected from ≥30 different positions within each spot. Masses frequently detected that arose from the matrix, trypsin, or known contaminants (e.g. keratins) were not analyzed.
MS and MS/MS spectra were subjected to database search employing MASCOT (www.matriscience.com) and NCBI sequence database (NCBInr 20130101) restricted to Arabidopsis (35375 sequences; 14477754 residues). As for differential total protein identification, the algorithm was set to use trypsin as the enzyme, allowing for one missed cleavage site and assuming carbamidomethyl as a fixed modification of cysteine and oxidized methionine as a variable modification. As for differential phosphoprotein identification, the algorithm was set to use trypsin as the enzyme, allowing for one missed cleavage site and assuming carbamidomethyl as a fixed modification of cysteine, oxidized methionine as a variable modification, and phosphorylation as a variable modification for serine/threonine and tyrosine.
For protein mass fragment (PMF) data, peptide mass tolerance was set to ±0.3 Da. For MS/MS database searches, mass tolerance of precursor ions and fragment ions was set to 150 ppm and ±0.4 Da. Protein hits were considered identified if the Mascot score was greater than 60 and matched at least four peptides for peptide mass fingerprinting and 37 for MS/MS analysis (significance level, p < 0.05). If more than one protein was identified in a spot, the single protein member with the highest score (top rank) was chosen from the multiprotein family.
2.5. Bioinformatic analysis
Proteins were distinguished functionally by a step-by-step classification and each protein was placed in only one category. The proteins were first scored according to their function reported in the literature and the Kyoto Encyclopedia of Genes and Genomes database (Release 52.0, http://www.genome.jp/kegg/): proteins that could not be defined were sought in the PIR database (Release 15.9, http://pir.georgetown.edu/). Proteins were also scanned for phosphorylation by plant protein phosphorylation database, P(3)DB (Release 3.0, http://www.p3db.org/index.php).
3. Results
3.1. Arabidopsis root growth under salt stress
Arabidopsis is a typical glycophyte that is not very salt tolerant, but it is always best model plant to unravel plant salt tolerance mechanisms. To select an appropriate concentration for salt stress prior to proteomic analysis, a root elongation dose response assay was performed under different NaCl concentrations (Figure S1). The results indicated 200 mM NaCl treatment almost inhibited the root growth, but 250 mM NaCl treatment caused the death of seedlings. Therefore, 200 mM was selected as the treatment concentration to be used in the present study.
3.2. Proteomic and phosphoproteomic profilings of Arabidopsis roots under salt stress
To investigate the differential expression of total proteins and phosphorylated proteins in Arabidopsis roots under salt stress, two-dimensional electrophoresis analyses from three biologically independent replicate experiments were carried out. The representative gels were shown in . Approximately 600 Coomassie Brilliant Blue R-350-stained spots and 150 Pro-Q Diamond-stained spots were reproducibly detected in Arabidopsis roots under salt stress. Quantitative image analysis revealed 43 total protein spots and 17 phosphorylated protein spots that changed their abundance (vol%) significantly (p < 0.05) by more than 2.0-fold.
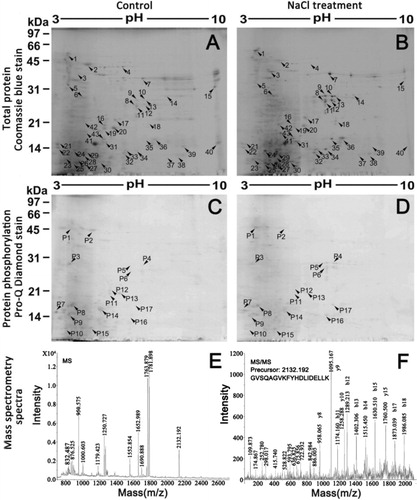
3.3. Identification of differential proteins by MS analysis in Arabidopsis roots
Among the 32 significantly upregulated total protein spots in the salt treatment group, 22 spots corresponding to 13 different gene products were identified, and among the 11 significantly downregulated total protein spots in the salt treatment group, 6 spots were identified to represent 5 different gene products. Among the 7 significantly upregulated phosphorylated protein spots in the salt treatment group, 5 spots corresponding to 2 different gene products were identified, and among the 10 significantly downregulated phosphorylated protein spots in the salt treatment group, 8 spots were identified to represent 6 different gene products. Totally, 17 unique proteins within 60 gel spots that differentially changed in abundance, phosphorylation, or both in response to NaCl stress were successfully identified by MS analysis (). They are V-type proton ATPase subunit B1, TNF receptor associated factor (TRAF)-like family protein, START/RHO_alpha_C/PITP/Bet_v1/CoxG/CalC (SRPBCC) ligand-binding domain-containing protein, PYK10-binding protein 1, peroxidase 22, nitrile-specifier protein 1, MLP-like protein 328, leucine aminopeptidase 1, jacalin-like lectin domain-containing protein heat shock 70 kDa protein 1/8, granulin repeat cysteine protease family protein, glyceraldehyde-3-phosphate dehydrogenase, calmodulin 5, calmodulin 2, beta-glucosidase 23, beta-glucosidase 22, and ATPase subunit 1. also shows the PMF and MS/MS spectra of a differential protein (beta-glucosidase 23).
Table 1. Identification of differential total proteins and phosphoproteins of Arabidopsis thaliana roots under salt treatment by MS analysis.
3.4. Bioinformatic analysis of differential proteins by MS analysis in Arabidopsis roots
Functional classification analysis according to gene ontology (GO) annotations and PubMed references revealed that the proteins were clustered into several categories (). Those 31 differential proteins corresponding to 17 unique proteins were involved in binding (13%), catalysis (39%), signal transduction (13%), metabolisms of cell wall (3%), and energy (13%), and reactive oxygen species (ROS) scavenging and defense (19%). Kyoto encyclopedia of genes and genomes (KEGG) analysis indicated biosynthesis of phenylpropanoids is the most enriched pathway, which included three differential proteins: heat shock cognate 70 kDa protein 1, peroxidase 22, and glyceraldehyde-3-phosphate dehydrogenase.
The biological process term of GO showed only 6 proteins (peroxidase 22, and glyceraldehyde-3-phosphate dehydrogenase, granulin repeat cysteine protease family protein, SRPBCC ligand-binding domain-containing protein, beta-glucosidase 23, and beta-glucosidase 22) involved in the response to salt stress in the literatures. The left 11 differential proteins were first reported salt-responsive proteins. Among seven unique phosphorylated differential proteins, calmodulin 2, PYK10-binding protein 1, and TRAF-like family protein have been recorded in P(3)DB, and the left 4 phosphorylated differential proteins (beta-glucosidase 23, granulin repeat cysteine protease family protein, jacalin-like lectin domain-containing protein, and SRPBCC ligand-binding domain-containing protein) were first reported.
4. Discussion
When plant encountered with salt stress, not only proteome but also phosphoproteome would change for the tolerance and adaption to salinity. After the perception of the salt stress, signal would be transferred from cell surface to the nucleus, and then responsive proteins would be translated. All these are controlled by protein phosphorylation (Kersten et al. Citation2009). As for low abundance of phosphoproteins, Pro-Q Diamond Phosphoprotein stain is a good choice and has been widely used (Chitteti & Peng Citation2007). Arabidopsis represents the most successful model with many advantages: a small and well-annotated genome; easy growth and manipulation. It was believed that the gained information could be applicable to most plant species and especially the major corps of the world (Guo et al. Citation2012). Here, we performed proteome and phosphoproteome of Arabidopsis roots under salt stress in order to throw new light on the plant physiology of salt tolerance.
Ten differential spots were common between 28 differential total protein spots and 13 differential phosphoprotein spots. Granulin repeat cysteine protease family protein and PYK10-binding protein 1 were upregulated in total proteins but downregulated in phosphoproteins. For 16 unique differential total proteins, 4 (25%) proteins corresponded to more than 2 spots. They are glyceraldehyde-3-phosphate dehydrogenase (2 spots), heat shock 70 kDa protein 1/8 (2 spots), SRPBCC ligand-binding domain-containing protein (2 spots), and beta-glucosidase 23 (10 spots). The spots of former three proteins were all upregulated. But for beta-glucosidase 23, eight spots were upregulated, and two spots were inverse. For seven unique differential phosphoproteins, three proteins corresponded to more than two spots. They are jacalin-like lectin domain-containing protein (two downregulated spots), SRPBCC ligand-binding domain-containing protein (two upregulated spots), and beta-glucosidase 23 (three up-regulated spots and two downregulated spots). Nonsynchronous differences might be caused by protein synthesis, proteolysis, post-translational modifications, and isoforms. The similar phenomena have been reported in the literature (Jiang et al. Citation2007).
The most differential spots were identified as beta-glucosidase 23, which might play an important role in the salt adaption. Beta-glucosidase 23 (Pyk10) is a root and hypocotyl specific myrosinase from Arabidopsis thaliana (Nitz et al. Citation2001), and belongs to glycoside Hydrolase (GH) Family 1, which was implicated in many diverse processes including lignification, hydrolysis of cell wall-derived oligosaccharides, and control of active phytohormone levels (Xu et al. Citation2004). PYK10-binding protein 1 (PBP1) has some effect on the activation of PYK10, and PBP1 may act like a molecular chaperone that facilitates the correct polymerization of PYK10 when tissues are damaged and subcellular structures are destroyed (Nagano et al. Citation2005). Those two proteins were all identified as differential total proteins and phosphoproteins. Another cell wall-related protein (peroxidase 22) also increased its expression, which was involved in auxin catabolism, and participated in the cross-linking of compounds to form a physical barrier against the water loss (Degenhardt & Gimmler Citation2000). The above results suggested the remodeling of plant cell wall might play a key role in root responses to NaCl.
During salt stress, plant undergoes cellular and morphological changes to survive. The proteins involved in energy metabolism were indispensable. Here, three energy-related differential proteins were found and upregulated. They were glyceraldehyde-3-phosphate dehydrogenase (glycolysis), ATPase subunit 1 (electron transport chain), and V-type proton ATPase subunit B1 (proton-transporting ATPase complex). Glyceraldehyde-3-phosphate dehydrogenase was a vital member of glycolysis and salt increased in the roots of many plants (Zhao et al. Citation2013). A mitochondrial ATP synthase subunit alpha produces ATP from ADP in the presence of a proton gradient across the membrane which is generated by electron transport complexes of the respiratory chain. In the previous report, ATP synthase delta chain was found to be responsive to NaCl stimulus (Jiang et al. Citation2007). V-type proton ATPase subunit B1 is a the vacuolar-type H+-ATPase, which acidifies intracellular compartments and generates a proton electrochemical gradient for the further Na+ sequestration. It is a cost-effective strategy to reduce the Na+ concentration in the plant cytosol (Sze et al. Citation2002).
5. Conclusions
In this study, proteome and phosphoproteome were used to disclose the differential salt-responsive proteins in Arabidopsis roots under salt treatment and some potential important proteins were found. Those proteins were distributed in the two-dimensional electrophoresis with significant heterogeneity, and might be involved in many biological processes. Stresses have the characterization of cross talk with each other and share similar response pathways. Therefore, an understanding for salt stress can be helpful to understand other stresses. The discovery of these proteins contributed to a better understanding of plant root response to salt, and provided new potential targets for further work.
Supplemental_Material.doc
Download MS Word (359 KB)Acknowledgements
The study was supported by grants from Shandong Provincial Natural Science Foundation, China (ZR2010CQ024) and A Project of Shandong Province Higher Educational Science and Technology Program (J10LC72).
Notes
1. Supplemental Content may be viewed online at http://dx.doi.org/10.1080/17429145.2013.845262.
References
- AgrawalGK, ThelenJJ. 2006. Large scale identification and quantitative profiling of phosphoproteins expressed during seed filling in oilseed rape. Mol Cell Proteomics. 5:2044–2059. doi:10.1074/mcp.M600084-MCP200
- ChittetiBR, PengZ. 2007. Proteome and phosphoproteome differential expression under salinity stress in rice (Oryza sativa) roots. J Proteome Res. 6:1718–1727. doi:10.1021/pr060678z
- DegenhardtB, GimmlerH. 2000. Cell wall adaptations to multiple environmental stresses in maize roots. J Exp Bot. 51:595–603. doi:10.1093/jexbot/51.344.595
- GorgA, WeissW, DunnMJ. 2004. Current two-dimensional electrophoresis technology for proteomics. Proteomics. 4:3665–3685. doi:10.1002/pmic.200401031
- GothamSM, FryerPJ, PatersonWR. 1988. The measurement of insoluble proteins using a modified Bradford assay. Anal Biochem. 173:353–358. doi:10.1016/0003-2697(88)90199-6
- GuoML, YangAH, ZhouCX, LiuX. 2012. The new understanding of Arabidopsis thaliana proteins associated with salinity. J Plant Interact. 7:348–355. doi:10.1080/17429145.2011.640438
- JiangY, YangB, HarrisNS, DeyholosMK. 2007. Comparative proteomic analysis of NaCl stress-responsive proteins in Arabidopsis roots. J Exp Bot. 58:3591–3607. doi:10.1093/jxb/erm207
- KerstenB, AgrawalGK, DurekP, NeigenfindJ, SchulzeW, WaltherD, RakwalR. 2009. Plant phosphoproteomics: an update. Proteomics. 9:964–988. doi:10.1002/pmic.200800548
- KumarathasanP, MohottalageS, GoeganP, VincentR. 2005. An optimized protein in-gel digest method for reliable proteome characterization by MALDI-TOF-MS analysis. Anal Biochem. 346:85–89. doi:10.1016/j.ab.2005.06.004
- NaganoAJ, MatsushimaR, Hara-NishimuraI. 2005. Activation of an ER-body-localized beta-glucosidase via a cytosolic binding partner in damaged tissues of Arabidopsis thaliana. Plant Cell Physiol. 46:1140–1148. doi:10.1093/pcp/pci126
- NitzI, BerkefeldH, PuzioPS, GrundlerFM. 2001. Pyk10, a seedling and root specific gene and promoter from Arabidopsis thaliana. Plant Sci. 161:337–346. doi:10.1016/S0168-9452(01)00412-5
- RampitschC, BykovaNV. 2012. The beginnings of crop phosphoproteomics: exploring early warning systems of stress. Front Plant Sci. 3:144. doi:10.3389/fpls.2012.00144
- SchulenbergB, GoodmanTN, AggelerR, CapaldiRA, PattonWF. 2004. Characterization of dynamic and steady-state protein phosphorylation using a fluorescent phosphoprotein gel stain and mass spectrometry. Electrophoresis. 25:2526–2532. doi:10.1002/elps.200406007
- ShavrukovY. 2013. Salt stress or salt shock: which genes are we studying? J Exp Bot. 64:119–127. doi:10.1093/jxb/ers316
- SzeH, SchumacherK, MüllerML, PadmanabanS, TaizL. 2002. A simple nomenclature for a complex proton pump: VHA genes encode the vacuolar H(+)-ATPase. Trends Plant Sci. 7:157–161. doi:10.1016/S1360-1385(02)02240-9
- WangW, VinocurB, AltmanA. 2003. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 218:1–14. doi:10.1007/s00425-003-1105-5
- XuZ, Escamilla-TrevinoL, ZengL, LalgondarM, BevanD, WinkelB, MohamedA, ChengCL, ShihMC, PoultonJ, EsenA. 2004. Functional genomic analysis of Arabidopsis thaliana glycoside hydrolase family 1. Plant Mol Biol. 55:343–367. doi:10.1007/s11103-004-0790-1
- YadavS, IrfanM, AhmadA, HayatS. 2011. Causes of salinity and plant manifestations to salt stress: a review. J Environ Biol. 32:667–685.
- ZhaoQ, ZhangH, WangT, ChenS, DaiS. 2013. Proteomics-based investigation of salt-responsive mechanisms in plant roots. J Proteomics. 82:230–253. doi:10.1016/j.jprot.2013.01.024
