Abstract
The presence of high cyanogenic glycoside concentrations may predispose plant to the tapping panel dryness (TPD). This study aimed to verify the involvement of cyanogenesis in the reduction of latex stability and in the establishment of TPD. The following parameters were evaluated in rubber tree trunk bark: concentration of cyanogenic glycosides with determination of cyanogenic potential (HCNp) and latex stability with lutoid bursting index (LBI). The study of the relationship between cyanogenesis and TPD was performed by semiquantitative comparison of hydrogen cyanide (HCN) gas released from the trunk bark under the following conditions: without (0%) and with (100%) TPD. The positive correlations between HCNp values and LBI indicate that cyanogenic glycosides present in the bark reduce latex stability, resulting in low yield due to the short duration of flow during tapping. The largest amount of HCN released by trunk bark tissues when the plant exhibits TPD symptoms strengthens the evidence of the involvement of this compound in the establishment of this condition.
1. Introduction
Rubber tree (Hevea brasiliensis Müll. Arg.) is a tropical perennial tree species which produces the polymer cis-1,4-polyisoprene, widely used in industries, known as natural rubber (Dall'Antonia et al. Citation2006; Obianga et al. Citation2009). The molecules of this polymer are produced, aggregated, and packaged in the latex vessels (laticifers) of rubber trees (Li et al. Citation2010). Latex, a cytoplasmic component of the laticifers, is a colloidal suspension that contains from 25% to 50% dry matter, from which 90% is rubber (Priyadarshan Citation2011). Laticifers form a network of anastomosed latex vessels arranged in concentric circles in the trunk of rubber trees (Kongsawadworakul & Chrestin Citation2003).
Latex extraction is carried out by tapping rubber trees, defined as a process of controlled wounding and removal of thin shavings of trunk bark to induce the flow of latex. Since this procedure leads to the loss of plant metabolites, it is important to interrupt the flow after a certain time to prevent excessive loss and entry of pathogens (Priyadarshan Citation2011). After tapping, this flow stops as a result of a complex phenomenon, which causes coagulation of latex particles and, consequently, obstruction of the laticifers in the area of the tapping cut. However, the progressive obstruction of laticifers gradually reduces the amount of latex exuded and, therefore, becomes an important limiting factor of rubber yield (Priyadarshan Citation2011).
Several hypotheses have been proposed to explain latex coagulation, and some of them emphasize the role of vacuolar structures called lutoids (D'Auzac et al. Citation1982). During this process, rubber particles are surrounded by a negatively charged protein layer, which preserves the colloidal stability of latex. Lutoids keep their integrity due to the presence of a membrane, and they naturally burst during the flow because of the friction of this movement, releasing proteins and Mg2 + and Ca2 + ions, capable of destabilizing latex by the agglutination of rubber particles (Kongsawadworakul & Chrestin Citation2003; Wititsuwannakul et al. Citation2008). In some studies, a positive correlation between flow duration, and consequently yield, and the capacity to maintain the integrity of lutoids is reported (Gomez Citation1983; D'Auzac Citation1989; Moraes & Moraes Citation1995).
Certain compounds, such as thiol radicals, protect membranes and therefore favor a low lutoid bursting index (LBI) (D'Auzac et al. Citation1982; D'Auzac Citation1989). Moraes et al. (Citation2001) observed that the presence of cyanide in latex accelerates the natural bursting process of lutoids, reducing flow duration and yield. Most Hevea species accumulate cyanogenic glycosides in the vacuoles of their cells, and when the tissues are ruptured, as it occurs during tapping, they are in direct contact with the apoplastic enzyme β-glucosidase, releasing cyanide, which is toxic (Lieberei Citation2007). Hydrogen cyanide (HCN) inhibits a large number of key enzymes such as: cytochrome c peroxidase, cytochrome P-450, Rubisco, peroxidase, nitrate reductase, superoxide dismutase, and catalase, affecting several metabolic processes such as respiration and photosynthesis (Solomonson Citation1981).
The total amount of HCN which can be liberated from the plant tissue is named hydrocyanic acid potential (HCN-p). The combination of HCN-p and HCN metabolizing enzyme activities (β-glucosidase) is referred to as HCN capacity (HCN-c). As both factors contribute positively to HCN liberation and both factors vary independently, they were combined by multiplication (Lieberei Citation1988).
Studying graft incompatibility between Hevea species, Moraes et al. (Citation2002) budded crown clones resistant to Microcyclus ulei (P. Henn). v. Arx, the causative agent of South American leaf blight (SALB), on productive panels. The authors established connections between the translocation of cyanogenic glycosides from the budded crown to the panel and the development of symptoms similar to tapping panel dryness (TPD), a physiological disorder whose immediate cause remains unknown. TPD syndrome affects latex biosynthesis and causes great loss to rubber producers worldwide (Venkatachalam et al. Citation2009). According to Moraes et al. (Citation2002), the symptoms that they observed started with destabilization of latex and developed to its coagulation in laticifers and trunk bark necrosis. The damage by releasing toxic HCN in tissue of stem during tapping, such as cell death and tissue necrosis, could also facilitate the establishment of pathogens. Recently, a low-molecular-weight RNA (LMW RNA) similar to viroid RNA was isolated from TPD-affected samples of rubber tree. The LMW RNA isolated from TPD-affected trees was found to cause infections on seedling of tomato cv. Pusa Ruby (Kumar et al. Citation2013).
The present study aimed to elucidate the effects of the relationship between cyanogenesis and latex stability on TPD and suggest further research lines to solve the main problem of rubber production on a commercial scale.
2. Materials and methods
The plants evaluated were grown in the experimental field of Embrapa Western Amazon (CPAA), in the municipality of Manaus (3°8′S, 59°52′W), in the state of Amazonas, in a clayey (790 g kg−1 clay) Xanthic Ferralsol [Oxisol or Yellow Latosol (Brazilian classification)] with low natural fertility (Moreira & Fageria Citation2009). The predominant climate in the region is tropical humid, Afi type according to Köppen classification, with abundant rainfall for 10 months (>2000 mm) and a two-month dry season (Antonio Citation2010).
To study the relationship between cyanogenesis and latex stability, the cyanogenic potential (HCNp) was determined in the trunk bark and latex stability was assessed by LBI in six-year-old plants resulting from the panel clone CNS AM 7905 (H. brasiliensis) combinations with the following crown clones resistant to SALB: CPAA C 06 and 11 (hybrids of H. pauciflora × H. rigidifolia); CPAA C 01, 13, 14, 15, 17, 18, 20, and 33 (hybrids of H. pauciflora × H. guianensis var. marginata); CBA 2 (H. pauciflora); CPAA C 64 and 65 (H. nitida), using five replicates of the same crown, represented by one plant each.
HCNp was measured in trunk bark samples collected at 1.2 m above ground level using an iron punch (3 cm diameter). In the laboratory, the samples were washed and the crushed margins of the trunk disks were cut out using a sharp penknife. After that, the samples were reduced to a 2.0-mm-wide layer from the inner to the outer layers of cambium, which constituted the tissue evaluated. The method used was described by Lieberei (Citation1986) and modified by Moraes et al. (Citation2002). To obtain the extracts, a 1.0-g aliquot of fresh tissue was macerated and homogenized with 4.0 mL Na2HPO4 (0.067 mol L−1), centrifuged at 20,000 × g for 14 min, between 0 and 6°C, and the supernatant was transferred to capped vials.
For total release of cyanide, the reaction media, composed of 0.05 mL of supernatant and 0.45 mL of NaH2PO4 (0.067 mol L−1), received 0.1 mL of the enzyme linamarase (β-glycosidase). The enzyme was extracted from rubber tree young leaflets (growth stage C, Hallé et al. Citation1978) in phosphate buffer pH 6.5, 10 mmol L−1, precipitated with (NH4)2SO4, desalinated by washing with phosphate buffer and successively filtering in Millipore membranes (type PTGC), at a constant pressure of 2.5 bar. The activity of the partially purified enzyme so obtained was determined based on the release of ρ-nitrophenol, using ρ-nitrophenyl-glucopyranoside as the substrate (Lieberei Citation1986).
After 20 min incubation, 0.6 mL of NaOH (0.2 mol L−1) and 3.8 mL of distilled water were added to the reaction media, to a total of 5.0 mL, followed by chloramine (50 mg), 2,3-dimethylbarbituric acid (34 mg) and pyridine (0.1 mL), producing a blue-violet hue. The absorbance was measured at 585 nm between 5 and 15 min after adding the reagents.
Latex stability was assessed by LBI according to the method described by Ribaillier (Citation1968) and Moraes and Moraes (Citation2004), which establishes a relationship between the activity of acid phosphatase released when lutoids burst during tapping (free acid phosphatase – FAP) and the activity of this enzyme in intact lutoids (total acid phosphatase – TAP).
The reaction of the activity of acid phosphatase was performed in the field, immediately after collecting 2 samples of 12 drops of latex each, using ρ-nitrophenyl-phosphate in acetate buffer pH 5.0 as the substrate. To preserve the lutoids, mannitol was added to the sample collected to assess FAP. The sample collected to assess TAP received the addition of detergent (Triton X114 0.1%) aiming to rupture lutoid membranes to release all enzymes. Rubber clogs were removed and the incubated latex serum aliquots were diluted with distilled water and treated with 0.1 mol L−1 NaOH to evidence the yellow hue of ρ-nitrophenol. LBI was calculated using the following formula:
After defining this color scale, we selected five plants with TPD and five plants without TPD of panel clone Fx 4098 (PB 86 × FB 74 – H. brasiliensis) combined with three crown clones of H. pauciflora (PA 31, CNS G 112, and CNS G 124). The trunk bark of these plants underwent the same procedures of young leaflet disks. Posteriorly, they were observed 20, 40, 50, 60, 70, and 80 min after their contact with Feigl–Anger paper and classified by comparison with the pre-established shades of blue.
To evaluate whether HCN release depends more on the activity of the enzyme that breaks down the cyanogenic glycoside or on the amount of this compound in the plant tissue, the cyanogenic capacity (HCNc) was determined using the expression HCNp × activity of β-glucosidase (Lieberei Citation1988). The evaluations of HCNp, already described for the study of the relationship between cyanogenesis and latex stability, and the activity of β-glucosidase (linamarase) were performed.
For the determination of the activity of β-glucosidase, 1.0 g of trunk bark was collected using the same procedures employed to obtain the semiquantitative values of HCN. The sample was homogenized in a precooled porcelain mortar, with 4.0 mL of NaH2PO4 0.067 mol L−1. The extract was decanted after compression of the fibrous residue and ground with 5.0 mL of NaH2PO4 0.067 mol L−1. The final extract, free from most part of the fibrous residue, was centrifuged at 20,000 × g for 60 min, between 0 and 6°C. The supernatant was washed three times with phosphate buffer (pH 6.5, 10 mmol L−1), filtered in Millipore membranes (type PTGC), at a constant pressure of 2.5 bar, to eliminate pigments and potential enzyme inhibitors, and in the last wash the final volume of the extract was reduced to 5.0 mL.
The activity of the partially purified enzyme was measured by quantifying the ρ-nitrophenol released by the substrate ρ-nitrophenyl-glucopyranoside in the middle of the incubation with the following initial composition: 1.0 mL of a 10.0 mmol L−1 ρ-nitrophenyl-glucopyranoside solution diluted in 3.9 mL of 0.1 mol L−1 acetate buffer pH 5.5 and 0.1 mL of β-glucosidase solution, to a total volume of 5.0 mL. The incubation was performed at 30°C for 10 min. After this period, an aliquot of 0.5 mL of the mixture was transferred to a flask containing 1.0 mL of precooled 0.5 mol L−1 NaCO3, and 2.5 mL of distilled water to interrupt the enzyme activity and evidence the color of ρ-nitrophenol released. The absorbance was measured at 400 nm and the amount of ρ-nitrophenol released was expressed in g of fresh matter min−1.
The results obtained for HCNp, β-glucosidase, and HCNc underwent the test of normality, analysis of variance, F test, Tukey's test for comparison of contrasts between means at a 5% level of probability, and correlation of LBI and HCNp in the trunk bark (p ≤ 0.05).
3. Results and discussion
HCNp values determined in the trunk bark of panel CNS AM 7905 under the different crowns resistant to SALB ranged from 77.7 to 445.2 µg g−1 and presented significant correlation () with the results found for LBI. This strongly evidences that the decrease in latex stability and consequent coagulation immediately after tapping (), the main depressing factor of crown clones on yield, is due to cyanide released by the mechanical wounding caused to trunk bark tissues during tapping (Moraes & Moraes Citation1995). The decrease in latex stability confirms the in vitro results reported by Moraes et al. (Citation2001) with low concentrations of cyanide, applied as KCN, to a determined volume of latex produced by panel clone IPA 1.
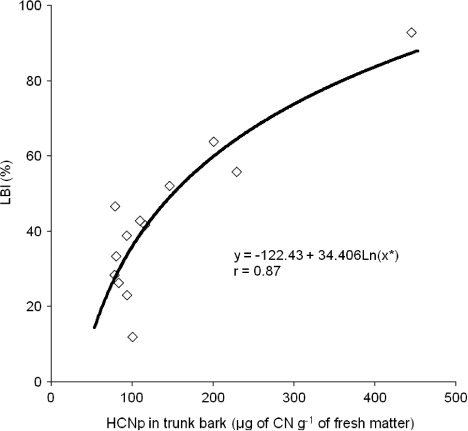

The high value found for LBI, because of the higher amount of HCNp released, affects latex stability due to a higher percentage of lutoids that burst during tapping, progressively plugging laticifers (D'Auzac et al. Citation1982). Since lutoids contain divalent cations and hydrolytic enzymes, they can destabilize latex, causing its gelation and flocculation and, eventually, producing microclogs (D'Auzac & Jacob Citation1989).
Wititsuwannakul et al. (Citation2008) studied an Hevea latex lecitin-like protein (HLL), derived from the lutoid membrane, which presented hemagglutination activity that induced coagulation of latex taken from several other non-Hevea latex-producing plants, indicating a universal role of this type of protein. Also, the authors demonstrated that glycoproteins inhibit HLL, whereas Ca2 + ions increase it. These findings reinforce the importance of lutoids in the aggregation of rubber particles, leading to the formation of clogs that plug laticifer ends stopping the flow of latex upon tapping.
In plants of panel clone Fx 4098 combined with three crown clones of H. pauciflora (PA 31, CNS G 112, and CNS G 124), the maximum color intensity of Feigl–Anger paper (5) was reached 50 or 60 min after its contact with the crushed trunk bark samples (). Among the plants without TPD, only one sample of the combination crown/panel CNS G 112/Fx 4098 reached the maximum color intensity, at 70 min.
Table 1. Color scale composed of five shades of blue (1–5) to test hydrogen cyanide (HCN) release, using Feigl–Anger paper, measured in trunk bark samples collected at 1.2 m above ground level from plants with tapping panel dryness (TPD, 100%) and without TPD (0%) of panel clone Fx 4098 (PB 86 × FB 74 – H. brasiliensis) combined with three crown clones of H. pauciflora (PA 31, CNS G 112, and CNS G 124).
The highest difference between plants with and without TPD was found in plants with the combination crown/panel CNS G 124/Fx 4098, with three samples reaching intensity 3, one reaching intensity 2 and the last one showing intensity 1 (). The present results pointed to higher cyanogenic activity in plants with TPD.
Comparing the values of HCNp in plants with and without TPD, significant statistical differences were observed (p ≤ 0.05) among the panel clone Fx 4098 budded with the three crown clones (), corroborating the results obtained with the test using Feigl–Anger paper (). De Faÿ et al. (Citation2010) also studied the relationship between cyanogenesis in trunk bark and TPD. The authors reproduced TPD symptoms by applying linamarin solution, the most abundant cyanogenic glycoside in the genus Hevea, in the trunk bark of rubber tree plants. The histological assessment of the treated material showed certain alterations typical of TPD in tissues of the conducting phloem and laticifers, such as presence of tylosoids, abundance of tannin cells, and obstruction of phloem vessels.
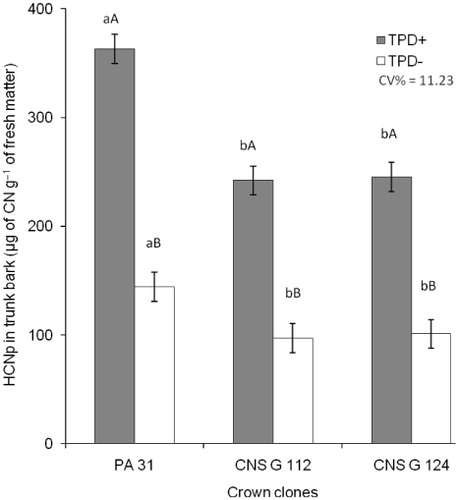
Analyzing the mean HCNp of the panel trunk bark (with and without symptoms) between the three crown clones, the influence of the crown on panel cyanogenesis is clear, the highest values reached under crown PA 31 (). In a trial under the same edafoclimatic conditions, Moraes et al. (Citation2012) also reported significant differences of HCNp in the trunk bark under different crowns, even if the lack of correlation between HCNp in the crown and in the trunk invalidated their hypothesis that the crown budded with different species of Hevea is the main source of cyanogenic glycosides in the trunk.
Kongsawadworakul et al. (Citation2009) and Moraes et al. (Citation2011) affirmed that the presence of cyanogenic glycosides in the trunk of rubber tree is related to the reserves of nitrogen and energy (glucose), which can be used by these plants to produce latex. However, the tapping cut allows a quick exposure of cyanogenic glycosides to β-glucosidase, and the activity of this glycolytic enzyme may greatly influence the release of high amounts of toxic HCN, preventing its use as a nitrogen source by the enzymes involved in the detoxification process, which present lower activities than β-glucosidase (Blumenthal et al. Citation1968). The cyanogenic glycoside linamarin is quantitatively accumulated in the apoplasm. It excludes the occurrence of intact linamarin in the apoplasmic space and thus any apoplasmic transport of this monoglucoside (Gruhnert et al. Citation1994).
The activity of β-glucosidase was not influenced by the crown/panel combinations (), and the results found for this parameter were considered high and nonlimiting to the release of high amounts of HCN. Moreover, these results are in accordance with those reported by Moraes et al. (Citation2002) for the same panel clone (Fx 4098) and by Kongsawadworakul et al. (Citation2009) for panel clones RRIM 600, RRIT 251, PR 107, and PB 217, both studies involving clones of H. brasiliensis.
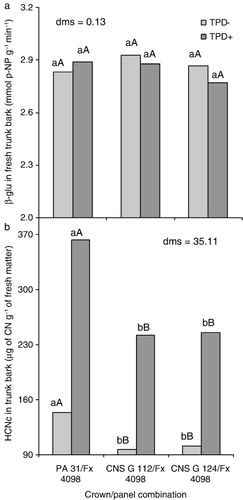
The lack of variation in the activity of β-glucosidase reflects on cyanogenic capacity (HCNc) (), which presented the same tendency observed for HCNp (). Given that the activity of the enzyme was neither affected by the different crowns of H. pauciflora used (PA 31, CNS G 112, and CNS G 124) nor the conditions with and without TPD, we suggest that the high amount of HCNp is the key element to develop the symptoms observed, as stated by Moraes and Moraes (Citation2003) when studying panel clones of H. brasiliensis Fx 3899 and Fx 4098. The HCNc can also be defined as the amount of toxic HCN which can be released per unit time (Ballhorn et al. Citation2005).
HCN liberation is governed not only by the β-glucosidase, but by the enzymes hydroxynitrile lyase that decompose the cyanohydrine released by β-glucosidase and β-cyanoalanine synthase which leads to fixation and detoxification. Although in alkaline pH the cyanohydrines are decomposed rapidly but in pH ranges lower than 6.5 the hydroxinitrile lyase becomes an essential catalytical factor for HCN liberation (Lieberei Citation1988).
4. Conclusions
Rubber tree plants with high amounts of HCNp present latex with high LBI, an indication that the release of HCN in trunk bark tissues causes a decrease in latex stability, which leads to its coagulation in laticifers, resulting in low yield due to the short duration of latex flow during tapping. The higher amount of HCN released by trunk bark tissues when the plant presents symptoms of TPD reinforces the evidence that this compound is involved in the onset of this condition. The activity of β-glucosidase is not influenced by the crown/panel combinations and the HCNc is directly related to HCNp in rubber tree trunk bark tissues.
Acknowledgments
The authors are thankful to Sérgio de Araújo Silva and Francisco Exigidras Leite Magalhães, from Embrapa Amazônia Ocidental, for their support in sample collection and analyses.
References
- Antonio IC. 2010. Boletim agrometeorológico 2009: estação agroclimatológica da Embrapa Amazônia Ocidental, no km 29 da Rodovia AM 010. [Agrometeorological bulletin 2009: agroclimatology station at Embrapa Amazônia Ocidental, km 29 of the Road AM 010]. Manaus: Embrapa Amazônia Ocidental. ( Embrapa Amazônia Ocidental. Documentos, 83).
- Ballhorn DJ, Lieberei R, Ganzhorn JU. 2005. Plant cyanogenesis of Phaseoulus lunatus and its relevance for herbivore plant interaction: the importance of quantitative data. J Chem Ecol. 31:1445–1473. doi:10.1007/s10886-005-5791-2
- Blumenthal SG, Hendrickson HR, Abrol YP, Conn EE. 1968. Cyanide metabolism in higher plants. III. The biosynthesis of β-cyanoalanine. J Biol Chem. 243:5302–5307.
- Dall'Antonia AC, Martins MA, Moreno RMB, Mattoso LHC, Job AE, Ferreira FC, Gonçalves PS. 2006. Avaliação de clones de borracha natural crua por ensaios padrão e análise dinâmico-mecânica. [Evaluation of raw natural rubber clones using standard tests and dynamic mechanical properties]. Polímeros16:239–245.
- D'Auzac J. 1989. Factors involved in the stopping of flow after tapping. In: D'auzac J, Jacob JL, Chrestin H, editors. Physiology of rubber tree latex. Boca Raton (FL): CRC Press; p. 257–280. doi:10.1590/S0104-14282006000300015
- D'Auzac J, Chrestin H, Marin B, Lioret C. 1982. A plant vacuolar system: the lutoids from Hevea brasiliensis latex. Phisiol Vég. 20:311–331.
- D'Auzac J, Jacob JL. 1989. The composition of latex from Hevea brasiliensis as a laticiferous cytoplasm. In: D'Auzac J, Jacob JL, Chrestin H, editors. Physiology of rubber tree latex. Boca Raton (FL): CRC Press; p. 59–96.
- De Faÿ E, Moraes LAC, Moraes VHF. 2010. Cyanogenesis and the onset of tapping panel dryness in rubber tree. Pesq Agropec Bras. 45:1372–1380.
- Gomez JB. 1983. Physiology of latex (rubber) production. Kuala Lumpur (Malaysia): Malaysian Rubber Research and Development Board.
- Gruhnert C, Bierhl B, Selmar, D. 1994. Compartmentation of cyanogenic glucosides and their degrading enzymes. Planta195: 36–42. doi:10.1007/BF00206289
- Hallé F, Olderman RAA, Tomlinson PB. 1978. Tropical trees and forest: an architectural analysis. Berlin: Springer-Verlag. doi:10.1007/BF00206289
- Kongsawadworakul P, Chrestin H. 2003. Laser diffraction: a new tool for identification and studies of physiological effectors involved in aggregation–coagulation of the rubber particles from Hevea latex. Plant Cell Physiol. 44:707–717. doi:10.1093/pcp/pcg085
- Kongsawadworakul P, Viboonjun U, Romruensukharom P, Chantuma P, Ruderman S, Chrestin H. 2009. The leaf, inner bark and latex cyanide potential of Hevea brasiliensis: evidence for involvement of cyanogenic glucosides in rubber yield. Phytochemistry70:730–739.
- Kumar A, Pandey DM, Abraham T, Mathew J, Ramachandran, P, Malathi VG. 2013. Determination of biotic aetiology of tapping panel dryness (TPD) syndrome of rubber tree (Hevea brasiliensis) by return-polyacrylamide gel electrophoresis (R-PAGE) technique. Arch Phytopathol Plant Protect. 46:710–720. doi:10.1016/j.phytochem.2009.03.020
- Li D, Deng Z, Chen C, Xia Z, Wu M, He P, Chen S. 2010. Identification and characterization of genes associated with tapping panel dryness from Hevea brasiliensis latex using suppression subtractive hybridization. BMC Plant Biol. 10:140–151. doi:10.1186/1471-2229-10-140
- Lieberei R. 1986. Cyanogenesis of Hevea brasiliensis during infection with Microcyclus ulei. J Phytopathol. 115:134–146. doi:10.1186/1471-2229-10-140
- Lieberei R. 1988. Relationship of cyanogenic capacity (HCN-c) of the rubber tree Hevea brasiliensis to susceptibility to Microcyclus ulei the agent causing South American Leaf Blight. J Phytopathol. 122:54–67. doi:10.1111/j.1439-0434.1988.tb00990.x
- Lieberei R. 2007. South American leaf blight of the rubber tree (Hevea spp.): new steps in plant domestication using physiological features and molecular markers. Ann Bot. 100:1125–1142. doi:10.1093/aob/mcm133
- Moraes LAC, Moraes VHF, Castro PRC. 2001. Aplicação de KCN e linamarina e a incompatibilidade de enxertia por translocação no clone de seringueira IPA 1. [KCN and linamarin applications and the translocation incompatibility of crown clones of Hevea sp. budded onto IPA 1]. Sci Agric. 58:717–723. doi:10.1590/S0103-90162001000400011
- Moraes LAC, Moraes VHF, Moreira A. 2002. Efeito da cianogênese na incompatibilidade entre clones de copa de seringueira e o clone de painel IPA 1. [Effect of cyanogenesis on the incompatibility of crown clones of Hevea spp. budded onto IPA 1]. Pesq Agropec Bras. 37:925–932.
- Moraes LAC, Moreira A, Cordeiro ER, Moraes VHF. 2012. Translocation of cyanogenic glycosides in rubber tree crown clones resistant to South American Leaf Blight. Pesq Agropec Bras. 47:906–912. doi:10.1590/S0100-204X2012000700005
- Moraes LAC, Moreira A, Tsai SM. 2011. Estado nutricional e teor de glicosídeos cianogênicos em plantas de seringueira. [Nutritional status and glycoside cyanogenic concentration in rubber tree plants]. Bragantia70:402–408. doi:10.1590/S0006-87052011000200022
- Moraes VHF, Moraes LAC. 1995. Diagnóstico do látex em sangria precoce de seringueira com copas enxertadas: possibilidades de emprego na seleção precoce de clones de copa e de painel. [Latex diagnosis in an early tapping test of rubber three-part-trees: prospects for use in the early selection of crown and panel clones]. Agrotrópica7:49–62.
- Moraes VHF, Moraes LAC. 2003. A possible role of cyanogenesis in the onset of tapping panel dryness of rubber (Hevea brasiliensis). Agrotrópica. 15:103–106.
- Moraes VHF, Moraes LAC. 2004. Características fisiológicas do látex do clone de Hevea brasiliensis Fx 4098 sob diferentes copas enxertadas de Hevea pauciflora. [Physiologic characteristics of the latex produced by the clone of Hevea brasiliensis Fx 4098 crowned with Hevea pauciflora]. Rev Cienc Agrar. 42:97–107.
- Moreira A, Fageria NK. 2009. Soil chemical attributes of Amazonas State, Brazil. Commun Soil Sci Plant Anal. 40:2912–2925. doi:10.1080/00103620903175371
- Obianga NLE, Sallé G, Eschbach JM, Salomon M. 2009. Susceptibility of rubber trees to Loranthaceae in Gabon. J Plant Interact. 4: 233–240. doi:10.1080/17429140902962613
- Priyadarshan PM. 2011. Biology of Hevea rubber. Wallingford (UK): CAB International.
- Ribaillier D. 1968. Action in vitro de certain íon minéraux et composés organiques sur la stabilité des lutoïds du latex d′Hevea. Rév Génér Caoutchoucs Plast. 45:1395–1398.
- Solomonson LP. 1981. Cyanide as a metabolic inhibitor. In: Vemesland B, Con EE, Knoubles CJ, Westley J, Wissing F, editors. Cyanide in biology. London: European Molecular Biology Organization; p. 11–28.
- Venkatachalam P, Thulaseedharan A, Raghothama K. 2009. Molecular identification and characterization of a gene associated with the onset of tapping panel dryness (TPD) syndrome in rubber tree (Hevea brasiliensis Muell.) by mRNA differential display. Mol Biotechnol. 41:42–52. doi:10.1007/s12033-008-9095-y
- Wititsuwannakul R, Pasitkul P, Kanokwiroon K, Wititsuwannakul D. 2008. A role for a Hevea latex lectin-like protein in mediating rubber particle aggregation and latex coagulation. Phytochemistry69:339–347. doi:10.1016/j.phytochem.2007.08.019
