Abstract
Salvinia natans L. response to hydrogen peroxide (H2O2) induced oxidative stress through physiological activities was evaluated. The plants were incubated with varying concentrations (0, 50, 100 µM) of H2O2 and 100 µM of H2O2 supplemented with 1 mM putrescine (Put) in hydroponic culture. This is observed with the decline in proline content and its biosynthetic enzymes viz. γ-glutamyl kinase and γ-glutamyl phosphate reductase activity. Protein carbamylated derivative by protein oxidation was another trait for oxidative damages by H2O2. The antioxidative enzymes like guaiacol peroxidase (GPX), glutathione reductase (GR), and catalase (CAT) recorded to express through in-gel staining with the H2O2 exposure. On nuclear level, plants were sensitive to H2O2 where the DNA disintegration was studied with comet assay and maximum comet tail observed at 100 µM H2O2 treatment. Application of Put reduced the generation of protein oxidation and comet tail length as well as moderated the enzyme activity as revealed through in-gel staining.
Introduction
Irrespective of the stresses both abiotic and biotic, the most detrimental effects on cellular integrity of plants are generated when redox regulation is hampered in plants (Suzuki et al. Citation2012). This happens due to some misfiring of electrons from electron transport chain, particularly in chloroplast, mitochondria, and other organelle harboring the redox reactions. In that case, the molecular oxygen is reduced and a number of oxygen radicals are formed; in general it is called as Reactive Oxygen Species (ROS) including superoxide radical (O2 −), single oxygen (½ O2), hydrogen peroxide (H2O2), and hydroxyl radical (OH−) (Halliwell & Gutteridge Citation1999). More so, H2O2 becomes quite reactive in presence of the transition metals (iron and copper being the most important) through the reaction of homolysis of H2O2 into two molecules of OH−, most harmful free radicals in plant system (Ghosh et al. Citation2011). Under this condition, H2O2 becomes more effective than other ROS to destabilize the cellular redox system and establishes the oxidative stress. On the other hand, pretreatment of H2O2 could be useful in alleviation of oxidative damages induced by paraquat as documented in pea plants (Moskova et al. Citation2009). In addition, wilting of leaf, a morphological syndrome of paraquat-induced oxidative stress, has also been reversed by exogenously applied H2O2, as pretreatment (Moskova et al. Citation2011). Now, in response to oxidative stress, a number of elicitor moieties have been recognized for their efficacy to moderate the generated ROS. Among the elicitors, polyamine is one of the integral component that involved in alleviation of various abiotic stresses like salinity, drought, chilling, oxidative stress, metal toxicity, and paraquat (Fariduddin et al. Citation2013). This study also showed that putrescine (Put), a di-amine, which is a straight chain aliphatic compound (Farooq et al. Citation2009), diversed the cellular responses like osmotic adjustment, membrane functioning, modulation of metabolic pathways, and gene activation for detoxifications of ROS as reported in plant system (Ding et al. Citation2010). With the ongoing achievements for polyamine over expression, it is imperative to determine the efficacy of exogenously applied polyamine under the condition that initiates oxidative stress. This approach has been an effective measure for controlling the oxidative damages in plant tissues. Moreover, the interaction and alleviation of any kind of abiotic stresses with tri-amine (spermidine) and tetra-amine (spermine) are more common in comparison to di-amine (putrescine). Few reports are there particularly in Salvinia species where Put have been documented to be competent in metal stress alleviation (Mandal et al. Citation2013) but hardly any reports were found in case of H2O2 treatment. With this rational we hypothesized that Put could be interacting in similar fashion to moderate the H2O2 induced oxidative stress in Salvinia. Therefore, the principle behind Put-mediated H2O2 metabolism deserves to be studied. Most of the angiospermic plants, particularly the crop species, have been the test sample to elucidate the pathways of antioxidation under various abiotic stresses. However, some non-angiospermic plants, particularly those of fern, are reported with their in-built tolerance to accommodate ROS (Mostafa & Tammam Citation2012; Satapathy et al. Citation2012). Chinese brake fern is a citing example for hyperaccumulation of heavy metals and its tolerance to ROS has already been demonstrated (Xie et al. Citation2009). Salvinia, a common free-floating aquatic fern species, is widely grown in contaminated area or water bodies abundant with industrial effluents, which contain different toxic materials including oxides and hydroxides of heavy metals (Prado et al. Citation2010). Due to high diffusion of H2O2 in water, those contaminated areas become toxic for other plants to survive. Still, Salvinia is a common habitat of those contaminated areas and very often exhibits rapid growth under such condition (Dhir et al. Citation2011). With this view, a study was undertaken to reveal the impact of H2O2 on Salvinia plants with reference to oxidative stress and its possible interaction with polyamine. In this communication, we described the cellular responses of Salvinia plants to oxidative stress directly by H2O2 application for protein oxidation, osmolites and its biosynthetic enzymes, DNA disintegration, and expression of antioxidative enzymes.
Materials and methods
Salvinia natans L., an aquatic fern belonging to the family Salviniaceae of class Pteridopsida of the division Pteridophyta, was taken as the material for the present experiment. Plants collected from unpolluted pond or marshy land were washed with running water to remove adhering salts/debris, if any. Though there are no true roots in Salvinia, rhizoids-like structure developed from third leaf of each whorl (Gifford & Foster Citation1989). After completion, plants were transferred into ¼ strength of MS media (Murashige & Skoog Citation1962) for seven days for acclimatization. After that, 10 matured plants were taken and incubated in each concentrations of 0, 50, 100 µM of H2O2, and 100 µM of H2O2 supplemented with 1 mM Put. All the sets were kept in a dark chamber for 6 hrs (Sairam & Srivastava Citation2000).
Determination of protein oxidation
For the protein oxidation, the carbonyl content of sample was measured in a reaction with dinitrophenyl hydrazine (DNPH) (Verbeke et al. Citation2000). 1 g sample was thoroughly crushed in 6% (w/v) sodium lauryl sulphate (SDS) followed by incubation at 37°C for 30 min. To this 10 mM DNPH in 1.5 mM trichloro acetic acid (TCA) was added and further gently agitated at every 10 min for 1 h. The protein was precipitated by TCA and protein pellet was recovered by centrifugation at 15,000× g followed by re-extraction with 20% (w/v) TCA. The pellet was suspended completely in 0.2 M sodium phosphate buffer (pH 7) and the absorbance was read at 360 nm. Finally, the protein-carbonyl complex content was calculated using the molar extinction coefficient (530 M−1 cm−1) of DNPH and expressed in µM DNPH mg−1 protein. The protein content of the sample was determined with Bradford reagent (Bradford Citation1976).
Detection of DNA damage by comet assay
Comet assay was performed to detect the damage of DNA (Achary et al. Citation2008). The nuclear suspension was collected in phosphate buffer (pH 7.4) under cold condition. Grease free slide was precoated with 1% normal agarose solution and layered with the mixture of nuclear suspension with 0.6% low melting agarose. The slide was further coated with 0.6% low melting agarose under cold condition. The nuclei were run on an electrophoresis system in a buffer (10 N NaOH in 200 mM ethylene diamine tetra acetic acid (EDTA), pH ≥ 12) for 20 min at 25 V and 300 mA. The slides were neutralized with 0.4 M Tris-EDTA (pH 8.0) followed by methanol for 15 min. The slides were then stained with 0.1% ethidium bromide and observed under fluorescence microscope with an excitation filter of BP 546/10 nm and a barrier filter of 590 nm.
Estimation of proline
Proline was extracted from 1 g of fresh sample by homogenizing in 3% aqueous sulfosalicylic acid and the homogenate was filtered through Whatman filter paper (#2) followed by reaction of filtrate with acid-ninhydrin solution and glacial acetic acid for 1 h at 100°C. The reaction was terminated in an ice bath, mixed well with toluene. The chromophore containing toluene was aspirated from the aqueous phase, kept at room temperature. The proline content was measured at 520 nm, expressed as µM g−1 of fresh tissue (Bates et al. Citation1973).
Assay of γ-glutamyl kinase (γ-GK) and γ-glutamyl phosphate reductase (γ-GPR) activity
γ-GK (EC 2.7.2.11) activity was assayed according to Jaleel et al. (Citation2009). 1 g of plant sample was extracted with 50 mM Tris-HCl buffer (pH-6.8) and centrifuged at 20,000× g for 30 min at 4°C. 0.1 mL of extract was added to reaction buffer containing 0.1 mM of adenosine triphosphate (ATP), incubated at 37°C for 30 min subsequently added with 2 mL of stop solution. γ-GK activity was measured spectrophotometrically at 535 nm and expressed as unit (U) defined as µg of γ-glutamyl hydroxamate formed min−1 mg−1 protein (Wang et al. Citation2010). Similar extraction buffer was used for γ-GPR (EC:1.2.1.41) activity under identical condition except 0.1 M cysteine was used to stabilize the protein. 50 µg of enzyme protein was incubated for assay of γ-GPR in an assay mixture: 100 mM Tris-HCl pH 7.2, 1.5 mm MgCl2, 5 mM glutamine, 5 mM ATP, and 0.2 mM NAD(P)H. The oxidation of NAD(P)H was read at 340 nm and activity was expressed as µM NAD(P)H oxidized mg protein−1min−1 (Jaleel et al. Citation2008)
Determination of ascorbic acid
For detection of ascorbic acid (AsA), 1 g of fresh tissue was extracted in 5% metaphosphoric acid containing 0.1 mM bovine serum albumin (BSA) followed by centrifugation at 20,000× g for 15 min at 4°C. The supernatant was saved and reacted in an assay mixture with 1 mM 2,2′-dipyridyl dissolved in 50 mM phosphate buffer (pH 7), 0.2 mM ferric chloride, and 0.1 mM dithiotheitrol (DTT). The mixture was incubated for half an hour. The excess DTT was removed with N-ethyl malemide and absorbance was read at 265 nm. The ascorbate (AsA) content was calculated with extinction coefficient of ascorbic acid (14 mM−1 Cm−1) as suggested by Dipierro et al. (Citation2005).
Determination of glutathione content
GSH (Glutathione reduced) was assayed according to Paradiso et al. (Citation2008). 1 g plant sample was homogenized in 6% (w/v) meta-phosphoric acid containing 1 mM EDTA. The homogenate was centrifuged at 11,500× g at 4°C for 15 min. After that, 0.4 mL supernatant was added with 0.5 M potassium phosphate buffer (pH 7.5). This was incubated in an assay mixture containing 10 mM BSA, 10 mM 5,5′-dithio-bis (2 nitrobenzoic acid) (DTNB), 0.5 mM NADH for 15 min at 37°C. Absorbance was read at 412 nm.
Glutathione oxidized (GSSG) assayed with the same assay mixture but with the addition of 2-vinyl pyrrolidine to remove the GSH and reacted the same way as suggested by Yu et al. (Citation2003). Synthetically prepared glutathione, reduced (GSH) and oxidized (GSSG), was used to prepare the standard.
Assay of antioxidative enzymes through in-gel
To study the enzymatic antioxidative pathway of Salvinia plants under control and different concentration of H2O2 treatment, three enzymes viz. guaiacol peroxidase (GPX), catalase (CAT), and glutathione reductase (GR) were assayed (Ghosh et al. Citation2012). For extraction of enzyme 1 g of tissue was crushed in liquid nitrogen followed by homogenizing with extraction buffer: 1 mM Tris-HCl (pH 7.7), 10 mM MgCl2, 1 mM DTT, 0.1 mM phenylmethanesulfonyl fluoride (PMSF), 0.1 mM EDTA, 0.1 mM leupeptin, 0.1 mM BSA, and 2% PVP under cold condition. That was centrifuged at 15,000× g, at 4°C for 15 min. The supernatant containing the crude enzyme was precipitated by 80% ammonium sulphate cut followed by dialysis in suitable buffer (10 mM KCl, 0.1 mM EDTA, 1 mM PMSF, 1 mM β-ME) under 4°C. The purified protein was estimated by Bradford reagent (Bradford Citation1976) and equal amount of protein was used for in-gel activity of GPX, CAT, and GR.
The activity of GPX (EC 1.11.1.7) was measured spectrophotometrically where O-dianisidine was used as electron donor and H2O2 as substrate according to Hu et al. (Citation2009). 1 g of leaf tissue was homogenized thoroughly and enzyme extract was prepared. Equivalent amount of enzyme extract containing 50 µg of protein was run in a 10% polyacrylamide gel at 10 V/lane and the isozymes of GPX shows separate banding pattern after incubation in an mixture containing 50 mM potassium phosphate buffer, 0.5 mM O-dianisidine, and 0.5% H2O2 (Ammar et al. Citation2008).
For in-gel staining of CAT (E.C. 1.11.1.6), the volume of supernatant equivalent to 50 µg of protein sample was loaded in a non-denaturing 10% polyacrylamide gel at 10 V/lane under cold condition. After running the gel, for the development of bands, it was incubated in 0.05% H2O2 solution followed by solution of (1% w/v) potassium ferricyanide and 1% (w/v) ferric chloride for 5 min and then fixed with 1% hydrochloric acid (Shah & Nahakpam Citation2012).
The in-gel staining of GR (EC 1.6.4.2) activity was assayed by adding 50 µg of enzyme protein loaded in each lane of 10% non-denaturing polyacrylamide native gel at cold condition. The isoforms of GR was detected by developing the bands on the gel after incubating it for 10 min in a mixture containing 0.5 mM NAD(P)H, 3.4 mM GSSG, 0.5 mM EDTA, 25 mM Tris-HCl (pH 7.6), dichlorophenol indophenol (DCPIP), 3-(4,5-dimethyl-2 thiazolyl 2,5 diphenyl- tetrazolium bromine) (MTT) (Mandal et al. Citation2013).
Statistical analysis
All the observations were recorded with three replications (n = 3) and data were expressed as mean ± SE. The statistical analysis was performed by one-way ANOVA analysis, taking P ≤ 0.05 as significant.
Results
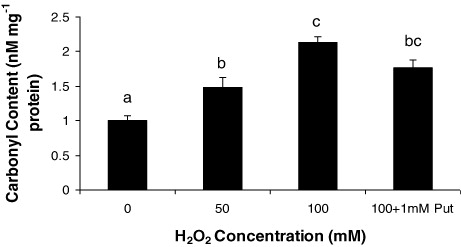
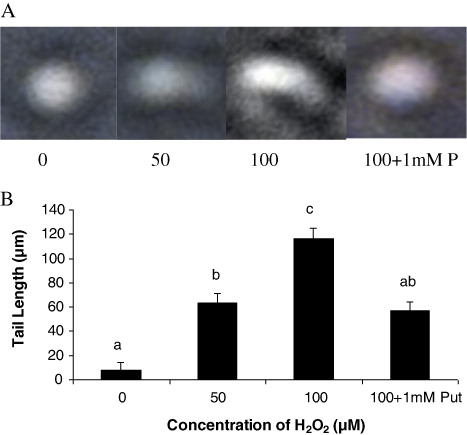
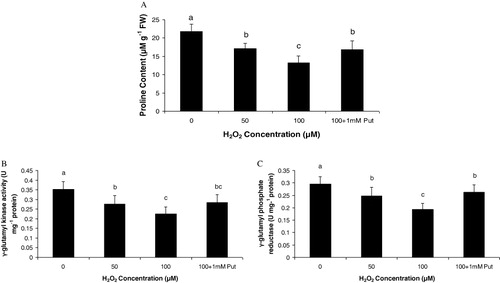
From the observations of Salvinia plants grown under H2O2 (50 and 100 µM) and highest dose of H2O2 supplemented with Put (100 µM + 1 mM Put), we recorded some interesting findings. It is obvious that plants had been prone to oxidative damages induced by H2O2 directly or H2O2 mediated development of other ROS, if any. As expected, Put as a polyamine had also been evident as a modulator of the response of plant under oxidative stress. This is because we have already confirmed from our earlier works that metals could sufficiently be able to promote H2O2 generation in Salvinia plants (data not shown here). Moreover, Put has been evident to moderate the content of peroxide as also revealed from this experiment. Therefore, it is quite expected that a direct exposure of H2O2 could elevate the total endogenous peroxide content in plant tissues. The oxidative stress preliminary induces an irreversible damage of the cellular membrane leading to significant loss of lipid and proteins. In the present experiment, the later was determined in terms of protein carbamylation from the leaves of the Salvinia plants. In comparison to control, the plants under different dosages of H2O2 and along with Put recorded a significant variation in accumulation of carbamylated derivatives. The variations were recorded as 1.48 fold and 2.13 fold under 50 µM and 100 µM H2O2, respectively, over control. The proteolytic activity of Salvinia plants were moderated by 1 mM Put applied with highest concentration of H2O2 (i.e. 100 µM). Thus, we record a down regulation in protein oxidation by 17.37% when compared with highest concentration of H2O2 i.e. 100 µM (). Since H2O2 is a potent ROS in cellular system, it targets most of the macromolecules and nucleic acid. Generally, H2O2 disintegrates the nuclear membrane by peroxidation reaction and produces the fragmented DNA molecules in comet shape. In the present study, DNA disintegration was measured by comet assay technique, based on alkaline lysis of DNA and its separation in low melting agarose. A distinct variation was recorded according to the concentration of H2O2 being maximum at 100 µM H2O2 (). A critical analysis of comet was done by measuring the comet tail and it recorded 7.87 fold and 14.5 fold increases under 50 µM and 100 µM H2O2, respectively, as compared to control. Moreover, Put acted as a reliever to down regulate the oxidative damage of nuclei by minimizing the comet tail by 50.8% over highest concentration of H2O2 (). It is obvious that plants are suffered from sort of osmotic stress under the stress condition those induces oxidative damages. Proline, an imino acid, happens to be one of the osmolytes in plant systems. Thus, we measure the proline accumulation along with its biosynthetic pathway in relation to assays of two enzymes: γ-GK and γ-GPR. The concentration of proline in the Salvinia plants was recorded: 21.55% and 38.99% over control, respectively, when incubated with 50 µM and 100 µM H2O2. On the contrary, Put alleviates the proline content by 1.26 fold than highest concentration (100 µM) of H2O2 treatment (). The important enzyme for proline biosynthesis is γ-GK. The γ-GK activity has decreased to a large extent in the H2O2 stressed Salvinia plants. Those were recorded 21.87% and 35.79% over control, respectively, under 50 µM and 100 µM H2O2 treatments. Put improved the γ-GK by 1.26 fold than highest concentration of H2O2 treatment (100 µM) (). On the other hand, second important enzyme for proline biosynthesis is γ-GPR which is regarded as rate limiting enzyme for the pathway. The activity of γ-GPR recorded 15.93% and 34.57% reduced from control (0 µM). However, the incubation of Put significantly (P < 0.05) induced the activity as recorded 1.35 fold over highest concentration (100 µM) of H2O2 treatment ().
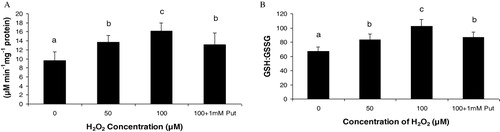
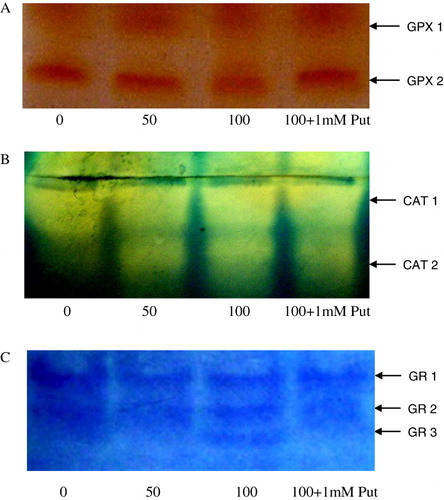
In the present experiment for antioxidative pathways, we found a significant regulation of non-enzymatic pathways for strategies in Salvinia plants. AsA and GSH being the predominant antioxidant moieties in plant system were evaluated under H2O2 induction. Salvinia plants recorded a linear increase of AsA through the H2O2 treatments and those were 1.43 fold and 1.69 fold at 50 µM and 100 µM of H2O2 treatments, respectively, over the control. Moreover, application of Put had diminished the AsA content by 18.51% in respect to 100 µM H2O2 treatment (). Another antioxidant in plant system more frequently exercised is GSH. This moiety with its two alternative form (reduced GSH and oxidized GSSG) are maintained in precise ratio for balancing the cellular redox. Thus in the present experiment the GSH:GSSG was recorded to be increased by 1.24 fold and 1.52 fold under 50 µM and 100 µM of H2O2 concentration. This showed the recruitment of GSH to sustain the reducing redox in the plant under oxidative condition. Application of Put, interesting to note had substituted the involvement of GSH in maintenance of redox. Thus the activity of GSH:GSSG was minimized by 15.13% under application of Put as compared to highest concentration of H2O2 treatment (100 µM) (). Responses to oxidative stress are maximally manifested into expression of antioxidative enzymes. In the present experiment, H2O2 being a potent ROS had been able to induce the important antioxidative enzymes. GPX, CAT, and GR activity expressions are detected by activity staining in-gel that had revealed the antioxidative responses differentially. Two possible isoforms of GPX (viz GPX1 and GPX2) were resolved after running the isolated and separated protein in native polyacrylamide gel electrophoresis (PAGE) and followed by consequent reaction with H2O2 and orthodianisidine. It is interesting to note that H2O2 treatment undoubtedly changed the expressions of GPX, though not in band numbers but in intensities; GPX2 was more expressed than GPX1 under H2O2 treatments in Salvinia (). Another peroxide hydrolyzing enzyme, CAT, requires no phenolic residue as electron donor as recorded significant variation under H2O2 treatment. The in-gel study of CAT showed two distinct isoforms (CAT1 and CAT2) and those were variable in expression among the treatments. This is clear that expression of CAT was in a dose-dependent manner as compared to control. This is clear at highest intensity under 100 µM of H2O2. This goes with some subdued effect when 1 mM Put was applied to Salvinia plant along with 100 µM of H2O2 (). Therefore, the appearance of this band may possibly suggest the polymorphism linked to oxidative stress. Another antioxidative enzyme, which is required to steady maintenance of GSH level, is the GR. For the expression profile of GR, significant variations recorded according to concentration of treatments. For GR, three distinct isozymic bands (GR1, GR2, and GR3) recorded (). This clearly indicates a possibility of induction and regulation of GR isozymes and their expression through in-gel study.
Discussion
The cellular content of H2O2 undoubtedly established the oxidative damages of Salvinia plants in the present experiment. Some reports are established that H2O2 could be able to accelerate or/and induce oxidative stress in many plant species with higher concentrations (Sairam & Srivastava Citation2000; Upadhyaya et al. Citation2007). This attitude of H2O2 is also variable according to tissue specificity and plant species. Therefore, it is still to be ascertained how this H2O2 could behave for other non-angiospermic plants like Salvinia as in the present case. However, the polyamines are ubiquitous molecules in functioning for physiological responses under abiotic stresses as already established in many studies (Alcazar et al. Citation2010). Higher polyamines like spermidine and spermine have been reported frequently to furnish resistance mechanism in many crop species and guaranteed their efficacy in frequent use. Hence, diamine (Put) had been chosen to monitor the oxidative exposure in our experiment. In our earlier findings it was recorded that Put could be sufficient to minimize the oxidative damages induced indirectly by metals (Mandal et al. Citation2013). Herein, the more understandable role of Put expected to be revealed when Salvinia plants interact directly with H2O2. From the results of our experiment, we recorded that the Put alleviated the H2O2 induced oxidative stress efficiently. The decrease in proline content under H2O2 treatment could be attributed by stabilizing osmotic status (Cheeseman Citation2006). Proline biosynthesis is a multistep pathway facilitated by a number of enzymes (Celik & Atak Citation2012). However, the two distinct committed steps are regarded as rate limiting and two enzymes are γ-GK and γ-GPR. These two enzymes are operative in a sequential manner for biosynthesis of proline and have been evident to relate the redox balance under osmotic stress (Thippeswamy et al. Citation2010). Recently, the effects of cadmium and zinc stress on these enzyme activities have been studied and revealed the regulatory property of proline in homeostasis of ions (Wang et al. Citation2010). However, in the present experiment, the activity of γ-GK and its down regulation under H2O2 were recorded. On the other hand, Put could relieve the γ-GK activity and thereby acted as a positive modulator for proline biosynthesis in relation to H2O2 induced osmotic stress. This holds the conformity for the oxidative exposure by H2O2 leading to development of compatible solutes like proline through regulation of rate limiting enzymes (Pavlikova et al. Citation2008). On the other hand, polyamine being a polycationic in nature could bind with the negatively charged domain of the membrane and thereby may shield the proteins from its oxidation by ROS. Thereby, the ROS-induced protein carbamoylation was alleviated by the polyamines. It is understood that polyamine could sustain the transportation of ions through membrane by stabilizing the membrane bound proteins (Groppa et al. Citation2007). The effect of ROS on DNA damage is another common consequence of the oxidative stress in plants. More specifically, it is the H2O2 itself or/and hydroxyl radical (OH−) readily generated by one electron reduction of H2O2 are most potent for DNA breakage (Rodriguez et al. Citation2011). In this regard, we have tested the mitigation of DNA damages by application of Put and found reduction in comet tail length in the Salvinia plants.
Under the ambient condition of cellular system, in-built antioxidative property is sufficient against the normal oxidation in the tissues. Likewise, POD, CAT, and GR are those enzymes involved in antioxidative cascade and often show fair correlation with stress tolerance. We have already tested the potentiality of Salvinia plants for their induced activities of those enzymes in in vitro condition under Al treatments (Mandal et al. Citation2013). Thus, the present experiment deals with the isozymic variation of those enzymes differentially expressed as polypeptide bands when resolved in in-gel staining. The partially purified protein when separated in non-denaturing gel and reacted with guaiacol shows some variations for isozymes. The extent of variations in isozymic expression is the resultant of antioxidation activities of GPX pool in the cells. In general, the tolerant varieties are more expressive in different sub-cellular components under oxidative stress and polyamine could modulate their expression (Yang et al. Citation2012). For the expression of CAT, Salvinia plants responded well to Put application. Previously, oxidative stress through direct metal exposure had significantly curtailed the CAT activities (Roychoudhury et al. Citation2012). Physiologically, plant tissues are required to maintain a reducing status under condition of oxidative environment. This is accomplished by the synthesis of some compounds like GSH, ASA, etc., acting as antioxidants (Ding et al. Citation2012). GSH with its two convertible forms, oxidized (GSSG) and reduced (GSH), plays an important role to maintain the cellular redox. The conversion of GSSG into GSH is carried out by GR. In the present experiment, the activity of GR and its modulation by Put are reflected with activity staining in-gel and thus it could be attributed in sustenance of a reduced state of redox at the cellular level. The isoforms of GR resolved as distinct bands when run in native gel and, in actual, are a composite effect of each individual isoenzymic proteins, some of those are differentially expressed in plants under various conditions inductive to oxidative stress (Hall Citation2002). Therefore, expressions of GR bands varying in intensities are also effected for Salvinia plants under H2O2 induced oxidative stress. Though H2O2 is not a typical free radical, still, it is enough for cellular redox transformation in plants, particularly, under various kinds of stresses. AsA is another non-enzymatic component of antioxidation stabilizing cellular redox (Loscos et al. Citation2008). In general, oxidation of AsA in stressed tissues is operated through two sequential steps via mono-dehydroascorbate (MDHA) and dehydroascorbate (DHA) following Halliwell Asada pathway (Sharma et al. Citation2012). MDHA converted into DHA and AsA spontaneously, whereas AsA is reduced by APX. Furthermore, this DHA could be replenished by a reductase using reducing equivalence of GSH. Therefore, AsA and GSH can complement the antioxidant demand of the tissues. In the present experiment, we find a steady state ratio of GSH to GSSG which might reflect the potentiality of the plants under H2O2 induction (Mandal et al. Citation2013). Put is thus supposed to be a modulator for maintaining the cellular redox and thus the activities of AsA and GSH were relieved. Therefore, polyamine might be credited as an effective elicitor when plants are directly exposed to ROS.
Conclusion
From the facts and the figures of the present investigation it is clearly revealed that the cellular responses of Salvinia plants under H2O2 induced oxidative stress have been detrimental in nature. H2O2 being an inducer of many cellular responses undoubtedly established the oxidative damage over the tolerable range of plants as evident from this study. Plants mostly are effected with cellular disintegration, protein carbamylation, DNA breakage, etc. under such condition. Put effectively has been capable in mitigation of those oxidative damages. Moreover, in combination with H2O2, Put had significantly modulated the antioxidation pathways, mostly by enzymatic activities. Therefore, Salvinia, being a non-angiospermic, pteridophytic plant species, was found to interact with polyamine at cellular level. However, a precise or short-term effect of H2O2 exposure, especially under in-vitro condition, may not necessarily unravel the exact behavior of plants as compared to natural condition of environment. Therefore, further in-depth study should be compulsory with higher H2O2 concentration to properly characterize the Salvinia plants and to interact with polyamine against oxidative stress.
Acknowledgments
This work is financially supported by DST-PURSE programme, Govt. of India, Department of Science and Technology, New Delhi
References
- Achary VMM, Jena S, Panda KK, Panda BB. 2008. Aluminium induced oxidative stress and DNA damage in root cells of Allium cepa L. Ecotoxicol Environ Saf. 70:300–310. 10.1016/j.ecoenv.2007.10.022
- Alcazar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C, Carrasco F, Tiburcio AF. 2010. Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta. 231:1237–1249. 10.1007/s00425-010-1130-0
- Ammar WB, Nouairi I, Zarrouk M, Ghorbel MH, Jemal F. 2008. Antioxidative response to cadmium in roots and leaves of tomato plants. Biologia Plantarum. 52:727–731. 10.1007/s10535-008-0140-2
- Bates LS, Waldren RP, Teare ID. 1973. Rapid determination of free proline for water stress studies. Plant Soil. 39:205–207. 10.1007/BF00018060
- Bradford MM. 1976. Rapid and sensitive method for quantitation of micro gram quantities of protein utilizing the principle of protein-binding dye. Analytical Biochem. 72:248–254. 10.1016/0003-2697(76)90527-3
- Celik O, Atak C. 2012. Evaluation of proline accumulation and Δ1-pyrroline-5-carboxylate synthetase (P5CS) gene expression during salinity stress in two soybean (Glycine max L. Merr.) varieties. Polish J Environ Stud. 21:559–564.
- Cheeseman JM. 2006. Hydrogen peroxide concentrations in leaves under natural conditions. J Experiment Bot. 57:2435–2444. 10.1093/jxb/erl004
- Dhir B, Sharmila P, Pardha Saradhi P, Sharma S, Kumar R, Mehta D. 2011. Heavy metal induced physiological alterations in Salvinia natans. Ecotoxicol Environ Saf. 74:1678–1684. 10.1016/j.ecoenv.2011.05.009
- Ding S, Lei M, Lu Q, Zhang A, Yin Y, Wen X, Zhang L, Lu C. 2012. Enhanced sensitivity and characterization of photosystem II in transgenic tobacco plants with decreased chloroplast glutathione reductase under chilling stress. Biochimica Biophysica Acta. 1817:1979–1991. 10.1016/j.bbabio.2012.06.003
- Ding C, Shi G, Xu X, Yang H, Xu Y. 2010. Effect of exogenous spermidine on polyamine metabolism in water hyacinth leaves under mercury stress. Plant Growth Regul. 60:61–67. 10.1007/s10725-009-9419-3
- Dipierro N, Mondelli D, Paciolla C, Brunetti G, Dipierro S. 2005. Changes in the ascorbate system in the response of pumpkin (Cucurbita pepo L.) roots to aluminium stress. J Plant Physiol. 162:529–536. 10.1016/j.jplph.2004.06.008
- Fariduddin Q, Varshney P, Yusuf M, Ahmad A. 2013. Polyamines: potent modulators of plant responses to stress. J Plant Interact. 8:1–16. 10.1080/17429145.2012.716455
- Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA. 2009. Plant drought stress: effects, mechanisms and management. Agron Sustain Develop. 29:185–212. 10.1051/agro:2008021
- Ghosh N, Adak MK, Ghosh PD, Gupta S, Sengupta DN, Mandal C. 2011. Differential responses of two rice varieties to salt stress. Plant Biotech Report. 5:89–103. 10.1007/s11816-010-0163-y
- Ghosh N, Das SP, Mandal C, Gupta S, Das K, Dey N, Adak MK. 2012. Variations of antioxidative responses in two rice cultivars with polyamine treatment under salinity stress. Physiol Mol Biol Plant. 18:301–313. 10.1007/s12298-012-0124-8
- Gifford EM, Foster AS. 1989. Morphology and evolution of vascular plants. New York: Freeman.
- Groppa MD, Ianuzzo MP, Tomaro ML, Benavides MP. 2007. Polyamine metabolism in sunflower plants under long-term cadmium or copper stress. Amino Acid. 32:265–275. 10.1007/s00726-006-0343-9
- Hall JL. 2002. Cellular mechanism for heavy metal detoxification and tolerance. J Experiment Bot. 53:1–11. 10.1093/jexbot/53.366.1
- Halliwell B, Gutteridge J. 1999. Free radicals in biology and medicine. 3rd ed. Oxford: Oxford University Press; p. 936.
- Hu Y, Ge Y, Zang C, Zu T, Cheng W. 2009. Cd toxicity and translocation in rice seedlings are reduced by hydrogen peroxide treatments. Plant Growth Regul. 5:51–61. 10.1007/s10725-009-9387-7
- Jaleel CA, Azooz MM. 2009. Exogenous calcium alters pigment composition, γ-glutamyl kinase and proline oxidase activities in salt-stressed Withania somnifera. Plant Omics J. 2:85–90.
- Jaleel CA, Gopi R, Gomathinayagam M, Panneerselvam R. 2008. Calcium chloride effects on metabolism of Dioscorea rotundata exposed to sodium chloride-induced salt stress. Acta Biologica Cracov Series Bot. 50:63–67.
- Loscos J, Matamoros MA, Becana M. 2008. Ascorbate and homoglutathione metabolism in common bean nodules under stress conditions and during natural senescence. Plant Physiol. 146:282–1292.
- Mandal C, Ghosh N, Maiti S, Das K, Gupta S, Dey N, Adak MK. 2013. Antioxidative responses of Salvinia (Salvinia natans Linn.) to aluminium stress and it's modulation by polyamine. Physiol Mol Biol Plant. 19:91–103. 10.1007/s12298-012-0144-4
- Moskova I, Todorova D, Alexieva V, Ivanov S, Sergiev I. 2009. Effect of exogenous hydrogen peroxide on enzymatic and nonenzymatic antioxidants in leaves of young pea plants treated with paraquat. Plant Growth Regul. 57:193–202. 10.1007/s10725-008-9336-x
- Moskova I, Todorova D, Alexieva V, Sergiev I. 2011. Leaf morphology and histology changes of pea plants treated with hydrogen peroxide and paraquat. Compt Rend Acad Bulg Sci. 64:1695–1700.
- Mostafa EM, Tammam AA. 2012. The oxidative stress caused by NaCl in Azolla caroliniana is mitigated by nitrate. J Plant Interact. 7:356–366. 10.1080/17429145.2011.628452
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Planta. 15:473–497. 10.1111/j.1399-3054.1962.tb08052.x
- Paradiso A, Berardino R, de-Pinto M, di-Toppi LS, Storelli FT, de-Gara L. 2008. Increasing ascorbate-glutathione metabolism as local as precocious systemic responses induced by cadmium in durum wheat plants. Plant Cell Physiol. 49:362–374. 10.1093/pcp/pcn013
- Pavlikova D, Pavlik M, Staszkova L, Motyka V, Szakova J, Tlustos P, Balik J. 2008. Glutamate kinase as a potential biomarker of heavy metal stress in plants. Ecotoxic Environ Saf. 70:223–230. 10.1016/j.ecoenv.2007.07.006
- Prado C, Rodríguez-Montelongo L, Gonzalez JA, Pagano EA, Hilal M, Prado FE. 2010. Uptake of chromium by Salvinia minima: effect on plant growth, leaf respiration and carbohydrate metabolism. J Hazard Mater. 177:546–553. 10.1016/j.jhazmat.2009.12.067
- Rodriguez E, Azevedo R, Fernandes P, Santos C. 2011. Cr (VI) induces DNA damage, cell cycle arrest and polyploidization: a flow cytometric and comet assay study in Pisum sativum. Chem Res Toxicol. 24:1040–1047. 10.1021/tx2001465
- Roychoudhury A, Basu S, Sengupta DN. 2012. Antioxidants and stress-related metabolites in the seedlings of two indica rice varieties exposed to cadmium chloride toxicity. Acta Physiol Planta. 34:835–847. 10.1007/s11738-011-0881-y
- Sairam RK, Srivastava. GC. 2000. Induction of oxidative stress and antioxidant activity by hydrogen peroxide treatment in tolerant and susceptible wheat genotypes. Biologia Planta. 43:381–386. 10.1023/A:1026730008917
- Satapathy P, Achary VMM, Panda BB. 2012. Aluminum-induced abiotic stress counteracts Fusarium infection in Cajanus cajan (L.). Millsp. J Plant Interact. 7:121–128. 10.1080/17429145.2011.584133
- Shah K, Nahakpam S. 2012. Heat exposure alters the expression of SOD, POD, APX and CAT isozymes and mitigates low cadmium toxicity in seedlings of sensitive and tolerant rice cultivars. Plant Physiol Biochem. 57:106–113. 10.1016/j.plaphy.2012.05.007
- Sharma P, Jha AB, Dubey RS, Pessarakli M. 2012. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot.
- Suzuki N, Koussevitzky S, Mittler R, Miller G. 2012. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 35:259–70. 10.1111/j.1365-3040.2011.02336.x
- Thippeswamy M, Chandraobulreddy P, Sinilal B, Shivakumar M, Sudhakar C. 2010. Proline accumulation and the expression of Δ1-pyrroline-5-carboxylate synthetase in two safflower cultivars. Biologia Planta. 54:386–390. 10.1007/s10535-010-0070-7
- Upadhyaya H, Khan MH, Panda SK. 2007. Hydrogen peroxide induces oxidative stress in detached leaves of Oryza sativa L. Gen Appl Plant Physiol. 33:83–95.
- Verbeke P, Siboska GE, Clark BFC, Rattan SIS. 2000. Kinetin inhibits protein oxidation and glycoxidation in vitro. Biochem Biophy Res Commun. 276:1265–1270. 10.1006/bbrc.2000.3616
- Wang Q, Yu L, Yu CA. 2010. Cross-talk between mitochondrial malate dehydrogenase and the cytochrome bc1 complex. J. Biol Chem. 285:10408–10414. 10.1074/jbc.M109.085787
- Xie QE, Yan XL, Liao XY, Li X. 2009. The arsenic hyperaccumulator fern Pteris vittata L. Environ Sci Technol. 43:8488–8495. 10.1021/es9014647
- Yang ZB, Eticha D, Albacete A, Rao IM, Roitsch T, Horst WJ. 2012. Physiological and molecular analysis of the interaction between aluminium toxicity and drought stress in common bean (Phaseolus vulgaris). J Experiment Bot. 63:3109–3125. 10.1093/jxb/ers038
- Yu CW, Murphy TM, Lin CH. 2003. Hydrogen peroxide-induces chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct Plant Biol. 30:955–963. 10.1071/FP03091
