Abstract
This study examined the effects of exogenous nitric oxide (NO) on physiological characteristics of peanut (Arachis hypogaea L.) growing on calcareous soil. Sodium nitroprusside (SNP), a NO donor, was root application (directly; slow-release bag; slow-release capsule; slow-release particle) and foliar application. The results showed that SNP application alleviated iron (Fe) deficiency-induced chlorosis, increased the yield of peanut and increased the Fe concentration in peanut grain. SNP, especially supplied by slow-release particle improved the available Fe in soil by reducing pH of soil and increasing available Fe of soil. Furthermore, SNP application significantly increased the H+-ATPase and Fe3+ reductase activities and increased the total Fe concentration in the leaves. Meanwhile, SNP application, especially foliar application enhanced the availability of Fe in the plant by significantly increasing the active Fe content and chlorophyll content in the leaves. In addition, SNP also increased the antioxidant activities, but decreased the superoxide anion (O2•−) generation rate and malondialdehyde content, which protected peanut against the Fe deficiency-induced oxidative stress. Therefore, these results support a physiological action of SNP on the availability, uptake and transport of Fe in the plant and foliar application SNP had the best effects in leaves and SNP supplied by slow-release particle had the best effects in roots. In addition, on the whole, the effects of SNP supplied by slow-release ways were better than directly supplied into the soil.
| Abbreviations | ||
| APX | = | ascorbate peroxidase |
| Ca | = | calcium |
| CAT | = | catalase |
| CRF | = | controlled release fertilizer |
| Fe | = | iron |
| FCR | = | ferric-chelate reductase |
| FRO | = | Fe3+ reductase oxidase |
| GPX | = | glutathione peroxidase |
| GR | = | glutathione reductase |
| MDA | = | malondialdehyde |
| NO | = | nitric oxide |
| O2•− | = | superoxide anion radical |
| POD | = | peroxidase |
| ROS | = | reactive oxygen species |
| SNP | = | sodium nitroprusside |
| SOD | = | superoxide dismutase. |
1. Introduction
Iron (Fe) is an important micro-nutrient for living organisms. Therefore, Fe deficiency severely impairs plant growth and is a widespread human dietary problem, with particularly high numbers of affected children and females (Bashir et al. Citation2010). Because over one-third of the world's soils are considered Fe-deficient, it is very important for safe crop production (Zuo & Zhang Citation2011). Peanut is one of the major oilseed crops in China. It accounts for 30% of the cropped area and 30% of the total oilseeds production in the country (Zuo et al. Citation2000). Therefore, increasing the Fe content of peanut grain has great potential in combating Fe deficiency and will have a significant impact on human health. However, increasing the Fe content of peanut is a difficult task for several reasons. Although Fe is abundant in soil, the availability of Fe is often very limited because of the insoluble oxidized form (Xiong et al. Citation2012). The fact that calcareous soils with a high pH account for 30% of the world's cultivated soils makes the situation worse. Thus, plants have developed sophisticated mechanisms to absorb Fe from soil and to transport it from root to shoot and grain. In Fe-deficient conditions, Strategy I is characterized by enhanced excretion of protons and increased activity of Fe3+ reductase to solubilize Fe3+ oxides to Fe2+ chelates. In most cases, Strategy I plants secrete chelating/reducing compounds mainly organic acide and phenolic (Abadía et al. Citation2002; Jin et al. Citation2007). After solubilization, Fe3+ is reduced to Fe2+ by a membrane-bound Fe3+ reductase oxidase (FRO). Then Fe2+ is taken up through an iron-regulated transporter (IRT1) (Eide et al. Citation1996).
In northern China, Fe deficiency-induced chlorosis was still a severe and common problem in peanut producing areas on calcareous soils, which greatly suppresses the overall growth and yield of peanut (Gao & Shi Citation2007). Peanut is considered as strategy I plant. Therefore, it employed a range of responses to Fe deficiency stress to acquire Fe from the soil, including: induction of both a plasmalemma ferric-chelate reductase (FCR) (Zhang et al. Citation2012) and plasmalemma FeII transporter in root cells; enhanced release of protons and reductants such as phenolic compounds into the rhizosphere (Jin et al. Citation2007); changes in root architecture, including enhanced root branching and subapical root hair development (Chen et al. Citation2010). However, when Fe availability is under a threshold level, those are not sufficient to support the Fe requirement for plant development, and the stress symptoms become evident. How to alleviate chlorosis induced by Fe deficiency still need further study.
Nitric oxide (NO) is a signaling molecule that plays an important role in a variety of physiological processes such as germination, growth, and Fe availability (Lamattina et al. Citation2003). In relation to abiotic stresses, it was shown that the application of the sodium nitroprusside (SNP), a NO donor, reduces the harmful effects of heavy metals and salinity on plants (Lopez-Carrion et al. Citation2008; Xu et al. Citation2013). In addition, NO has previously been associated with Fe homeostasis in plants (Graziano & Lamattina Citation2005). Sun et al. (Citation2007) reported that NO could protect maize plants from Fe deficiency-induced oxidative stress by reacting with reactive oxygen species (ROS) directly or by changing the activities of ROS-scavenging enzymes. In addition, Fe deficiency-induced endogenous NO accumulation in the roots epidermis of tomato (Graziano & Lamattina Citation2007). NO can be a key signaling molecule in signal transduction pathways. Our previous study also proved that SNP or FeSO4 added into controlled release fertilizer (CRF) or sprayed on leaves could alleviate Fe deficiency-induced chlorosis of peanut plants growing on calcareous soil (Zhang et al. Citation2012). However, the FeSO4 which was added into the CRFs and sprayed on leaves was apt to be oxidized, and added SNP into CRFs is a big work. Therefore, we adopted a more simple and effective method by adding SNP directly or through different slow-release ways or foliar application. If SNP supply could alleviate peanut chlorosis induced by Fe deficiency on calcareous soil, it may be able to solve human dietary problem and provide theoretical basis for developing new Fe nutrition preparation for crops.
Therefore, the aim of this experiment was to investigate (1) whether SNP could alleviate Fe deficiency-induced chlorosis on calcareous soil and what is the mechanism; (2) which way of SNP added into the soil is the best.
2. Materials and methods
2.1. Plant material and pot experiment conditions
A pot experiment was conducted on a calcareous soil at a farm near Dezhou City in northern China, where the Fe-deficiency chlorosis in peanut already appeared in previous years. The soil parent material was the sediments from the Yellow River, and the soil texture was sandy with the following properties: organic carbon (C) 8.78 g kg−1, total nitrogen (N) 0.89 g kg−1, Olsen phosphorus (P) 54.18 mg kg−1, available potassium (K) 74.35 mg kg−1, diethylene triamine pentacetic acid (DTPA)-Fe 3.30 mg kg−1, calcium carbonate (CaCO3) 13.6%, and pH (H2O) 8.2.
Seeds of peanut (Arachis hypogaea L.) were seeded on 14 April 2012. Plants were grown in plastic pots (six plants per container) with a capacity of 2.5 kg air-dried soil. Pots were arranged in randomized block designs with three replicates. During the growing season, plants were managed under commonly used agronomic and irrigation practices. The fertilizer (N-P2O5-K2O 15-15-15) which provided by Chinese National Engineering Research Center for Slow/Controlled Release Fertilizers was 6.35 g per container as base fertilizer supplied into soil directly. The SNP was supplied into soil directly or by the slow-release ways or foliar application. Six treatments were established as follows: (CK) the calcareous soil without supplying SNP; (T1) 35.8 mg SNP applied into calcareous soil directly; (T2) 35.8 mg SNP added into a slow-release capsule (cap length: 6.0 ± 0.30 mm, body length: 9.5 ± 0.30 mm) (SRC), then applied into calcareous soil; (T3) 35.8 mg SNP made into slow-release particles (diameter: 3–4 mm) (SRP), then applied into calcareous soil; (T4) 35.8 mg SNP added into a slow-release bag (length: 10 mm, width: 10 mm) (SRB), then applied into calcareous soil; (T5) foliar application of peanut leaves with 1.0 mM SNP every three days and proceeded for three successive times at each critical growth stage (seedling, flowering, podding, and maturity) with 10 mL every time.
At each growth stage after emergence (seedling, flowering, podding, and maturity), the chemical and biochemical analysis were carried out on young leaves of control and treated plants. The crop was harvested at maturity stage, and the pod yield and the Fe concentration in peanut grain were determined.
2.2. Determination of plant growth and mineral element concentrations
Samples of peanut were taken at harvest stage. Roots were soaked in 20 mM Na2-ethylenediaminetetraacetic acid (EDTA) for 15 min to remove metal ions adhering to root surfaces, and rinsed with deionized water several times. The peanut plants were divided into roots and shoots and measured plant height, root length, and fresh weight. The root volume was determined by the water displacement. The samples were oven-dried for 30 min at 105°C, and then at 70°C till the materials reach their constant weights and weighted. The samples were mineralized by wet open digestion in HNO3: H2SO4: HClO4 (5:1:1, 2–5 mL for 0.5–1.5 g fresh weight). The concentration of Fe and calcium (Ca) were estimated by atomic absorption spectrophotometer (SHIMADZU AA-6300). One gram peanut grain was also mineralized by wet open digestion in HNO3: H2SO4: HClO4 (5:1:1, 2–5 mL for 0.5–1.5 g fresh weight). The content of Fe in peanut grain was estimated by atomic absorption spectrophotometer (SHIMADZU AA-6300).
2.3. Determination of root activity
Root activity was determined by triphenyl tetrazolium chloride (TTC) method according to Zhang and Di (Citation2003). Roots were first washed with deionized water, cut into small pieces and then 1–2 g fresh root was weighed. Roots were then placed into test tubes and mixed with 5 mL 0.4% (w/v) TTC and 5 mL 66 mM Na2HPO4–NaH2PO4 (pH 7.0). Incubate the mixture at 37 °C for 3 h and then 2 mL 1 M H2SO4 was added. Six milliliters of ethyl acetate was added and the root was grinded with mortar and pestle then filtered with whatman filter paper. Four milliliters of ethyl acetate was added to the filtrate and shake on mechanical shaker for 1 min. It was then measured with spectrophotometer (Shimadzm UV-2210) at wavelength of 485 nm. Root activity was expressed as absorbance per unit gram root fresh weight.
2.4. Determination of chlorophyll content
The chlorophyll content was determined according to the method of Knudson et al. (Citation1977). Fresh young peanut leaves (0.5 g) were extracted in 2 mL 95% (v/v) ethanol for 24 h in the dark, and the extracted solution was analyzed. The amounts of chlorophyll a, b, and carotenoid were determined spectrophotometrically (SHIMADZU UV-2450, Kyoto, Japan), by reading the absorbance at 665, 649, and 470 nm. The chlorophyll content results are expressed as unit's mg per gram-fresh weight (mg g−1 FW).
2.5. Determination of active Fe content
Active Fe content was determined according to the procedure of Takker and Kaur (Citation1984). Fresh young leaves were cut into pieces and 2.00 g was weighed and extracted with 20 mL 1 N HCl (in 1:10 tissue: extractant), shaken for 5 h and filtered, and the subsequent Fe concentration in the filtrate was measured with atomic absorption spectrophotometer (PE-2100B, Perkin Elmer Co. Ltd., Wellesley, MA, USA).
2.6. Isolation of plasma membrane and the measurement of H+-ATPase and Ca2+-ATPase activities in PMs
A membrane fraction enriched in plasma membrane vesicles was prepared as described by Briskin et al. (Citation1987) with minor changes. Excised roots were homogenized (1/2, w/v) with a mortar and pestle in a cold grinding medium containing: 25 mM HEPES-Tris (pH 7.2), 250 mM mannitol, 5 mM EDTA, 5 mM ethylene glycol tetraacetic acid (EGTA), 1 mM dithiothreitol (DTT), and 1.5% (w/v) PVP. The whole isolation procedures were carried out at 4 °C. The homogenate was filtered through four layers of cheesecloth and centrifuged at 560× g for 12 min, then the supernatant was centrifuged at 10,000× g for 15 min, and the supernatant was centrifuged at 60,000× g for 30 min to yield a crude membrane fraction. The resulting pellet was resuspended with 1 mL in a gradient buffer containing: 20 mM HEPES-Tris (pH 7.5), 5 mM EDTA, 0.5 mM EGTA. The supernatant was layered on top of a step gradient consisting of 1 mL 45%, 33%, and 15% (w/w) sucrose, respectively, and then centrifuged for 2 h at 70,000× g.
ATP hydrolysis assays were performed as described by Briskin et al. (Citation1987) in 0.5 mL the reaction medium containing: 36 mM Tris–Mes (pH 6.5), 30 mM ATP-Na2, 3 mM MgSO4, 1 mM NaN3, 50 mM KNO3, 1 mM Na2MoO4, 0.02% (w/v) Triton A-100, in the presence or absence of 2.5 mM Na3VO4. The reaction was started by adding 50 µL PM vesicles. After 30 min incubation at 37 °C, the reaction was quenched by the addition of 50 µL 55% (w/v) trichloroacetic acid (TCA). The H+-ATPase activity was determined by measuring the release of Pi (Ohinishi et al. Citation1975).
Additionally, ATP hydrolysis assays were performed as described by Briskin et al. (Citation1987) in 0.5 mL the reaction medium containing: 50 mM Tris–Me (pH 7.6), 250 mM sucrose, 1.0 mM DTT, 2.0 mM ATP-Na2, 0.1 mM (NH4)2 MoO4, 0.02% TritonX-100 (v/v), 300 mM NaNO3, 1.0 mM NaN3, in the presence or absence of 2.0 mM Ca(NO3)2. The reaction was started by adding 50 µL PM vesicles. After 30 min incubation at 37 °C, the reaction was quenched by the addition of 50 µL 55% (w/v) TCA. The Ca2+-ATPase activity was determined by measuring the release of Pi (Ohinishi et al. Citation1975).
2.7. Determination of the pH of the soil and available Fe content of the soil
Soil samples of root zone were taken at flowering and maturity stages, and the soil pH was determined (soil:water 1:2.5). The available Fe was extracted by DTPA (pH 7.3) and quantified by atomic absorption spectrophotometry (Lindsay & Norvell Citation1978).
2.8. Assay of Fe3+ reductase activity
Plants roots which rinsed several times in distilled water were transferred to a beaker with saturated CaSO4 under vigorous aeration. After 5 min, the plant was rinsed several times in distilled water, then placed with its root system in a beaker with nutrient solution which contained 0.1 mM Fe-EDTA and 0.4 mM 2,2′-bipyridyl. After 2 h, Fe3+ reductase activity by the roots was determined by measuring the concentration of Fe2+-dipyridyl complex formed at A523 in a spectrophotometer (Shimadzm UV-2210) (Xu et al. Citation1998).
2.9. Antioxidant enzymes and malondialdehyde extraction and assay
The second fully expanded leaves of the plants were sampled for enzymatic analysis. Samples were homogenized in 0.05 mM phosphate buffer (pH 7.8) by grinding with a mortar and pestle under chilled condition with liquid nitrogen. The homogenate was filtered through four layers of muslin cloth and centrifuged at 12,000× g for 10 min at 4 °C. All spectrophotometric analysis was conducted on a SHIMADZU UV-2450 spectrophotometer (Kyoto, Japan). Superoxide dismutase (SOD) activity was assayed by measuring its ability to inhibit the photochemical reduction of nitro blue tetrazolium following the method of Stewart and Bewley (Citation1980). Catalase (CAT) activity was measured as the decline in absorbance at 240 nm due to the decrease of extinction of H2O2 according to the method of Patra et al. (Citation1978). Peroxidase (POD) activity was measured by the increase in absorbance at 470 nm due to guaiacol oxidation (Nickel & Cunningham Citation1969). The level of lipid peroxidation in fresh leaf was measured in terms of malondialdehyde (MDA) content by the thiobarbituric acid reaction method (Heath & Packer Citation1968). MDA content was expressed as nmol g−1 FW.
2.10. Determination of the O2 •−generation rate
For the measurement of O2 •−germination rate, 0.3 g fresh leaves were ground in liquid N2 and extracted in 3mL of ice cold 50 mM sodium phosphate buffer (PBS) (pH 7.0). One milliliter supernatant of fresh leaves extraction was added to 0.9 mL 65 mM phosphate buffer solution (pH 7.8) and 0.1 mL 10 mM hydroxylammonium chloride. The reaction was incubated at 25 °C for 35 min. 0.5 mL solution from the above reaction mixture was then added in 0.5 mL 17 mM sulfanic acid and 0.5 mL 7.8 mM α-naphthylamine solution. After 20 min of reaction, 2 mL of ether was added into the above solution, and then mixed well. The solution was centrifuged at 1500× g at 4 °C for 5 min. The absorbance of the pink supernatant was measured at 530 nm with the spectrophotometer. Absorbance values were calibrated to a standard curve generated with known concentrations of HNO2 (Shi & Zhu Citation2008).
2.11. Statistical analyses
Statistical analyses were carried out by analysis of variance (ANOVA) using SAS software (SAS Institute, Cary, NC, USA). Differences between treatments were separated by the best significant difference (LSD) test at a 0.05 probability level.
3. Results
3.1. Plant growth, yield of peanut, and the Fe concentration in peanut grain
As shown in , SNP supplied into soil directly had no effect on shoot height, fresh weight, and root/shoot ratio. However, SNP supplied by slow-release ways and foliar application, significantly improved plant growth compared with CK at flowering stage. SNP supplied by slow-release particle had the best effect. It significantly increased shoot height, root length, fresh weight, and root/shoot ratio by 45.12, 32.03, 53.50, and 28.57% compared with CK. In addition, SNP supplied into soil directly or by slow-release ways or foliar application all significantly increased the yield of peanut and the Fe concentration in peanut grain. SNP supplied by slow-release particle was the most effective, and foliar application was second. They enhanced the yield of peanut and the Fe concentration in peanut grain by 46.90, 36.79% (yield) and 26.82, 24.28% (Fe concentration), respectively.
Table 1. Effects of exogenous SNP on plant growth of peanut at flowering stage, pod yield of each plant and Fe concentration in peanut grain in maturity stage.
3.2. Chlorophyll content
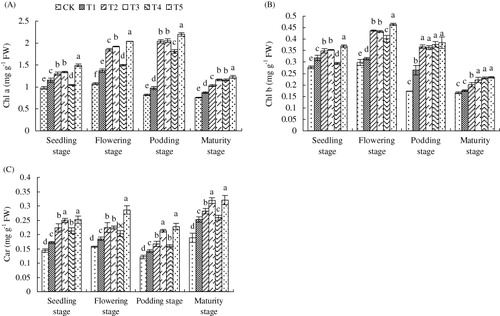
The content of photosynthetic pigment was significantly influenced by applied SNP as shown in . SNP supplied by direct and slow-release ways increased chlorophy a, b, and carotenoids at any growth stage. Foliar SNP application had the best alleviation effect on Fe deficiency-induced chlorophyll content, which was reflected that chlorophyll a, b, and carotenoids were increased by 59.98, 33.38, and 75.54% compared with CK at seedling stage. At later stage, this treatment further exhibited its advantage.
Note: Different lower case letters indicate a significant difference (P < 0.05) between the treatments. The values are means ± SD of three replicate samples per treatment. CK: the calcareous soil without supplying SNP; T1: 35.8 mg SNP applied into calcareous soil directly; T2: 35.8 mg SNP added into a slow-release capsule (cap length: 6.0 ± 0.30 mm, body length: 9.5 ± 0.30 mm) (SRC), then applied into calcareous soil; T3: 35.8 mg SNP made into made into slow-release particles (diameter: 3–4 mm) (SRP), then applied into calcareous soil; T4: 35.8 mg SNP added into a slow-release bag (length: 10 mm, width: 10 mm) (SRB), then applied into calcareous soil; T5: foliar application of peanut leaves with 1.0 mM SNP every three days and proceeded for three successive times at each critical growth stage (seedling, flowering, podding, and maturity) with 10 mL every time.
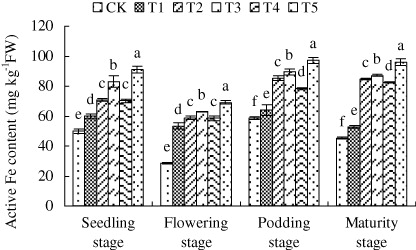
3.3. Active Fe content
Flowering stage is the critical stage for Fe consumption. SNP supplied by direct or by slow-release ways or foliar application all markedly increased the active Fe content at this stage. Foliar SNP application and root application by slow-release particle increased the active Fe content by 2.44 and 2.22-fold respectively at this stage. And at any growth stage, the efficiency of foliar application with 1.0 mM SNP was significantly better than other treatments ().
Note: Different lower case letters indicate a significant difference (P < 0.05) between the treatments. The values are means ± SD of three replicate samples per treatment. CK: the calcareous soil without supplying SNP; T1: 35.8 mg SNP applied into calcareous soil directly; T2: 35.8 mg SNP added into a slow-release capsule (cap length: 6.0 ± 0.30 mm, body length: 9.5 ± 0.30 mm) (SRC), then applied into calcareous soil; T3: 35.8 mg SNP made into made into slow-release particles (diameter: 3–4 mm) (SRP), then applied into calcareous soil; T4: 35.8 mg SNP added into a slow-release bag (length: 10 mm, width: 10 mm) (SRB), then applied into calcareous soil; T5: foliar application of peanut leaves with 1.0 mM SNP every three days and proceeded for three successive times at each critical growth stage (seedling, flowering, podding, and maturity) with 10 mL every time.
3.4. Root activity, H+-ATPase and Ca2+-ATPase activities, and the concentrations of Fe and Ca
Root activity reflects the status of roots growth and development of plant and it is the comprehensive evaluation index of root vitality. As shown in , SNP supplied by slow-release ways or foliar application significantly increased the root activity compared with CK, while SNP directly supplied into soil had no effect. However, SNP supplied by direct or slow-release ways or foliar all significantly increased the H+-ATPase and Ca2+-ATPase activities and the Fe and Ca concentrations in the roots. SNP supplied by slow-release particle had the best effect, significantly increasing the activities of H+-ATPase and Ca2+-ATPase by 58.48 and 135.22%, and the concentrations of Fe and Ca by 70.81 and 36.32% compared with CK. And the effects were found to be in a general trend of slow-release particle > foliar application > slow-release capsule, slow-release bag > direct.
Table 2. Effects of exogenous SNP on root activity, H+-ATPase activity and Ca2+-ATPase activity in roots, and the concentrations of Fe and Ca in leaves of peanut at flowering stage.
3.5. The pH and available Fe content in soil, and Fe (3+)-chelate reductase activity of peanut at flowering and podding stages
shows SNP supplied by slow-release ways and foliar application significantly decreased the pH in soil and increased available Fe in soil compared with control at flowering and podding stages. And they significantly increased FCR activity at flowering and podding stages. The way of SNP supplied by slow-release particle had the best effect on available Fe content in soil and FCR activity in roots. It increased the available Fe content in soil by 116.87 and 107.75% compared with control, and improved FCR activity by 66.91 and 93.45% compared with control at flowering and podding stages.
Table 3. Effects of exogenous SNP on pH and available iron in soil, and Fe3+ chelate reductase activity of peanut at flowering and podding stage.
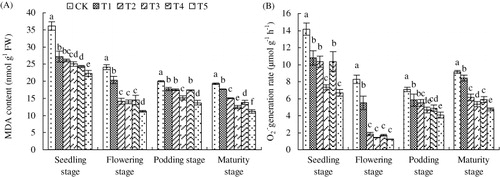
3.6. The MDA content and O2 •−generation rate
When plants were subjected to environmental stress, oxidative damage resulted in membrane lipid peroxidation, which could be estimated by MDA content. As shown in , SNP supplied by direct or by slow-release ways or foliar application all significantly decreased the MDA content. Foliar SNP application had the best effect. It strikingly decreased the MDA content by 38.65, 53.37, 31.33, and 42.22% at seedling, flowering, podding, and maturity stages. Similar to MDA change, SNP supply, especially foliar application, also significantly decreased O2 •−generation rate at each growth stage.
Note: Different lower case letters indicate a significant difference (P < 0.05) between the treatments. The values are means ± SD of three replicate samples per treatment. CK: the calcareous soil without supplying SNP; T1: 35.8 mg SNP applied into calcareous soil directly; T2: 35.8 mg SNP added into a slow-release capsule (cap length: 6.0 ± 0.30 mm, body length: 9.5 ± 0.30 mm) (SRC), then applied into calcareous soil; T3: 35.8 mg SNP made into made into slow-release particles (diameter: 3–4 mm) (SRP), then applied into calcareous soil; T4: 35.8 mg SNP added into a slow-release bag (length: 10 mm, width: 10 mm) (SRB), then applied into calcareous soil; T5: foliar application of peanut leaves with 1.0 mM SNP every three days and proceeded for three successive times at each critical growth stage (seedling, flowering, podding, and maturity) with 10 mL every time.
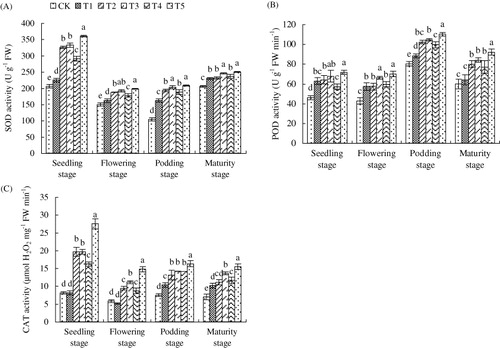
3.7. Antioxidant enzymes activities
Activities of several representative antioxidant enzymes, including SOD, POD, and CAT were measured to assess the mechanism of SNP application in regulation of these antioxidant enzymes upon Fe deficiency stress. As shown in , SNP increased the activities of SOD, POD, and CAT at entire growing stage. The effect of SNP supplied by slow-release ways was better than SNP supplied directly into soil and foliar SNP application had the best effect, increasing SOD activity by 75.05, 32.11, 100.70, and 21.64%, POD activity by 56.08, 64.03, 37.12, and 53.76%, and CAT activity by 238.37, 152.46, 118.48, and 120.88% at seedling, flowering, podding, and maturity stages.
Note: Different lower case letters indicate a significant difference (P < 0.05) between the treatments. The values are means ± SD of three replicate samples per treatment. CK: the calcareous soil without supplying SNP; T1: 35.8 mg SNP applied into calcareous soil directly; T2: 35.8 mg SNP added into a slow-release capsule (cap length: 6.0 ± 0.30 mm, body length: 9.5 ± 0.30 mm) (SRC), then applied into calcareous soil; T3: 35.8 mg SNP made into made into slow-release particles (diameter: 3–4 mm) (SRP), then applied into calcareous soil; T4: 35.8 mg SNP added into a slow-release bag (length: 10 mm, width: 10 mm) (SRB), then applied into calcareous soil; T5: foliar application of peanut leaves with 1.0 mM SNP every three days and proceeded for three successive times at each critical growth stage (seedling, flowering, podding, and maturity) with 10 mL every time.
4. Discussion
Iron (Fe) deficiency has been a widespread problem in peanut grown on calcareous soils of northern China and has resulted in significant yield losses (Zuo et al. Citation2000; Gao & Shi Citation2007). A lot of researches have been done and many measures such as foliage spray Fe fertilization, the use of coated slow-release Fe fertilizer and intercropping with gramineae crops (Zuo et al. Citation2000; Zuo & Zhang Citation2008) have been taken to solve Fe deficiency-induced chlorosis on calcareous soil. However, Fe fertilization is easy to oxidation and intercropping with gramineae crops is a heavy workload. The ideal solution has not found. In the present study, SNP supplied by direct or by slow-release ways or by foliar application all significantly increased the yield of peanut compared with CK, especially by slow-release particle and foliar application. On one hand, this may be because SNP supplication increased root activity () which can reflect the situation of root growth and the level of metabolite in plant, and directly affect the shoot growth, nutrition status, and production of plant. On the other hand, this may be because SNP improved the availability of Fe in the soil by improving the activity of H+-ATPase and reducing the pH of soil (), and increased the active Fe content in the plant (), resulting in improved plant growth, increased the yield of peanut, and increased the Fe concentration in peanut grain ().
In natural environments, during slow growth stages of plant, such as the seedling stage, the nutrient requirement is low and the nutrient concentration in rhizosphere soil is relatively higher. Thus, nutrient content in plant tissues is also usually higher. However, when plant growth rate is accelerated, along with the slow diffusion of Fe3+ in the soil, Fe in the rhizosphere soil solution can be easily exhausted. Therefore, in general, the visual chlorosis in young leaves appeared at about 20 days after emergence, became more severe at flowering and needling stages (from days 35 to 65 after emergence) and alleviated at late growth stages (Gao & Shi Citation2007). In our previous experiment, Fe deficiency significantly decreased chlorophyll concentration in peanut leaves (Zhang et al. Citation2012). Such a large decrease in chlorophyll concentration can be ascribed to the role of Fe in the formation of δ-aminolevulinic acid and protochlorophyllide, the precursors of the chlorophyll biosynthesis (Molassiotis et al. Citation2005). NO was suggested to be involved in plant responses triggered by Fe deficiency and to mediate Fe deficiency responses as a signal molecule (Leonor et al. Citation2011). In this experiment, SNP supplied by different ways, especially by foliar application, increased the chlorophyll concentration and alleviated Fe deficiency-induced chlorosis. This may be because SNP increased the active Fe content in the leaves of peanut (), where the active Fe participates in various physiological actions inside the plants, such as the electron transport, chlorophyll biosynthesis. Zhang (Citation2012) showed that SNP application improved net photosynthesis; this could be attributed to the role of SNP in improving the functional state of the photosynthetic machinery in plants either by the mobilization of internal tissue nitrate or by chlorophyll biosynthesis. Therefore, SNP application increased the chlorophyll concentration and increased the yield of peanut ().
In most soils with a pH range between 7 and 9, the concentration of free Fe2+ and Fe3+ in soil solutions is extremely low, not exceeding 10−10 molL−1. This is why Fe deficiency in plants occurs mostly on calcareous and alkaline soils, especially under arid and semi-arid conditions. The Fe3+ reduction in the apoplast was greatly influenced by the apoplast and medium pH. High pH in the root medium depresses the reduction of Fe3+ complexes. Calcareous soils are characterized by low organic matter, high pH (7.5–8.5) and high levels of bicarbonate. The reactivity of soil carbonate affects the solubility of Fe, and especially, the concentration of the bicarbonate ion, which is a strong buffer and may neutralize the H+ release by proton pumps of the root plasmalemma (Šrámek & Dubský Citation2011). The bicarbonate ion can influence Fe reduction in the roots during Fe uptake and Fe transport or metabolism within the plant (Zuo et al. Citation2000). Decrease in FCR activity in response to bicarbonate has been reported previously for conventional growth citrus (Chouliaras et al. Citation2004). It is well known that H+-ATPase in plasma membrane plays an important role in the transport of multiple ions (Palmgren & Harper Citation1999), and there are investigations indicating that NO could induce H+-ATPase activity (Cui et al. Citation2010). In this study, SNP decreased the soil pH. The reason may be because SNP increased the H+-ATPase activity and NO induced a great increase of organic acids secretion by root, especially the malate and citrate (Zhang Citation2012). The decreased pH is beneficial to increase the available Fe in soil and improve FCR activity. Ferric Fe is reduced to ferrous Fe at the root surface and then enters the root cytoplasm where it is rapidly reoxidized to ferric Fe, followed by translocation of Fe3+-citrate to the aerial portions of the plant via the xylem (Li et al. Citation2011). In the present study, we found that SNP increased the Fe concentration in the leaves (). Leaf cells uptake of Fe require Fe3+-chelate reductase: the ferric Fe is reduced to ferrous Fe in the leaves and is then able to carry out its physiological functions. Our experimental data also showed that SNP increased the active Fe content. This may be because SNP decreased the pH and increased FCR activity in the leaves. Therefore, we infer that SNP can improve the uptake and transportation of Fe in the plant.
Growing in their natural environment, plants often encounter unfavorable environmental conditions that interrupt normal plant growth and productivity (Jan et al. Citation2013). A common consequence of most abiotic and biotic stresses is the increased production of ROS, namely superoxide (O2 •−), hydrogen peroxide (H2O2), hydroxyl radicals (.OH), and singlet oxygen (1O2). During Fe deficiency-induced oxidative stress, two primary processes may be involved in the formation of ROS during photosynthesis. One is the direct photoreduction of O2 to the O2 •−radical by reduced electron transport components associated with PS I, and other is the reactions linked to the photorespiratory cycle in plant metabolism. These processes initiate chain reactions that produce more harmful oxygen radicals, which may destroy normal metabolism through oxidative damage of lipids, proteins, and nucleic acids when they are produced in excess (Molassiotis et al. Citation2005). In addition, when plants were subjected to environmental stress, oxidative damage resulted in the level of membrane lipid peroxidation, which could be estimated by MDA content. Fe deficiency significantly increased the MDA content and O2 •−generation rate. The involvement of (O2 •−) radicals in the electron transport to extracellular ferric chelates may be of significant importance for the acquisition of Fe by roots (Molassiotis et al. Citation2005). In addition, Graziano and Lamattina (Citation2005) proposed that the free radical NO as a candidate for the translation of the Fe deficiency signal and Zaharieva et al. (Citation2004) also suggested that responses to Fe deficiency may include a shift in the redox balance inside the root cell, along with an increased capacity of roots to reduce Fe. Therefore, it is important to keep redox balance. SNP reduced the accumulation of MDA and O2 •−generation rate induced by Fe deficiency indicates that SNP protect membrane lipids against oxidative damage.
Plants have evolved efficient systems for ROS removal, which include specific ROS-scavenging enzymes like SOD, ascorbate peroxidase (APX), CAT, glutathione reductase (GR), and glutathione peroxidase (GPX) and some small non-enzymatic molecules such as ascorbate, glutathione, tocopherol, flavonoids, anthocyanins, polyphenolic compounds, and carotenoids are also observed, that act as ROS scavenger (Türkan & Demiral Citation2009; Shaheen et al. Citation2013). It was found here that SNP supplied by slow-release ways influenced the SOD, POD, and CAT activities of Fe-deficient plant (). The SOD, POD, and CAT are all important enzymes involved in antioxidation process and presented in different organelles in plants, protecting plants from oxidative stress. SOD converts the O2 •−radical to the H2O2. Therefore, SNP supplied by slow-release ways, especially foliar application, significantly increased the SOD activity and decreased the O2•−generation rate ( and ). However, if the produced H2O2 cannot be scavenged efficiently, it can interact with O2 •−to form highly reactive.OH that are thought to be primarily responsible for oxygen toxicity in the cell. Therefore, the efficient scavenging of H2O2 is very important for normal metabolism of plant. CAT is unique among the H2O2-scavenger enzymes, in that it degrades H2O2, without consuming cellular reducing equivalents. Furthermore, CAT and POD are all heme-containing enzymes. Their activities may be affected by active Fe content. Therefore, in this experiment, SNP supplied by slow-release ways, and especially foliar application, increased the active Fe content and the activities of CAT and POD. The reason of foliar SNP application had the best effect in leaves and supplied SNP by slow-release particle had the best effect in roots, maybe because the released content of SNP at different stage by foliar application and slow-release particle in compliance with the rule of peanut needed content in the leaves and roots.
5. Conclusion
SNP can alleviate Fe deficiency-induced chlorosis, improve the yield of peanut and increase the Fe concentration in peanut grain. In addition, foliar SNP application had the best effect in leaves and the effects of SNP supplied by slow-release ways were better than directly supplied into soil. On the whole, the way of SNP supplied by slow-release particle had the best effect in roots. The mechanism of SNP alleviated Fe deficiency-induced chlorosis mainly by improving the availability of Fe in the soil, increasing the uptake and translocation of Fe, promoting the activation of Fe in the leaves of peanut, and protecting Fe deficiency-induced oxidative stress. The effect of SNP on soil enzymes activities, soil microbial and environmentally compatible will be further researched.
Acknowledgements
The authors thank English lecturer Xiujuan Wang (College of Foreign Languages, Shandong Agricultural University) for her critical reading and revision of the manuscript. The authors also thank Pingping Yang (College of Animal Science and Technology, Shandong Agricultural University, China) for her supplying instruments and patient guidance. Special acknowledgments are given to the editors and reviewers. And this work was supported by the Projects Shandong Provincial Natural Science Foundation, China (No. ZR2013CM003).
References
- Abadía J, López-Millán AF, Rombolà A, Abadía A. 2002. Organic acids and Fe deficiency: a review. Plant Soil. 241:75–86. 10.1023/A:1016093317898
- Bashir K, Ishimaru Y, Nishizawa NK. 2010. Iron uptake and loading into rice grains. Rice. 3:122–130. 10.1007/s12284-010-9042-y
- Briskin DP, Leonard RT, Hodges TK. 1987. Isolation of the plasma membrane: markers and general principles. Method Enzymol. 148:542–558.
- Chen WW, Yang JL, Qin C, Jin CW, Mo JH, Ye T, Zheng SJ. 2010. Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in responses to iron deficiency in Arabidopsis. Plant Physiol. 154:810–819. 10.1104/pp.110.161109
- Chouliaras V, Dimassi K, Therios I, Molassiotis A, Diamantidis G. 2004. Root-reducing capacity, rhizosphere acidification, peroxidase and catalase activities and nutrient levels of Citrus taiwanica and C. volkameriana seedlings, under Fe deprivation conditions. Agronomie. 24:1–6. 10.1051/agro:2003055
- Cui XM, Zhang YK, Wu XB, Liu CS. 2010. The investigation of the alleviated effect of copper toxicity by exogenous nitric oxide in tomato plants. Plant Soil Environ. 6:274–281.
- Eide D, Broderius M, Fett J, Guerinot ML. 1996. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci USA. 93:5624–5628. 10.1073/pnas.93.11.5624
- Gao L, Shi YX. 2007. Genetic differences in resistance to iron deficiency chlorosis in peanut. J Plant Nutr. 30:37–52. 10.1080/01904160601054965
- Graziano M, Lamattina L. 2005. Nitric oxide and iron in plants: an emerging and converging story. Trends Plant Sci. 10:4–8. 10.1016/j.tplants.2004.12.004
- Graziano M, Lamattina L. 2007. Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. Plant J. 52:949–960. 10.1111/j.1365-313X.2007.03283.x
- Heath RL, Packer L. 1968. Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 125:189–198. 10.1016/0003-9861(68)90654-1
- Jan AT, Singhal P, Mohd Q, Haq R. 2013. Plant abiotic stress: deciphering remedial strategies for emerging problem. J Plant Interact. 8:97–108. 10.1080/17429145.2012.702226
- Jin CW, You GY, He YF, Tang CX, Wu P, Zheng SJ. 2007. Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover. Plant Physiol. 144:278–285. 10.1104/pp.107.095794
- Knudson LL, Tibbitts TW, Edwards GE. 1977. Measurement of ozone injury by determination of leaf chlorophyll concentration. Plant Physiol. 60:606–608. 10.1104/pp.60.4.606
- Lamattina L, Garcia-Mata C, Graziano M, Pagnussat G. 2003. Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol. 54:109–136. 10.1146/annurev.arplant.54.031902.134752
- Leonor R, Marcela S, Irene M, Eduardo Z, Lorenzo L. 2011. Nitric oxide, nitrosyl iron complexes, frataxin: a well equipped team to preserve plant iron homeostasis. Plant Sci. 181:582–592. 10.1016/j.plantsci.2011.04.006
- Li LY, Cai QY, Yu DS, Guo CH. 2011. Overexpression of AtFRO6 in transgenic tobacco enhances ferric chelate reductase activity in leaves and increases tolerance to iron-deficiency chlorosis. Mol Biol Rep. 38:3605–3613. 10.1007/s11033-010-0472-9
- Lindsay WL, Norvell WA. 1978. Development of a DTPA soil test for Zn, Fe, Mn and Cd. Soil Sci Soc Am Proc. 42:421–428. 10.2136/sssaj1978.03615995004200030009x
- Lopez-Carrion AI, Castellano R, Rosales MA, Ruiz JM, Romero L. 2008. Role of nitric oxide under saline stress: implications on proline metabolism. Plant Biol. 52:587–591. 10.1007/s10535-008-0117-1
- Molassiotis AN, Diamantidis GC, Therios IN, Tsirakoglou V, Dimassi KN. 2005. Oxidative stress, antioxidant activity and Fe(III)-chelate reductase activity of five Prunus rootstocks explants in response to Fe deficiency. Plant Growth Regul. 46:69–78. 10.1007/s10725-005-6396-z
- Nickel RS, Cunningham BA. 1969. Improved peroxidase assay method using Ieuco 2,3,6-trichlcroindophenol and application to comparative measurements of peroxidase catalysis. Anal Biochem. 27:292–299. 10.1016/0003-2697(69)90035-9
- Ohinishi T, Gall RS, Mayer ML. 1975. An improved assay of inorganic phosphate in the presence of extralabile phosphate compounds: application to the ATPase assay in the presence of phosphocreatine. Anal Biochem. 69:261–267. 10.1016/0003-2697(75)90585-0
- Palmgren MG, Harper JF. 1999. Pumping with plant p-type ATPases. J Exp Bot. 50:883–893.
- Patra HL, Kar M, Mishre D. 1978. Catalase activity in leaves and cotyledons during plant development and senescence. Biochem Pharmacol. 172:385–390.
- Shaheen S, Naseer S, Ashraf M, Akram NA. 2013. Salt stress affects water relations, photosynthesis, and oxidative defense mechanisms in Solanum melongena L. J Plant Interact. 8:85–96. 10.1080/17429145.2012.718376
- Shi QH, Zhu ZJ. 2008. Effects of exogenous salicylic acid on manganese toxicity, element contents and antioxidative system in cucumber. Environ Exp Bot. 63:317–326. 10.1016/j.envexpbot.2007.11.003
- Šrámek F, Dubský M. 2011. Occurrence and correction of lime-induced chlorosis in petunia plants. Plant Soil Environ. 57:180–185.
- Stewart RC, Bewley JD. 1980. Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 65:245–248. 10.1104/pp.65.2.245
- Sun BT, Jing Y, Chen KM, Song LL, Chen FJ, Zhang LX. 2007. Protective effect of nitric oxide on iron deficiency-induced oxidative stress in maize (Zea mays). J Plant Physiol. 164:536–543. 10.1016/j.jplph.2006.02.011
- Takker PN, Kaur NP. 1984. HCl method for Fe2+ estimation to resolve iron chlorosis in plants. J Plant Nutr. 7:81–90. 10.1080/01904168409363176
- Türkan I, Demiral T. 2009. Recent developments in understanding salinity tolerance. Environ Exp Bot. 67:2–9. 10.1016/j.envexpbot.2009.05.008
- Xiong HC, Kobayashi T, Kakei Y, Senoura T, Nakazono M, Takahashi H, Nakanishi H, Shen HY, Duan PG, Guo XT, et al. 2012. AhNRAMP1 iron transporter is involved in iron acquisition in peanut. J Exp Bot. 63:4437–4446. 10.1093/jxb/ers117
- Xu LL, Dong YJ, Fan ZY, Kong J, Liu S, Bai XY. 2013. Effects of the application of exogenous NO at different growth stage on the physiological characteristics of peanut grown in Cd-contaminated soil. J Plant Interact. doi:10.1080/17429145.2013.830780
- Xu LZ, Zhang FS, Li CJ. 1998. 2,2′-bipyridyl-colorimetric method for measurement of Fe(III) reductase activity in roots of dicotyls. Plant Nutr Fert Sci. 4:63–66.
- Zaharieva TB, Gogorcena Y, Abadia J. 2004. Dynamics of metabolic responses to iron deficiency in sugar beet roots. Plant Sci. 166:1045–1050. 10.1016/j.plantsci.2003.12.017
- Zhang XW. 2012. Study on the effect and mechanism of exogenous nitric oxide regulates iron deficiency chlorosis of peanut [D] [master dissertation]. Beijing, China: CNKI (China National Knowledge Infrastructure); [cited 2012 Aug 27]. Available from: http://cdmd.cnki.com.cn/Article/CDMD-10434-1012487322.htm
- Zhang ZL, Di WJ. 2003. Laboratory guide for plant physiology [M]. Beijing, China: Higher Education Press.
- Zhang XW, Dong YJ, Qiu XK, Hu GQ, Wang YH, Wang QH. 2012. Exogenous nitric oxide alleviates iron-deficiency chlorosis in peanut growing on calcareous soil. Plant Soil Environ. 58:111–120.
- Zuo YM, Zhang FS. 2008. Effect of peanut mixed cropping with gramineous species on micronutrient concentrations and iron chlorosis of peanut plants grown in a calcareous soil. Plant Soil. 306:23–36. 10.1007/s11104-007-9484-1
- Zuo YM, Zhang FS. 2011. Soil and crop management strategies to prevent iron deficiency in crops. Plant Soil. 339:83–95. 10.1007/s11104-010-0566-0
- Zuo YM, Zhang FS, Li XL, Cao YP. 2000. Studies on the improvement in iron nutrition of peanut by intercropping with maize on a calcareous soil. Plant Soil. 220:13–25. 10.1023/A:1004724219988
