Abstract
The beneficial effects of trans-resveratrol on pathogen-response and its physiological mechanisms were studied in two wheat cultivars differing in the level of resistance against powdery mildew (Blumeria graminis (DC.) Speer). Resveratrol induced phenolics metabolism in a manner that lead to increased phenylalanine ammonia-lyase (PAL) and β-D-glucosidase activity in leaf regions showing no symptoms of pathogen infection. In less-resistant cultivar, similar alterations were additionally observed in regions invaded by the pathogen. Resveratrol also stimulated photosynthetic efficiency during pathogenesis and this effect was more prominent in the resistant cultivar. Regulatory function of resveratrol on wheat growth and development was also observed (stimulation of generative induction). High performance liquid chromatography (HPLC) analysis detected a low amount of compound with retention time corresponding to trans-resveratrol in healthy leaves and increased the content of this compound in infected foliage. The multidirectional properties of resveratrol activity in wheat plants are discussed.
Introduction
Resveratrol (trans-3,5,4′-trihydroxystilbene) was discovered in 1940s of the twentieth century. However, it gained on popularity only in the past 20 years. In the last decade, there was a fivefold increase in the number of articles describing different properties of trans-resveratrol (Kiselev Citation2011). Resveratrol has been shown to possess many health benefits for humans. It exhibits pleiotropic properties, such as anti-inflammatory and anticarcinogenic activities, and has beneficial effects against cardiovascular diseases (Vidavalur et al. Citation2006). In plants, resveratrol is the first member of the stilbene series synthesized by the enzyme stilbene synthase, via the shikimic pathway (Chong et al. Citation2009). In grapevine or peanut, resveratrol has proven to accumulate in leaves and its concentration correlates with the resistance level to fungi such as Botrytis cinerea Pers. (Sbaghi et al. Citation1995). The beneficial effects of resveratrol have been shown in the exogenous applications of this compound as well as in the transformed plants, in which resveratrol synthase genes were introduced. These transgenic plants displayed increased resistance against certain fungi, including gray mold and powdery mildew (Leckband & Lörz Citation1998). External application of resveratrol to harvested grapes resulted in the reduction of microbial flora growth and prolonged shelf life without affecting the nutritional quality of the fruits (Jiménez et al. Citation2005). These findings make resveratrol a natural fungicide. Despite the reported positive effects of resveratrol on pathogen-infected plants, little is known about the mechanisms of its action. It is known, however, that pathogen attack induces in plants a biochemical system of detection and response, recognizing pathogenic signals and triggering appropriate defenses. This biochemical response is based on the molecules such as salicylate, jasmonate, and ethylene, and generates the accumulation of pathogenicity-related proteins, i.e. phytoalexins, or other phenolic compounds (Dong Citation1998; Ahuja et al. Citation2012). Thus, the effect may also be achieved by the exogenous application of some substances. For example, resistance induction against Alternaria porri was observed in tomato after application of methyl jasmonate (Kępczyńska & Król Citation2012) and in pearl millet by oligosacharides of Trichoderma virens against downey mildew (Nandini et al. Citation2013). It is likely that resveratrol enters the plant metabolic network associated with cellular response to the pathogen.
We applied trans-resveratrol to wheat infected in early tillering stage with a common cereal pathogen – powdery mildew (Blumeria graminis (DC.) Speer). Powdery mildew fungi are biotrophic parasites invading epidermal cells. In cereals, powdery mildew causes formation of yielding spots lowering the photosynthetic productivity of green tissue. Due to the disease of crop plants, yield may be decreased up to 30% (Hűckelhoven Citation2005). The aim of this work was to investigate the mechanistic basis of resveratrol activity by studying pathogen-response-related components of phenolic metabolism, such as phenylalanine ammonia-lyase (PAL) and β-D-glucosidase activity, together with free phenolic content measurements. The photosynthetic efficiency in wheat leaves was also monitored as well as the impact of resveratrol on wheat growth and development. The presence of endogenous resveratrol in healthy leaves of wheat and leaves infected with powdery mildew was also assayed.
Material and methods
Plant material and treatments
Two cultivars of wheat (Triticum aestivum L.) were chosen for this study. Cv. Katoda is a spring wheat while cv. Monsun, in the Central European climate, may be sown during autumn or spring time. According to the Polish Research Centre for Cultivar Testing (COBORU), cv. Katoda is more resistant to powdery mildew (8.1 in 10-point scale) than cv. Monsun (7.3).
The experimental design of the pot experiment was fully randomized with three replications. Plants were cultivated in pots (9 plants per pot; pot diameter, 20 cm; height, 20 cm) in greenhouse (temperature 22°C d/n; natural light conditions: September/October; latitude: 50°03′ North, longitude: 19°55′ East). Two-week-old plants were sprayed with the solution of trans-resveratrol (Sigma–Aldrich, Poznan, Poland) in the concentration of 100 mg dm−3 (50 cm3 per plant). The solution of resveratrol was prepared as a water suspension according to Jiménez et al. (Citation2005) while control plants were treated with water. Four-week-old, control and resveratrol-treated plants were inoculated with powdery mildew (B. graminis (DC.) Speer) as described by Vanacker et al. (Citation1998). Measurements of leaf photosystem II (PSII) efficiency were performed first on the youngest fully developed leaves in two-week-old plants (before spraying with resveratrol) and then every week until the seventh week of experiment. Measurements were carried out avoiding leaf areas with disease spots (DS) of powdery mildew. The flag leaves were collected for biochemical analysis (total free phenolic content and enzymatic assays) at the ninth week of growth. For these assays, the tissue with DS and areas surrounding the spots as well as the tissue without spots (WS) were separately collected. Additionally, the impact of resveratrol on plant growth and development was also monitored. The length of the upper part of the plant was measured weekly between the third and seventh week of vegetation. Percentage of heading plants versus plants in vegetative stage was evaluated at the end of the experiment at ninth week of growth. The progress of infection was observed during plant vegetation and finally symptoms of infection were determined on flag leaves of nine-week-old plants. The percentage of flag leaf area covered by lesion spots was estimated using WinDIAS 3 Image Analysis System (Delta-T Devices Ltd Cambridge UK).
Separate experiment was conducted to obtain plant material for analysis of trans-resveratrol content in healthy leaves of wheat and leaves infected with B. graminis. Seeds of cv. Monsun were sown into pots with soil and cultured in greenhouse (temperature 22°C d/n; natural light conditions: October; latitude: 50°03′ North, longitude: 19°55′ East). Two-week-old seedlings were inoculated with powdery mildew (B. graminis (DC.) Speer) according to the method described by Vanacker et al. (Citation1998). The control plants were separated in another greenhouse chamber to prevent infection. First, visible symptoms of infection were noticed on the leaves after one week of inoculation. Aerial parts of four-week-old plants with massive appearance of powdery mildew symptoms were harvested for analyses of resveratrol content. Upper parts of noninfected four-week-old plants were also collected as a control.
Total free phenolic content
Total free phenolic content was measured colorimetrically with Folin-Ciocalteau reagent according to Singleton and Rossi (Citation1965). The 0.2 g samples were homogenized in 80% ethanol. The absorbance at λ = 760 nm was measured using the LKB Ultrospec II spectrophotometer. Total concentration of phenolic compounds was calculated using chlorogenic acid as the calibration standard.
Activity of PAL (EC 4.3.1.5)
The activity of PAL was measured as described by Peltonen and Karjalainen (Citation1995) with slight modifications. Leaf samples (0.2 g) were homogenized in extraction buffer (50 mM Tris–HCl [pH 8.5], 14.4 mM 2-mercaptoethanol, 5% w/v insoluble polyvinylpolypyrolidone) and centrifuged. The reaction mixture contained 0.2% solution of L-phenylalanine in 50 mM Tris–HCl (pH 8.5) and supernatant. The incubation lasted for 24 h at 38°C, then the absorbance was read at λ = 290 nm. The enzyme activity was expressed as mg of cinnamic acid per 1 mg of fresh weight.
Activity of β-D-glucosidase (EC 3.2.1.21)
The activity of β-D-glucosidase was measured using the method described by Nichols et al. Citation1980, with a slight modification. Leaf samples (0.2 g) were homogenized in 0.1 M phosphate buffer (pH 7.0). The extract was added to tubes containing 0.2 cm3 0.1 M phosphate buffer (pH 7.0) and 0.2 cm3 substrate p-nitrophenyl-β-D-glucopyranoside and tubes were incubated at 30°C for 1 h. The reaction was then stopped by the addition of 0.6 cm3 2 M Na2CO3, and absorbance was read at λ = 400 nm. One unit of enzyme activity was defined as the amount of reaction product (µM p-nitrophenyl) released from 1 g of fresh mass of the leaf.
All chemicals were purchased from Sigma–Aldrich (Poznan, Poland). Assays were performed in five replications.
Efficiency of PSII
Efficiency of PSII was estimated based on chlorophyll a fluorescence measurements using Plant Efficiency Analyzer (PEA) (Hansatech Ltd. King's Lynn, UK). Measurements were performed according to the methodology described by Skoczowski et al. (Citation2011). Obtained values of fluorescence were used to calculate the so-called phenomenological fluxes: energy absorption (ABS/CS = Fm); energy flux for trapping (TR0/CS = Fv/Fm (ABS/CS)); energy flux for electron transport (ET0/CS = (Fv/Fm) (1-VJ) Fm); energy dissipation (DI0/CS = (ABS/CS)–(TR0/CS)), where CS is the cross-section of the sample. Values of phenomenological fluxes informs about the specific energy flow in PSII per CS of the sample. Moreover, a parameter informing synthetically about leaf photosynthetic efficiency, performance index (PI) of PSII normalized for equal absorption (PIABS), was also extracted using the PEA (Rapacz Citation2007). Measurements of PSII efficiency were taken for both cultivars in nine replications.
Resveratrol detection
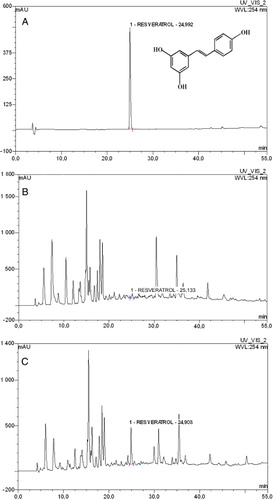
The analysis of the content of resveratrol was carried out on cut aerial parts of four-week-old wheat seedlings of cv. Monsun. Samples of healthy plants weighed 21 g while the fresh weight of plants infected with powdery mildew was 11.5 g (dry weight 5.85 and 2.99 g, respectively). Leaves were ground and extracted with methanol in a round bottom flask (80 cm3 of methanol per 10 g of tissue) in a water bath using reflux condenser at the boiling point of the solvent. The extraction was repeated twice. Each extraction lasted 2 h. Extracts were drained and appropriately combined and methanol was evaporated in a rotary evaporator under reduced pressure. Aqueous hydrochloric acid (5%, 20 cm3) was added to the residue in the flasks and heated in a steam bath for 1 h at 90°C. The products of hydrolysis were eluted from the acidic aqueous phase with ethyl acetate in two steps (10 cm3 each). Ethyl acetate phases were combined, rinsed with distilled water, and then concentrated to dryness. The residue was dissolved in 1 cm3 of methanol and this solution was analyzed by high performance liquid chromatography (HPLC). The analysis was performed on a liquid chromatograph (Dionex Corporation, USA) with the PDA 100 UV-VIS detector, the P 680 pump, the TCC 100 thermostat, and the ASI 100 autosampler, using the Chromeleon 6.60 software. Methanol solutions of hydrolysates were filtered through Nylon Membrane Titan HPLC filters (Church, Gloucester, UK) and applied to the chromatographic column with the autosampler. Stationary phase: Hypersil Gold C-18 column (5 µm, 250 × 4.6 mm; Thermo EC), Hypersil Gold C-18 precolumn (5 µm, 10 × 4 mm; Thermo EC). Parameters of analysis according to Justesen et al. (Citation1998): column temperature 20°C, injection volume 0.02 cm3, wavelength 254 nm and 285 nm. Mobile phase: solvent A – 1% formic acid in water and solvent B – acetonitrile. Flow rate programmed from 0 min A 95%, 52 min. A 40%; flow rate at 1 cm3 per minute, analysis time: 52 min. Three injections were performed (chemical replicates) on the samples of the control plants and mildewed plants. Exemplary chromatograms are shown on figure. The results were quantified based on the calibration curve prepared for the trans-3,5,4′-trihydroxystilbene (resveratrol).
Statistical approach
All calculations were carried out using STATISTICA 9.1 (StatSoft, Polska) software package. Data were analyzed by multifactor analysis of variance (ANOVA). Post hoc comparison was conducted using Duncan's multiple range test and Student's t-test (P ≤ 0.05). Statistical differences for data regarding generative development were estimated according to Pearson χ2 test, P ≤ 0.05. Graphs were plotted using means for each data point.
Results
Evaluation of symptoms of powdery mildew infection
The leaves inoculated with powdery mildew did not display visual symptoms of infection during first week of inoculation. After one week, first randomly distributed lesion spots became apparent on the surface of oldest leaves. Within next four weeks, these spots enlarged and became more conspicuous. Five weeks post-inoculation, lesion spots expanded and affected more leaves, and symptoms were observed even on the flag leaves ().

Flag leaves of cv. Katoda showed a large disparity between the control and resveratrol-treated plants (10.40% and 1.29% of leaf area, respectively, was covered by lesion spots). In the latter plants, lesions spots were hardly observed; however, the oldest leaves were infected. Flag leaves of resveratrol-treated plants of cv. Monsun were infected in 11.22% while in control plants it was 28.61%. What is more, in control plants of cv. Monsun the surface of oldest leaves was almost completely covered by lesion spots.
Enzymatic activity and phenolics content
Biochemical analyses revealed heterogenic reaction in regions free of DS and within developing lesions and surrounding tissue ().
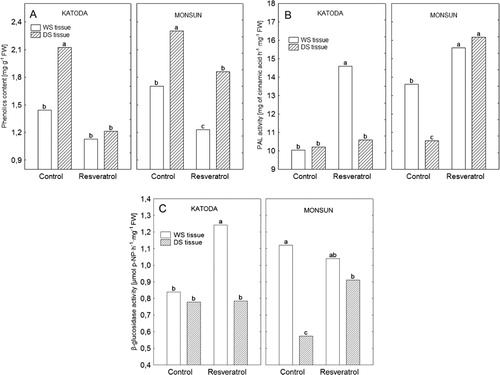
Higher level of total free phenolics was observed for control of both cultivars in DS tissue in comparison to WS tissue (). Phenolic level in cv. Katoda, after spraying with resveratrol, was similar in both WS and DS tissues and did not differ from WS tissue of control plants (). In resveratrol-treated plants of cv. Monsun, free phenolic content was lowered in WS and DS tissue in comparison to control ().
The activity of PAL in control plants of cv. Katoda did not differ significantly between DS and WS tissues (). Resveratrol significantly increased PAL activity (by about 31%), but only in WS tissue. In control plants of cv. Monsun, PAL activity in WS tissue was significantly higher than in DS tissue (by about 43%). While in resveratrol-treated plants, PAL activity highly increased in both WS and DS tissues.
Pattern of changes of β-D-glucosidase activity in both cultivars proceeded similar to the changes in PAL activity (). In cv. Katoda, enzyme activity raised by about 32% only in WS tissue, after spraying with resveratrol compared to control. In cv. Monsun, the activity of β-D-glucosidase after resveratrol treatment did not differ from control in WS tissue while in DS tissue it was higher by about 37% than in control.
Efficiency of PSII
Values of all parameters were changing during plants' development, showing general increasing tendency, especially in plants of cv. Katoda ( and ). External application of resveratrol (before inoculation) increased efficiency of an energy flux for electron transport and performance index in plants of cv. Katoda but not in cv. Monsun. An inoculation of four-week-old plants (cv. Monsun) with powdery mildew caused transient but significant drop in PSII efficiency between the fourth and sixth week of plant vegetation. After this period, a recovery of photosynthetic efficiency was noted in seven-week-old plants. As for cv. Katoda, no negative effect of pathogen infection on PSII efficiency was noted between the fourth and sixth week of growth while in seven-week-old plants, an increase of photosynthetic efficiency was observed.
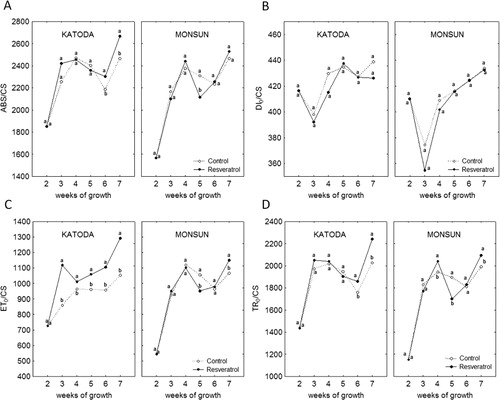
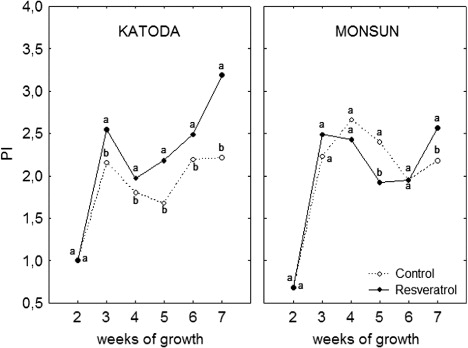
Resveratrol modified the efficiency of PSII of infected plants. In plants of cv. Katoda, the positive effect of resveratrol was constant from the third to the seventh week of growth. In seven-week-old plants of cv. Katoda, sprayed with resveratrol, values of ET0/CS were higher than in control by about 25%. Although the value of ET0/CS was significantly higher at all times, energy absorption (ABS/CS) and energy loss (DI0/CS) were not increased. Energy trapped in reaction centers (TR0/CS) was increased in the sixth and seventh week of growth. In case of plants of cv. Monsun, a transient drop in ET0 value was noted in the fifth week of growth. Eventually, seven-week-old plants had significantly higher values of TR0/CS, ET0/CS, and PI in comparison to control by about few percent. Simultaneously, the energy absorption (ABS/CS) was not increased.
Plant growth and heading
Resveratrol influenced plant growth between the third and seventh week of vegetation (). Growth stimulation of upper part of plants was observed in cv. Katoda after application of resveratrol. Plants treated with resveratrol were also higher than controls by about 20% in the third week of vegetation and 15% in the seventh week of vegetation. As for cv. Monsun, resveratrol application reduced the growth of plants. The effect was statistically significant from the third to the sixth week of growth and became negligible in the seventh week of culture. Application of resveratrol stimulated the development of plants of cv. Katoda. At the end of experiment (ninth week of culture), 29.4% of the control population were in the heading stage while in population treated with resveratrol, nearly 60% plants were heading. By contrast, none of the plants of cv. Monsun at the same final phase of experiment were found in the heading stage, either in control or resveratrol-treated groups ().
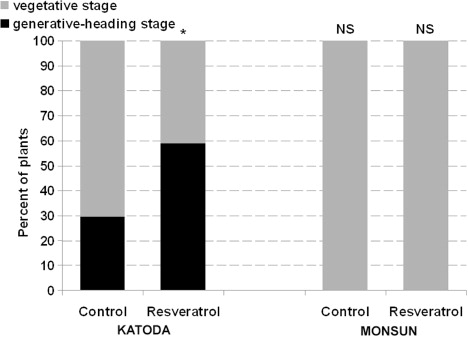
Table 1. The influence of resveratrol on plant growth. Mean values marked with the same letters do not differ significantly according to Duncan’s test; P ≤ 0.05.
Resveratrol content
Retention time of trans-resveratrol standard was assayed by HPLC analysis and amounted to 24.99 min (). A small peak with similar retention time as chemically synthesized resveratrol was found on chromatogram of sample extract of not infected leaves of wheat. Several times higher peak with retention time of 24.92 was detected in the extract of leaves of mildewed plants. Based on the standard curve, putative trans-resveratrol content was calculated at 4.1 µg g−1 FW and 40.2 µg g−1 FW for healthy and mildewed leaves, respectively.
Discussion
General overview on possible directions of resveratrol action in plants
This experiment studied the reactions of two varieties of wheat, infected with B. graminis, to exogenously applied resveratrol. In both varieties, a positive effect of resveratrol was obtained, reducing the degree of damage to the leaves. The flag leaves of resistant variety showed almost no signs of infection while lesion spots in more sensitive plants were significantly reduced. The biochemical analyses and physiological measurements that were carried out allowed to identify some of the mechanisms of action of resveratrol.
Overall, the effects of exogenously applied resveratrol can occur in three possible directions. The first one is the direct action of the antibiotic compound on the pathogen (Caruso et al. Citation2011). It seems, however, that this mechanism does not apply in our experiment. Such fungistatic effect on powdery mildew conidia should be universal, independent of the resistance resulting from genotypic differences.
The second of the possible courses of action of resveratrol stems from the antioxidant properties of this phenolic compound (He & Yan Citation2013). Infection of wheat plant with powdery mildew causes oxidative stress in cells and results in increased activity of antioxidative enzymes (Kovacs et al. Citation2011). If the system is unable to control the level of generated reactive oxygen species (ROS), it demands exogenous supplement of antioxidants to scavenge excessive ROS and restores the stable state of cellular ‘redox homeostasis’ (Foyer & Noctor Citation2005, He & Yan Citation2013). In this case, a direct antioxidative effect of resveratrol, resulting from its chemical properties such as the presence of three phenol groups or trans-isomery of the double bound, can be beneficial for the plant (He & Yan Citation2013). Some studies on the human cell mitochondria show that resveratrol treatment inhibits ROS production and attenuates superoxide generation (Shin et al. Citation2009).
The third way of resveratrol action in plants might be based on its regulatory function. Resveratrol may regulate of metabolism of other compounds, which determines defensive reactions. This effect may be manifested by an increase in the activity of some enzymes linked to plants resistance (e.g. enzymes of phenolic metabolism or antioxidative enzymes) as well as by the regulation of the production of certain hormones (polyamines, salicylic acid, jasmonic acid or ethylene).
Resveratrol modulates phenolic metabolism in wheat infected with powdery mildew
The increase of phenolic compounds is a common reaction observed after fungal pathogens attack (Dixon & Paiva Citation1995). The most important enzyme responsible for phenylpropanoids synthesis is PAL, which is considered a marker of plant reaction to environmental stress (Pociecha et al. Citation2008). High activity of PAL leads to an increase of many derivatives of hydroxycinnamic acid and alcohols. These compounds may represent direct fungistatic effect or may be utilized in reactions of strengthening the cell wall – natural barrier preventing pathogen penetration. Thus, the pool of wall-bound phenylpropanoid units acts as a reservoir, including phenols which are inactive in bound forms but can be quickly released following pathogen recognition. They are commonly sequestered in conjugated forms, usually with glycosidic attachments, in vacuoles or organelles of healthy plants and are released by hydrolyzing enzymes of plants (i.e. β-D-glucosidase). In our work, resveratrol increased the activity of enzymes involved in the phenolic metabolism (PAL and β-D-glucosidase). More resistant cultivar showed higher activity of enzymes in leaf regions showing no symptoms of pathogen infection. In less resistant cultivar, the same processes were altered but they were detected in regions with and without symptoms of pathogen invasion. Increased activity of the so-called defense enzymes, including PAL and β-1,3-glucanase, correlated with increased resistance to B. cinerea in tomato after L-arginine application was reported by Zheng et al. (Citation2011). In turn, Morkunas et al. (Citation2005) observed that infection with Fusarium oxysporum generally stimulated β-glucosidase activity in lupine. In the current study, control plants also showed accumulation of soluble phenolic compounds in pathogen-invaded tissue, despite the lack of increase in PAL and β-D-glucosidase activity. Similarly, a strong defensive reaction based on phenolic compounds accumulation and callose deposition was observed in regions invaded by pathogen and in adjacent cells of barley infected by B. graminis compared to noninvaded regions in the study by Swarbrick et al. (Citation2006). By contrast, no relationship between the soluble phenolics content and PAL activity was reported by Olenichenko and Zagoskina (Citation2005) in wheat and by Pociecha et al. (Citation2009) in festulolium where no correlation was detected between the PAL activity and soluble phenolic in reaction to cold. It is possible that in some cases phenolic monomers inhibit PAL and for that reason PAL activity is not related to production of phenolic compounds (Olenichenko & Zagoskina Citation2005). Furthermore, in infected tissue of resveratrol-treated plants, soluble phenolics level was also not correlated with PAL activity while in the invaded leaf regions of cv. Monsun, soluble phenolics level was higher than in cv. Katoda. Similar reaction was observed in the study by Hakulinen et al. (Citation1999), where phenolic content in willow clones that were highly resistant to Melampsora rust was lower than in less-resistant plants. These authors explained a decrease in phenolics concentration after infection by Melampsora rust by lignin biosynthesis. In our study, the use of resveratrol increased the activity of PAL and β-D-glucosidase (in resistant varieties only in uninfected leaf areas), and yet the content of phenolics is lower or similar to the phenolic content in the leaves of control plants. It can be assumed that a large amount of this phenolic pool is immediately utilized for cell wall reinforcement. This effect of resveratrol supports, at the same time, the previous hypothesis about the direct effect of this compound on the first line of defense associated with strengthening the cell walls.
Resveratrol affects photosynthesis in wheat infected and not infected with powdery mildew
The pathogenesis of powdery mildew significantly impairs the functioning of the photosynthesis (Magyarosy et al. Citation1976, Gordon & Duniway Citation1982). However, the impact of pathogen on the photosynthesis may depend on resistance of particular cultivars. In barley infected with B. graminis, photosynthesis of resistant leaves was unaffected by the fungus (Scott & Smillie Citation1966). Whereas the infection of susceptible leaves resulted in progressive decrease in the photosynthesis rate compared to noninfected leaves. These data are consistent with our observations in wheat plants. Values of the most important parameter of PSII efficiency, reflecting energy flux for electron transport (ET0/CS), decreased just after infection in cv. Monsun while remained unchanged in more resistant cv. Katoda.
To our knowledge, the influence of exogenous resveratrol on photosynthesis intensity has not been studied. In our experiment, cv. Katoda responded better to resveratrol application than cv. Monsun. Even before the infection of plants of cv. Katoda with mildew conidia, an increase in the efficiency of energy flux for electron transport was detected in resveratrol-treated plants compared to controls, despite maintaining a similar level of energy absorption and energy dissipation. This phenomenon, however, was not observed in the cv. Monsun.
The efficiency of electron transport (ET0/CS) and PI are the most sensitive parameters of fluorescence kinetics, reflecting plant response to environmental stimuli (Rapacz Citation2007). Monitoring of changes in these parameters for cv. Katoda and cv. Monsun during pathogenesis illustrates the differences between the cultivars in response to a pathogen and resveratrol. Despite the weaker initial response of cv. Monsun to resveratrol in the seventh week of vegetation, the plants of both varieties treated with resveratrol showed better PSII efficiency expressed by ET0/CS and PI values compared to control plants. At this stage, we can only hypothesize how resveratrol influences the efficiency of PSII. As we know, this efficiency is based on the proper functioning of the electron transport proteins located in the membranes of chloroplasts. Pathogen attack, in turn, is associated with the damage (peroxidation) of membrane lipids (El Zahaby et al. Citation1995), which may be one of the causes of dysfunction of these proteins. Meanwhile stilbenoids, similarly as sterols (Grunwald Citation1974) or steroids (Janeczko et al. Citation2013), may exert protective effects that stabilize membranes while acting as antioxidants preventing peroxidation of unsaturated lipids in the lipid bilayer (Brittes et al. Citation2010). There was no significant effect of resveratrol on the absorption of energy (accompanied by increased chlorophyll content), but resveratrol did influence the parameter of energy flux for electron transport. It suggests that the effect of resveratrol was likely connected with the improvement of stability and efficiency of the membranes, even though other effects cannot be ruled out. Action of resveratrol may also be associated with the stimulation of production of plant hormones such as polyamines and salicylic acid in particular. These hormones are linked to plant defense responses to pathogens (Davies Citation2010) as well as involved in functioning of the photosynthesis (Wang et al. Citation2010; Shu et al. Citation2012). The hypothesis that resveratrol may stimulate the synthesis of salicylic acid would be further supported by the fact that the effects of resveratrol exhibited in a decrease of flag leaf infection. Therefore, these effects were observed in the newly developed leaves, five weeks after the application of this compound in juvenile stage. This may be an evidence of activation of systemic resistance, which is mediated by salicylic acid (Davies Citation2010).
Regulation of wheat growth and development by resveratrol
The consequence of photosynthesis disturbances after pathogen attack is growth inhibition. After application of resveratrol, we observed growth stimulation despite the infection progress in more resistant to powdery mildew cv. Katoda. Simultaneously, resveratrol increased the percentage of heading plants. In cv. Monsun, transient inhibiting effect of resveratrol on plant growth was observed and there was no visible impact of this compound on plant heading within experimental time frame. It is very likely that the effect of resveratrol on plant growth and development could be relayed via the same pathways as for estrogens. It is known that estrogens stimulates wheat growth and generative development (Janeczko & Filek Citation2002, Janeczko unpublished data). Moreover, estrogen receptors are present in wheat cells (Janeczko et al. Citation2008). Simultaneously, resveratrol is classified as phytoestrogen (Gehm et al. Citation1997) with mild estrogenic activity in mammal cells (Sanoh et al. Citation2003). Future studies should investigate whether plants do have specific receptors for resveratrol or rather resveratrol is bound to other (estrogen) receptors.
Content of resveratrol in wheat – effect of pathogen infection
We have assayed the content of resveratrol in plants infected with powdery mildew and healthy plants to find the connection between the pathogenesis in wheat and resveratrol level in leaf tissue. In plants of the family Poaceae, resveratrol has been identified for the first time in 1994 (Powell et al. Citation1994). Resveratrol was present in the seeds and forage in range from 0.5 to as much as 2300 µg g−1 of plant material. The amount of resveratrol depended on the species of grass and source of origin. Moreover, an influence of endophyte infection on increased resveratrol content was also noticeable. In wheat, endogenous resveratrol (0.95 µg g−1 FW) was detected by Fettig and Hess (Citation1999) while Serazetdinova et al. (Citation2005) did not detect stilbene derivatives in the wild type wheat of cv. Combi. Our analysis showed the presence of a compound with standard retention time similar to resveratrol in uninfected plants in the amount of about 4 µg g−1 FW. Although this value is higher than that obtained by Fettig and Hess (Citation1999), according to Powell et al. (Citation1994) it is in the normal range for the grasses. Infection of wheat with powdery mildew caused 10-fold increase in the content of resveratrol. Increased production of resveratrol due to mildew is also described in other species, mainly grapes (Romero-Pérez et al. Citation2001). The phenomenon of increased production of resveratrol by wheat plants infected with powdery mildew has probably not been tested and our work is the first report indicating this issue. However, we believe that furthermore detailed studies are required, including experiments resolving the structure of the compound detected in the retention time of resveratrol. In addition, we cannot exclude at the moment that the detected compound, which content is rapidly increasing after the infection, is a product of fungal origin. There are fungi described that are able to produce stilbene derivatives (Shi et al. Citation2012). Nevertheless, it seems rather unlikely because resveratrol would facilitate defense reactions of the host-plant and thus reduced the pathogenicity of the fungus.
Practical aspects of resveratrol application in wheat
Transformation of wheat with stilbene synthase increased the content of resveratrol and improved resistance to fungi (Leckband & Lörz Citation1998, Fettig & Hess Citation1999, Serazetdinova et al. Citation2005). However, eliminating the risk of mildew and other pathogens by transgenesis of all the cultivated varieties seems like a daunting task. Especially that powdery mildew occurrence depends on many environmental factors, and there are years, when crops are infected incidentally. For this reason, the possibility of the application of exogenous resveratrol may still be a convenient solution, especially in the so-called ecological agriculture, where use of synthetic fungicides and transgenic plants is excluded. Modern methods of early detection of powdery mildew (Bürling et al. Citation2012) provide additional rapid identification of the disease and allow for taking countermeasures in the form of spraying.
Conclusion
To conclude, the current study characterized some of the mechanism of action of resveratrol in wheat plants. In parallel, several hypotheses have been proposed that will require experimental confirmation. Resveratrol showed multidirectional properties in wheat plants and was actively involved in the processes leading to the increased wheat resistance to B. graminis. In particular, resveratrol treatment induced phenolic metabolism by increasing activity of both PAL and β-D-glucosidase enzymes. Prior to pathogen infection, resveratrol stimulated photosynthesis intensity but this effect was cultivar-dependent (only in cv. Katoda). What is more, resveratrol increased the efficiency of photosynthesis in infected plants of both cultivars. According to our HPLC analysis, resveratrol was likely present in the spring wheat. Content of the compound, of similar retention time to chemically synthesized resveratrol, increased 10-fold as a result of pathogen infection. Based on the data obtained in our work, we can state that resveratrol has regulatory function in wheat plants. In addition to metabolic changes caused by resveratrol that led to increased resistance, this compound also influenced plant growth and might stimulated plant development.
References
- Ahuja I, Kissen R, Bones AM. 2012. Phytoalexins in defense against pathogens. Trends Plant Sci. 17:73–90. 10.1016/j.tplants.2011.11.002
- Brittes J, Lúcio M, Nunes C, Lima JL, Reis S. 2010. Effects of resveratrol on membrane biophysical properties: relevance for its pharmacological effects. Chem Phys Lipids. 163:747–754. 10.1016/j.chemphyslip.2010.07.004
- Bürling K, Hunsche M, Noga G. 2012. Presymptomatic detection of powdery mildew infection in winter wheat cultivars by laser-induced fluorescence. Appl Spectrosc. 66:1411–1419. 10.1366/12-06614
- Caruso F, Mendoza L, Castro P, Cotoras M, Aguirre M, Matsuhiro B, Isaacs M, Rossi M, Viglianti A, Antonioletti R. 2011. Antifungal activity of resveratrol against Botrytis cinerea is improved using 2-furyl derivatives. PLoS ONE. 6:e25421. 10.1371/journal.pone.0025421
- Chong J, Poutaraud A, Hugueney P. 2009. Metabolism and roles of stilbenes in plants. Plant Sci. 177:143–155. 10.1016/j.plantsci.2009.05.012
- Davies PJ. 2010. Plant hormones. Biosynthesis: signal transduction: action! Dordrecht, Heidelberg, London, New York: Springer.
- Dixon RA, Paiva NL. 1995. Stress-induced phenylpropanoid metabolism. Plant Cell. 7:1085–1097.
- Dong X. 1998. SA, JA, ethylene and disease resistance in plants. Curr Opin Plant Biol. 1:316–323. 10.1016/1369-5266(88)80053-0
- El Zahaby HM, Gullner G, Kiraly Z. 1995. Effects of powdery mildew infection of barley on the ascorbate-glutathione cycle and other antioxidants in different host-pathogen interactions. Phytopathol. 85:1225–1230. 10.1094/Phyto-85-1225
- Fettig S, Hess D. 1999. Expression of a chimeric stilbene synthase gene in transgenic wheat lines. Transgenic Res. 8:179–189. 10.1023/A:1008941607113
- Foyer CH, Noctor G. 2005. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 17:1866–1875. 10.1105/tpc.105.033589
- Gehm BD, McAndrews JM, Chien PY, Jameson JL. 1997. Resveratrol: a polyphenolic compound found in grapes and wine: is an agonist for the estrogen receptor. Proc Natl Acad Sci USA. 94:14138–14143. 10.1073/pnas.94.25.14138
- Gordon TR, Duniway JM. 1982. Effects of powdery mildew infection on the efficiency of CO2 fixation and light utilization by sugar beet leaves. Plant Physiol. 69:139–142. 10.1104/pp.69.1.139
- Grunwald C. 1974. Sterol molecular modifications influencing membrane permeability. Plant Physiol. 54:624–628. 10.1104/pp.54.4.624
- Hakulinen J, Sorjonen S, Julkunen-Tiitto R. 1999. Leaf phenolics of three willow clones differing in resistance to Melampsora rust infection. Physiol Plant. 105:662–669. 10.1034/j.1399-3054.1999.105410.x
- He S, Yan X. 2013. From resveratrol to its derivatives: new sources of natural antioxidant. Curr Med Chem. 20:1–13.
- Hűckelhoven R. 2005. Powdery mildew susceptibility and biotrophic infection strategies. FEMS Microbiol Lett. 245:9–17. 10.1016/j.femsle.2005.03.001
- Janeczko A, Budziszewska B, Skoczowski A, Dybała M. 2008. Specific binding sites for progesterone and 17beta-estradiol in cells of Triticum aestivum L. Acta Biochim Pol. 55:701–711.
- Janeczko A, Filek W. 2002. Stimulation of generative development in partly vernalized winter wheat by animal sex hormones. Acta Physiol Plant. 24:291–295. 10.1007/s11738-002-0054-0
- Janeczko A, Tóbiás I, Skoczowski A, Dubert F, Gullner G, Barna B. 2013. Progesterone moderates damage in Arabidopsis thaliana caused by infection with Pseudomonas syringae or P. fluorescens. Biol Plantarum. 57:169–173. 10.1007/s10535-012-0142-y
- Jiménez JB, Orea JM, Montero C, Ureña AG, Navas E, Slowing K, Gómez-Serranillos MP, Carretero E, De Martinis D. 2005. Resveratrol treatment controls microbial flora: prolongs shelf life: and preserves nutritional quality of fruit. J Agric Food Chem. 53:1526–30. 10.1021/jf048426a
- Justesen U, Knuthsen P, Leth T. 1998. Quantitative analysis of flavonols: flavones: and flavanones in fruits: vegetables and beverages by high-performance liquid chromatography with photo-diode array and mass spectrometric detection. J Chromatogr A. 799:101–110. 10.1016/S0021-9673(97)01061-3
- Kępczyńska E, Król P. 2012. The phytohormone metyl jasmonate as an activator of induced resistance against the necrotroph Alternaria porri f. sp. solani in tomato plants. J Plant Interact. 7:307–315. 10.1080/17429145.2011.645169
- Kiselev KV. 2011. Perspectives for production and application of resveratrol. Appl Microbiol Biotechnol. 90:417–25. 10.1007/s00253-011-3184-8
- Kovács V, Pál M, Vida G, Szalai G, Janda T. 2011. Effect of powdery mildew infection on the antioxidant enzyme activities in different lines of Thatcher-based wheat. Acta Biologica Szegediensis. 55:99–100.
- Leckband G, Lörz H. 1998. Transformation and expression of a stilbene synthase gene of Vitis vinifera L. in barley and wheat for increased fungal resistance. Theor Appl Genet. 96:1004–1012. 10.1007/s001220050832
- Magyarosy AC, Schürmann P, Buchanan BB. 1976. Effect of powdery mildew infection on photosynthesis by leaves and chloroplasts of sugar beets. Plant Physiol. 57:486–489. 10.1104/pp.57.4.486
- Morkunas I, Marczak L, Stachowiak J, Stobiecki M. 2005. Sucrose-induced lupine defense against Fusarium oxysporum sucrose-stimulated accumulation of isoflavonoids as a defense response of lupine to Fusarium oxysporum. Plant Physiol Biochem. 43:363–373. 10.1016/j.plaphy.2005.02.011
- Nandini B, Hariprasad P, Niranjana SR, Shetty HS, Geetha NP. 2013. Elicitation of resistance in pearl millet by oligosaccharides of Trichoderma spp. against downey mildew disease. J Plant Interact. 8:45–55. 10.1080/17429145.2012.710655
- Nichols EJ, Beckman JM, Hadwiger LA. 1980. Glycosidic enzyme activity in pea tissue and pea-Fusarium solani interactions. Plant Physiol. 66:199–204. 10.1104/pp.66.2.199
- Olenichenko NA, Zagoskina NV. 2005. Response of winter wheat to cold: production of phenolic compounds and L-phenylalanine ammonia-lyase activity. Appl Biochem Microbiol. 41:681–685. 10.1007/s10438-005-0109-2
- Peltonen S, Karjalainen R. 1995. Phenylalanine ammonia-lyase activity in barley after infection with Bipolaris sorokiniana or treatment with purified xylanase. J Phytopath. 143:239–245. 10.1111/j.1439-0434.1995.tb00606.x
- Pociecha E, Płażek A, Janowiak F, Waligórski P, Zwierzykowski Z. 2009. Changes in abscisic acid, salicylic acid and phenylpropanoid concentrations during cold acclimation androgenic forms of Festulolium Festuca pratensis × Lolium multiflorum in relation to resistance to pink snow mould (Microdochium nivale). Plant Breeding. 128:397–403. 10.1111/j.1439-0523.2009.01664.x
- Pociecha E, Płażek A, Janowiak F, Zwierzykowski Z. 2008. ABA level, proline and phenolic concentration, and PAL activity induced during cold acclimation in androgenic Festulolium forms with contrasting resistance to frost and pink snow mould (Microdochium nivale). Physiol Mol Plant P. 73:126–132. 10.1016/j.pmpp.2009.03.005
- Powell RG, TePaske MR, Plattner RD, White JF, Clement SL. 1994. Isolation of resveratrol from Festuca versuta and evidence for the widespread occurrence of this stilbene in the Poaceae. Phytochemistry. 35:335–338. 10.1016/S0031-9422(00)94759-9
- Rapacz M. 2007. Chlorophyll a fluorescence transient during freezing and recovery in winter wheat. Photosynthetica. 45:409–418. 10.1007/s11099-007-0069-2
- Romero-Pérez AI, Lamuela-Raventós RM, Andrés-Lacueva C, de La Torre-Boronat MC. 2001. Method for the quantitative extraction of resveratrol and piceid isomers in grape berry skins. Effect of powdery mildew on the stilbene content. J Agric Food Chem. 49:210–215. 10.1021/jf000745o
- Sanoh S, Kitamura S, Sugihara K, Fujimoto N, Ohta S. 2003. Estrogenic activity of stilbene derivatives. J Health Sci. 49: 359–367. 10.1248/jhs.49.359
- Sbaghi M, Jeande P, Faivre B, Bessis R, Fournioux JC. 1995. Development of methods using phytoalexin resveratrol. Assessment as a selection criterion to screen grapevine in vitro cultures for resistance to grey mould (Botrytis cinerea). Euphytica. 86:41–47. 10.1007/BF00035937
- Scott KJ, Smillie RM. 1966. Metabolic regulation in diseased leaves. I. The respiratory rise in barley leaves infected with powdery mildew. Plant Physiol. 41:289–97. 10.1104/pp.41.2.289
- Serazetdinova L, Oldach KH, Lörz H. 2005. Expression of transgenic stilbene synthases in wheat causes the accumulation of unknown stilbene derivatives with antifungal activity. J Plant Physiol. 162:985–1002. 10.1016/j.jplph.2004.11.005
- Shi JL, Zeng Q, Liu YL, Pan ZL. 2012. Alternaria sp MG1: a resveratrol-producing fungus: isolation: identification: and optimal cultivation conditions for resveratrol production. Appl Microbiol Biotechnol. 95:369–379. 10.1007/s00253-012-4045-9
- Shin SM, Cho IJ, Kim SG. 2009. Resveratrol protects mitochondria against oxidative stress through AMP-activated protein kinase-mediated glycogen synthase kinase-3beta inhibition downstream of polyADP-ribose.polymerase-LKB1 pathway. Mol Pharmacol. 76:884–895. 10.1124/mol.109.058479
- Shu S, Guo S-R, Yuan L-Y. 2012. A review: polyamines and photosynthesis. In: Dr Mohammad Najafpour, editor. Advances in photosynthesis – fundamental aspects. Rijeka: InTech; p. 439–464. ISBN: 978-953-307-928-8.
- Singleton VL, Rossi JAJr. 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagent. Amer J Enol Viticult. 16:144–153.
- Skoczowski A, Janeczko A, Gullner G, Tóbias I, Kornas A, Barna B. 2011. Response of brassinosteroid-treated oilseed rape cotyledons to infection with the wild type and HR-mutant of Pseudomonas syringe or with P. florescence. J Therm Anal Cal. 104:131–139. 10.1007/s10973-010-1204-z
- Swarbrick PJ, Schulze-Lefert P, Scholes JD. 2006. Metabolic consequences of susceptibility and resistance race-specific and broad spectrum in barley leaves challenged with powdery mildew. Plant Cell Environ. 29:1061–1076. 10.1111/j.1365-3040.2005.01472.x
- Vanacker H, Carver TLW, Foyer CH. 1998. Pathogen-induced changes in the antioxidant status of the apoplast in barley leaves. Plant Physiol. 117:1103–1114. 10.1104/pp.117.3.1103
- Vidavalur R, Otani H, Singal PK, Maulik N. 2006. Significance of wine and resveratrol in cardiovascular disease: French paradox revisited. Exp Clin Cardiol. 11:217–225.
- Wang LJ, Fan L, Loescher W, Duan W, Liu GJ, Cheng JS, Luo HB, Li SH. 2010. Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol. 10:34. 10.1186/1471-2229-10-34
- Zheng Y, Sheng J, Zhao R, Zhang J, Lv S, Liu L, Shen L. 2011. Preharvest L-arginine treatment induced postharvest disease resistance to Botrysis cinerea in tomato fruits. J Agric Food Chem. 59: 6543–6549. 10.1021/jf2000053
