Abstract
Cell suspension cultures of Linum album Kotschy ex Boiss. have been reported to produce anticancer podophyllotoxin and its related lignans. In the present study, we investigated the effect of culture filtrate of Fusarium graminearumon growth, and lignan and phenolic compounds in L. album cell culture. After 7 days of pre-culture, the cells were treated with 1% (v/v) of the culture filtrate. Cell growth was reduced, while phodophyllotoxin and lariciresinol production was stimulated reaching a maximum 0.0187 mg/g fresh weight (FW) and 0.0136 mg/g FW 5 days after the treatment, respectively. Also, our results provide evidence that the culture filtrate of F. graminearum can be effective on phenylalanine ammonia-lyase activity and phenolic compounds.
Introduction
Linum album Kotschy ex Boiss (Linaceae), an endemic species in Iran, is also known to accumulate podophyllotoxin (PTOX) and its related lignans and has been targeted as a possible alternative source of PTOX (Petersen & Alfermann Citation2001; Fuss Citation2003). Lignans are characterized as phenylpropanoid dimers which form a large group of secondary metabolites. These compounds have beneficial effects on human health (Westcott & Muir Citation2003).
In particular, PTOX obtained commercially from Podophyllum species is an important lignan used as an antiviral agent and a precursor for the semi-synthesis of anticancer drugs like etoposide, teniposide, and etopophose (Canel et al. Citation2001).
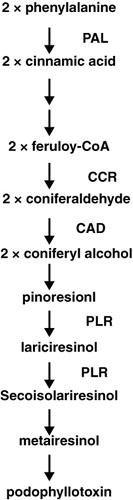
The phenylpropanoid pathway is linked to the biosynthesis of major secondary metabolites in plants, such as flavonoids, anthocyanins, lignins, and lignans (Sreelakshmi & Sharma Citation2008). Phenylalanine is deaminated by phenylalanine ammonia-lyase (PAL) to give cinnamic acid. PAL is one of the most important enzymes and has a key role in regulation of phenylpropanoid production in plants (Achnine et al. Citation2004). Hydroxylation by cinnamic acid 4-hydroxylase then leads to p-coumaric acid and finally coniferyl alcohol is formed by cinnamyl-alcohol-dehydrogenase after additional steps. Two molecules of coniferyl alcohol are directed toward the lignan pathway and coupled to produce pinoresinol. Pinoresinol is reduced via lariciresinol to secoisolariciresinol by pinoresinol-lariciresinol reductase and subsequently oxidized to matairesinol. The conversion of matairesinol into deoxypodophyllotoxin (DOP), the first aryltetralin lignan, has yet to be fully characterized, although the pathway for matairesinol to yatein, an intermediate of the matairesinol to PTOX biosynthesis, was reported. Furthermore, DOP-7-hydroxylase is thought to be responsible for the hydroxylation at positions 7 of DOP to give PTOX (Seidel et al. Citation2002; Federolf et al. Citation2004) ().
Lignans synthesis and accumulation in plant cell cultures can be stimulated by the application of elicitors (Zhao et al. Citation2005; Ionkova Citation2007). Lignans are very well known to play an important role in plant defense (Figgitt et al. Citation1989). Therefore, elicitation is used in plant cell cultures to enhance the lignan compounds. For instance, methyl jasmonate and salicylic acid were found to induce PTOX production in cell culture of L. album (Yousefzadi, Sharifi, Behmanesh, Moyano, & Palazon Citation2010). Furthermore, Baldi et al. (Citation2008) showed the increase of the PTOX production by co-culturing L. album cells with axenically arbuscular mycorrhiza-like fungi, Piriformospora indica and Sebacina vermifera. Also, Esmaeilzadeh et al. (Citation2011) found novel elicitatory activities of five fungal extracts on lignan accumulation in L. album cell cultures. They concluded that Fusarium graminearum cell extract induced the highest increase of PTOX. Thus, according to previous research in this study we investigated the elicitation potential of culture filtrate of F. graminearum on lignans and phenolic compounds in cell culture of L. album.
Materials and methods
Cell suspension cultures
Cell suspension cultures of L. album were prepared from leaf explants-derived calliin 50 mL of Murashige and Skoog (Citation1962) medium supplemented with 30 g/L sucrose, 2 mg/L naphthalene acetic acid, and 0.4 mg/L kinetin (Yousefzadi, Sharifi, Behmanesh, Moyano, & Palazon Citation2010). All suspension cultures were incubated on a gyratory shaker at 120 rpm in darkness at 25°C and subcultured every week. L. album cells cultured in mass spectrometry (MS) were treated for 3, 5, and 7 days. They were separated from medium by filtration under suction, weighted and immediately frozen in liquid nitrogen. Also, viability was determined by the Evans Blue staining technique (Baker & Mock Citation1994).
Elicitor preparation and addition to cell cultures
Cultures of F. graminearum were harvested in mid log phase. The fungal biomass was separated by passing through a filter paper and the medium was centrifuged at 5000 × g for 20 min to remove suspended particles. Culture was filtrated and sterilized through a 0.4-µm membrane filter and saved for elicitation. According to the previous research performed by Esmaeilzadeh et al. (Citation2011), we tested three concentrations of culture filtrate of F. graminearum (0.5–1–2)% v/v in L. album cell culture (data not shown) and cells were harvested for 5 days after elicitation. We found the optimal concentration of elicitor (1%) by measurement of growth and lignan contents. For elicitation, 2 g of fresh cells were transferred to 100 mL flasks containing 30 mL of the medium and were supplemented with 1% (v/v) of the fungal culture filtrate after 7 days of pre-culture. The incubation continued on a shaker at 120 rpm in darkness at 25°C. Cells were collected for analyses at 3, 5, and 7 days after elicitation.
Determination of lignans by high pressure liquid chromatography (HPLC)
Lignans were extracted from cells by sonication in methanol/water, as previously described (Yousefzadi, Sharifi, Behmanesh, Moyano, & Palazon Citation2010). The extract was dissolved in methanol again, and filtered through a Millex-LH filter with 0.45 µm pore size (Millipore, Bedford, MA, USA). The presence of lignans was verified by HPLC by comparing the Rt and UV spectral peaks of the sample with those of an authentic standard. UV spectra were measured online using a Thermo Quest. The HPLC system was equipped with a Spectra System KO 6000 LP photodiode array detector. A Grom-Sil 120 ODs-3 ST, 5 µm C18 (250 mm × 4.6 mm) was used to safeguard the analytical column. The detector was set at 290 nm. All injection volumes were 20 µL and all calculations for quantitative analysis were performed with linear regression external standardization by measurement of peak areas. A gradient system with acetonitrile (A%) and water (B%) was used as follows: (0 min): A (40), B (60), flow rate (0.8 mL/min); (17 min): A (67), B (33), flow rate (1 mL/min); (18 min): A (40), B (60), flow rate (1 mL/min); (20 min): A (40), B (60), flow rate (0.8 mL/min).
The presence of PTOX and lariciresinol in the samples was verified by MS with commercially available standards [Podophyllotoxin: Sigma Aldrich CAS Number: 518-28-5; Lariciresinol: Sigma Aldrich, CAS Number: 27003-73-2] (Esmaeilzadeh et al. Citation2011). All MS data were acquired by an instrument of positive-mode electrospray ionization quadrapole time-of-flight MS (Micromass-Waters) equipped with a nano flow probe.
Determination of total phenolics content
Folin-Ciocalteu reagent was used for analysis of total phenolics content according to Akkol et al. (Citation2008). One milliliter of methanolic extract or gallic acid (standard phenolic compound) was mixed with 5 mL Folin-Ciocalteu reagent and 4 mL sodium carbonate solution 7.0%. The mixtures were allowed to stand for 2 h before its absorbance was measured at 765 nm. Gallic acid was used as a standard for the calibration curve. Total phenol values are expressed in terms of mg equal gallic acid in 1 g FW.
Determination of flavonoids and flavonols
Total flavonoid and flavonol contents were estimated by the aluminum chloride method, according to Akkol et al. (Citation2008). For total flavonoid 1 mL of methanolic extract, 250 µL of aluminum chloride solution and 250 µL of potassium acetate were mixed and the samples remained at room temperature for 30 min. The absorbance of the reaction mixture was measured at 415 nm with a spectrophotometer. For total flavonol assay 1 mL of methanolic extract, 1 mL of aluminum chloride solution and 3 mL of sodium acetate were mixed and absorbance was read at 445 nm. Rutin was used as a standard for calibration curve. Total flavonoids and flavonols values are expressed in terms of mg equal rutin in 1 g FW.
Determination of lignin
Lignin content was measured via modified acetyl bromide procedure adapted from Iiyama and Wallis (Citation1988). In brief, 6 mg of fine-powdered, air-dried cell wall was treated with a mixture of 25% acetyl bromide (v/v inglacial acetic acid) and 0.1 mL of 70% HClO4 and incubated for 30 min at 70°C. Samples were rapidly cooled in ice, and mixed with NaOH and glacial acetic acid. The lignin content was determined by measuring absorbance at 280 nm using specific absorption coefficient value of 20 g/L/cm.
PAL activity assay
PAL was extracted from 500 mg of cells with 6.5 mL of 50 mM Tris–HCl buffer (pH 8.7) containing 15 mM of β-mercaptoethanol. The homogenate was centrifuged at 50,000 × g for 20 min, and the supernatant was collected for enzyme assay. PAL activity was determined based on the rate of cinnamic acid production, according to Wang et al. (Citation2006). One unit of enzyme activity was defined as the amount of PAL that produced 1 µmol of cinnamic acid within 1 min and was expressed as µmol cinnamic acid/mg protein/min. Total protein was assayed with bovine serum albumin as the standard using the dye-binding method of Bradford (Citation1976).
Statistical analysis
All analyses were conducted at least three times, each with three independent repetitions. The analysis of variance and the Duncan test (P ≤ 0.05) of mean comparison were performed using the MSTATC program ver. 1.4.
Results
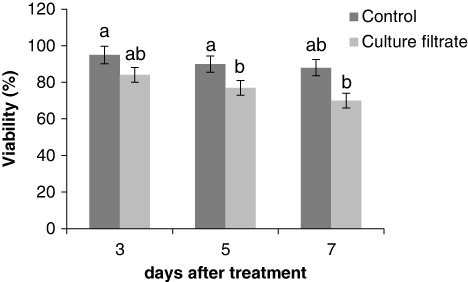
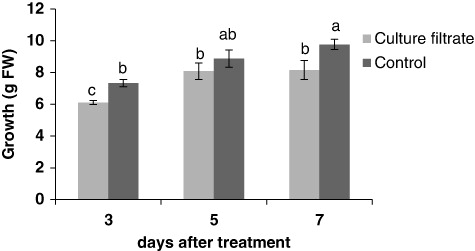
Effect of culture filtrate on cell viability and growth
L. album cell cultures grew during the linear growth phase until 7 days after subculturing, when cell viability was about 90%. As shown in , culture filtrate of F. graminearum reduces viability around 20% at 7 days after treatment compared to the control. The effects of the culture filtrate on cell growth were evaluated by measurement of the cell FW (). Culture filtrate was shown to decrease growth of cells after 3, 5, and 7 days compared with the control.
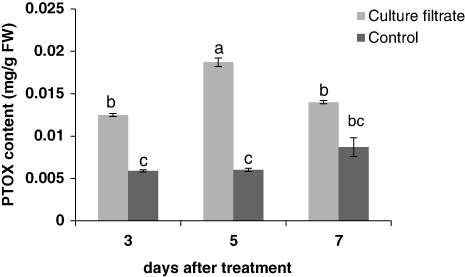
Effect of the culture filtrate on lignan production
Lignans content in samples was evaluated by HPLC and compared with an authentic standard. Formerly the presence of PTOX and lariciresinol in the samples was verified by MS (Esmaeilzadeh et al. Citation2011). The effects of culture filtrate of F. graminearum on lignan content are shown in and . The production of PTOX was significantly induced when the culture filtrate was added to the cell media. The PTOX content was 0.012 mg/g fresh weight (FW) at 3 days after treatment, whereas it significantly increased after 5 days and reached 0.018 mg/g FW ().
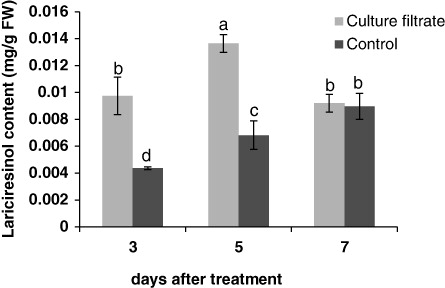
Lariciresinol content was significantly increased when the cells were subjected to fusarium culture filtrate for 3 and 5 days, but at 7 days after treatment there was no significant difference between the treated cell and controls (). At 5 days after treatment lariciresinol was greater than other times compared with its control.
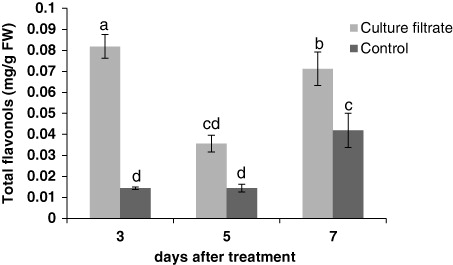
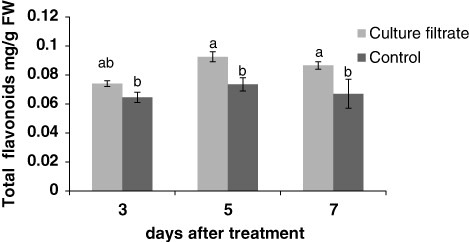
Effect of the culture filtrate on total phenolics, flavonoids, and flavonols
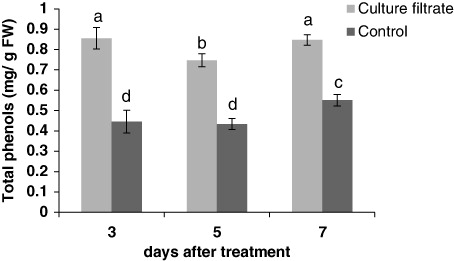
The results showed that culture filtrate of F. graminearum induced the total phenolic compounds (). The highest value of total phenolic content was 0.85 (mg/g FW) after 3 days. Flavonol and flavonoid levels were increased after treatment with culture filtrate ( and ). In general, flavonols content was very low in control samples compared with treated cells. The greatest increase in flavonols and flavonoids content was observed at 3 and 5 days after treatment, respectively.
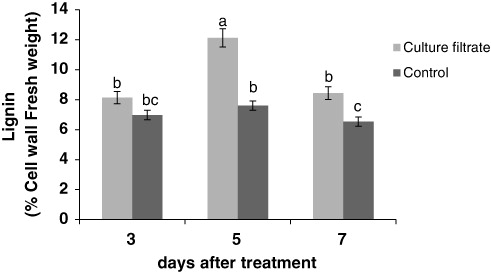
Effect of culture filtrate of F. graminearum on lignin
The results indicate that culture filtrate of F. graminearum can increase the amount of lignin in the cells compared with the control. Lignin content strongly enhanced after 5 days of treatment ().
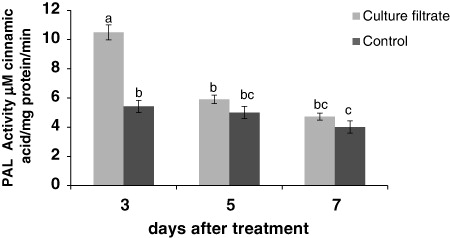
Effect of culture filtrate of F. graminearum on PAL activity
Our results showed that culture filtrate has effect on PAL activity. Under the treatment, PAL activity significantly increased. On the third day after treatment, this increase is significant (10.4 µmol cinnamic acid/mg protein/min). But the PAL activity obtained from 5 and 7 days after treatment were lower than the third day ().
Discussion
Elicitation of plant cells in culture represents a useful biotechnological tool to improve the production of valuable metabolites (Vasconsuelo & Boland Citation2007). The present study is the first report on the effects of culture filtrate of F. graminearum on growth and secondary metabolites production in L. album cells. There are few studies about the effect of fungal elicitors on lignan compounds in cell culture of L. album (Shams Ardakani et al. Citation2005; Esmaeilzadeh et al. Citation2011). For instance, Esmaeilzadeh et al. (Citation2012) reported that cell extracts of F. graminearum and Rhizopus stolonifer are potent elicitors for the PTOX and lariciresinol production among tested elicitors. In addition, Kumar et al. (Citation2012) reported that the culture filtrate of root endophytic fungus P. indica promotes the growth and lignan production of L. album hairy root cultures. Our results showed that the culture filtrate of F. graminearum leads to decrease the growth of L. album cells. Also, the decline in cell growth by fungal elicitation was reported by Chong et al. (Citation2005). The addition of the fungal elicitors to the suspension cultures also caused an enhancement in plant biomass (Baldi et al. Citation2008; Kumar et al. Citation2012). These diversities in response to elicitors could be explained by the variety in types of treatments and plant species and the concentration of each elicitor (Baldi et al. Citation2008).
Based on the results presented here, concentration of 1% (v/v) of culture filtrate of F. graminearum can increase accumulation of lignans, total phenolics, flavonols, and flavonoids.
These findings compared to other researches indicated that fungal elicitor is a more effective elicitor for lignans production than other elicitors (Esmaeilzadeh et al. Citation2011). The exposure time of an elicitor is important for its elicitation potential. Our investigations represent that the increment of PTOX and lariciresinol as well as the effect of the culture filtrate on the lignan pathway occurred within a low exposure time. Similarly, Kumar et al. (Citation2012) reported that an exposure time of 48 h of culture filtrate of P. indica was found to be optimum to induce a significant enhancement of lignan production in L. album hairy root cultures. However, by exposing the culture for a longer time (7 days), the lariciresinol was decreased. This reduction in the lariciresinol content could be due to its conversion, as a precursor, to other compounds, since the accumulation of PTOX would occur.
The phenylpropanoid pathway is linked to biosynthesis of major secondary metabolites in plants, such as flavonoids, lignins, and lignans. The close relationship between plant secondary metabolism and defense responses is widely recognized (Dixon & Paiva Citation1995). PAL catalyzes the first step of the phenylpropanoids pathway by which L-phenylalanine is deaminated to trans-cinnamic acid and is considered as an important regulation point between primary and secondary metabolism (Dixon & Paiva Citation1995). Plants respond to stresses through activating an array of defense mechanisms such as reactive oxygen species formation which is scavenged by production of phenolic compounds with antioxidant properties (Mishra et al. Citation2012; Shaheen et al. Citation2013). In our study, total phenolics, falvonoids, and flavonols were increased in response to the culture filtrate. With increasing in these compounds, also PAL activity was enhanced after elicitation. In other studies the PAL activity and plant phenolic compounds have been affected by many factors such as biotic stresses (Bidlack & Buxton Citation1995). Culture filtrate of F. graminearum could increase the amount of lignin content. Lignin known as a defensive layer in plants and also lignin increment has been reported in response to fungal elicitors (Hano et al. Citation2006; De Alwis et al. Citation2009).
Conclusion
Our results indicate that culture filtrate affects L. album cell cultures as enhancing accumulation of PTOX and lariciresinol as well as other phenolic compounds. The culture had a particularly variable effect on the accumulation of lignans and other phenolic compounds by the passage of time. The PTOX and lariciresinol increased significantly at 5 days after treatments. These responses in L. album cell culture seems to control by a highly complex mechanism for lignan and other phenolic compounds biosynthesis, which suggest further experiments to be understood. Also, characterization of active components in culture filtrate which induces the lignans and other phenolic compound production leads to ascertain the way for the elucidation of the mechanisms underlying plant cells–fungi and the resultant responses.
References
- Achnine L, Blancaflor EB, Rasmussen S, Dixon RA. 2004. Colocalization of L-phenylalanine ammonia-lyase and cinnamate 4-hydroxylase for metabolic channeling in phenylpropanoid biosynthesis. Plant Cell. 16:3098–3109. doi:10.1105/tpc.104.024406
- Akkol EK, Göger F, Kosar M, Baser HC. 2008. Phenolic composition and biological activities of Salvia halophila and Salvia virgata from Turkey. Food Chem. 108:942–949. doi:10.1016/j.foodchem.2007.11.071
- Baker CJ, Mock NM. 1994. An improved method for monitoring cell death in cell suspension and leaf disc assays using Evans Blue. Plant Cell Tiss Org. 39:7–12. doi:10.1007/BF00037585
- Baldi A, Jain A, Bisaria VC. 2008. Co-culture of arbuscular mycorrhiza-like fungi (Piriformospora indica and Sebacina vermifera) with plant cells of Linum album for enhanced production of podophyllotoxin: a first report. Biotechnol Lett. 30:1671–1677. doi:10.1007/s10529-008-9736-z
- Bidlack JE, Buxton DR. 1995. Chemical regulation of growth, yield, and digestibility of alfalfa and smooth brome grass. Plant Growth Regul. 14:1–7. doi:10.1007/BF00212639
- Bradford MM. 1976. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254. doi:10.1016/0003-2697(76)90527-3
- Canel C, Dayan FE, Ganzera M, Khan IA, Rimando A, Burandt CL Jr, Moraes RM. 2001. High yield of podophyllotoxin from leaves of Podophyllum peltatum by in situ conversion of podophyllotoxin 4-o-β-glucopyranoside. Planta Med. 67:97–99. doi:10.1055/s-2001-10636
- Chong TM, Abdulah MA, Lai OM, Nor'Aini FM, Lajis NH. 2005. Effective elicitation factors in Morinda elliptica cell suspension culture. Process Biochem. 40:3397–3405. doi:10.1016/j.procbio.2004.12.028
- De Alwis R, Fujita K, Ashitani T, Kuroda K. 2009. Induced monoterpene and lignin production in mechanically stressed and fungal elicited cultured Cupressus lusitanica cells. Plant Biotechnol Rep. 3:57–65. doi:10.1007/s11816-008-0074-3
- Dixon P, Paiva NL. 1995. Stress-induced phenylpropanoid metabolism. Plant Cell. 7:1085–1097. doi:10.1007/s11816-008-0074-3
- Esmaeilzadeh S, Sharifi M, Behmanesh M, Safaei N, Murata J, Araki R, Yamagaki T, Satake H. 2012. Time-course changes in fungal elicitor-induced lignan synthesis and expression of the relevant genes in cell cultures of Linum album. J Plant Physiol. 169:487–491. doi:10.1016/j.jplph.2011.12.006
- Esmaeilzadeh S, Sharifi M, Safaei N, Murata J, Araki R, Yamagaki T, Satake H. 2011. Increased lignan biosynthesis in the suspension cultures of Linum album by fungal extracts. Plant Biotechnol Rep. 5:367–373. doi:10.1007/s11816-011-0190-3
- Federolf K, Alfermann AW, Fuss E. 2004. Aryltetralin-lignan formation in two different cell lines of Linum album: deoxypodophyllotoxin 6-hydroxylase, a key enzyme for the formation of 6-methoxypodophyllotoxin. Phytochemistry 68:1397–1406. doi:10.1016/j.phytochem.2007.02.031
- Figgitt DP, Denever SP, Dewick PM, Jackson DE, Williams P. 1989. Topoisomerase II: a potential target for novel antifungal agents. Biochem Biophys Res Commun. 160:257–262. doi:10.1016/0006-291X(89)91649-5
- Fuss E. 2003. Lignans in plant cell and organ cultures: an overview. Phytochem Rev. 2:307–320. doi:10.1023/B:PHYT.0000045500.56476.f5
- Hano C, Addi M, Bensaddek L, Crônier D, Baltora-Rosset S, Doussot J, Maury S, Mesnard F, Chabbert B, Hawkins S, Lainé E, Lamblin F. 2006. Differential accumulation of monolignol-derived compounds in elicited flax (Linum usitatissimum) cell suspension cultures. Planta. 223:975–989. doi:10.1007/s00425-005-0156-1
- Iiyama K, Wallis A. 1988. An improved acetyl bromide procedure for determining lignin in woods and wood pulps. Wood Sci Technol. 22:271–280. doi:10.1007/BF00386022
- Ionkova I. 2007. Biotechnological approaches for the production of lignans. Pharmacogn Rev. 1:427–443.
- Kumar V, Rajauria G, Sahai V, Bisaria VS. 2012. Culture filtrate of root endophytic fungus Piriformospora indica promotes the growth and lignan production of Linum album hairy root cultures. Process Biochem. 47:901–907. doi:10.1016/j.procbio.2011.06.012
- Mishra AK, Sharma K, Misra RS. 2012. Elicitor recognition, signal transduction and induced resistance in plants. J Plant Interact. 7:95–120. doi:10.1080/17429145.2011.597517
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Planta. 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
- Petersen M, Alfermann AW. 2001. The production of cytotoxic lignans by plant cell cultures. Appl Microbiol Biotechnol. 55:135–142. doi:10.1007/s002530000510
- Seidel V, Windhövel J, Eaton G, Alferman AW, Aroo RR, Medarde M, Petersen M, Woolley JG. 2002. Biosynthesis of podophyllotoxin in Linum album cell cultures. Planta. 215:1031–1039. doi:10.1007/s00425-002-0834-1
- Shaheen S, Nasee S, Ashraf M, Akram AA. 2013. Salt stress affects water relations, photosynthesis, and oxidative defense mechanisms in Solanum melongena L. J Plant Interact. 8:85–96. doi:10.1080/17429145.2012.718376
- Shams Ardakani MR, Hemmati S, Mohagheghzadeh A. 2005. Effect of elicitors on the enhancement of Podophyllotoxin biosynthesis in suspension cultures of Linum album. DARU J Pharm Sci. 13:56–60.
- Sreelakshmi Y, Sharma R. 2008. Differential regulation of phenylalanine ammonia lyase activity and protein level by light in tomato seedlings. Plant Physiol Biochem. 46:444–451. doi:10.1016/j.plaphy.2008.02.001
- Vasconsuelo A, Boland R. 2007. Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci. 172:861–875. doi:10.1016/j.plantsci.2007.01.006
- Wang JW, Zheng LP, Wu JY, Tan RX. 2006. Involvement of nitric oxide in oxidative burst, phenylalanine ammonia-lyase activation and taxol production induced by low-energy ultrasound in Taxus yunnanensis cell suspension cultures. Nitric Oxide Biol Ch. 15:351–358. doi:10.1016/j.niox.2006.04.261
- Westcott ND, Muir AD. 2003. Flax seed lignan in disease prevention and health promotion. Phytochem Rev. 2:401–417. doi:10.1023/B:PHYT.0000046174.97809.b6
- Yousefzadi M, Sharifi M, Behmanesh M, Moyano E, Palazon J. 2010. Salicylic acid improves podophyllotoxin production in cell cultures of Linum album by increasing the expression of genes related with its biosynthesis. Biotechnol Lett. 32:1739–1743. doi:10.1007/s10529-010-0343-4
- Zhao J, Davis LC, Verpoorte R. 2005. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv. 23:283–333. doi:10.1016/j.biotechadv.2005.01.003