Abstract
Extensive development of canal irrigation system led to formation of unproductive sodic lands, which limited the cultivation of flower crop gladiolus in the basins of major rivers. The purpose of this study was to isolate native rhizospheric and endophytic bacteria from sodic environment and evaluate their growth enhancement and bio-ameloirant properties in gladiolus under sodic soils. Sixteen isolates of plant-growth-promoting rhizospheric and endophytic bacteria were isolated and screened for growth promotion potential. The promising strains were identified and evaluated for growth and production in gladiolus. The plants treated with strains CSR-G-1, CSR-B-2, and CSR-B-3 significantly produced marketable spikes and higher number of florets/spike. Also, the treated plants showed an increased activity of superoxide dismutase, phenyl alanine lyase, catalase, peroxidase, phenols, and proline than control. Further, the soil pH, total carbonates, and sodium adsorption ratio were lower in treated soils. The Na+/K+ ratio in leaves of treated plants was observed to be lower than control. In conclusion, these bio-inoculums can be used as growth enhancer and bio-ameliorant in sodic soils as an eco-friendly management strategy.
Introduction
The global trends warrant that in order to produce more food for the expanding world population, there will be an increase in the use of marginal-quality water and land resources (Bouwer Citation2000; Gupta & Abrol Citation2000). The problem of salinity and sodicity has been developed across the globe by the introduction of major irrigation schemes, which has reduced the agricultural productivity and sustainability. Over decades the northwest plains of the Indo-Gangetic basin and Yellow river basin of China have witnessed steady increase in sodic soils, which limited the production of agricultural crops (Gupta & Abrol Citation2000). Sodic soils are characterized by the soil with pH >8.5 and exchangeable sodium percent >15 with high concentration of free carbonates and bicarbonates. The hydraulic conductivity in such soils used to be poor and show high impedance to root growth (Qadir & Schubert Citation2002). These changes in soil properties affect the rhizospheric populations, thereby affecting the productivity of the commercial crops (Tank & Saraf Citation2010). Gladiolus is one such commercial monocotyledonous cut flower being cultivated for years along the river basins of major rivers of many countries (Wilfret Citation1992). It is grown well in the soils of pH 7.5–8.5 and is considered to be a highly remunerative crop for small and marginal farmers in developing countries. The development of sodicity in the river basins limited the cultivation of this crop due to its sensitivity toward sodicity (Damodaran et al. Citation2011). Chemical amendments such as mineral gypsum and pyrites being used in reclamation process ameliorated top 0–15-cm soil, leaving the physical properties like congestion of water and nutrients in rhizospheric soil unchanged (Damodaran et al. Citation2013). Many plant-growth-promoting rhizobacteria (PGPR) are known for providing protection to the plants from stressed environment (Zahir et al. Citation2008). Exploitation of epiphytic and endophytic bacteria in agricultural production depends on knowledge of plant–bacteria interaction and their potential to act as a beneficial micro-organism (Hallmann et al. Citation1997). Sometimes the interaction between the rhizospheric bacteria and plants can be unstable, where the repeatability of the good results obtained in laboratory is being limited under field condition (Cattelan et al. Citation1999; Zhender et al. Citation1999). Moreover, it is also known that the survival of micro-organism in stressed and contaminated habitat is only possible due to their ability of utilizing the resources available under that suitable niche (Madigan et al. Citation1998).
Therefore, with this background, the current research was undertaken to isolate the bacterial diversity associated with saline and sodic soils and evaluate their growth promotion potential, and also to identify the promising strains and assess their efficacy in the cut flower gladiolus, grown under sodic soil conditions.
Materials and methods
Collection of isolates
The soil rhizospheric and bacterial endophytes were isolated from roots, leaf, and culms of phyto-ameliorant grasses grown on the patches of undisturbed sodic soil with prominent salt efflorescence as described by Quadt-Hallmann et al. (Citation1997). Pure cultures of endophytes were maintained on nutrient agar slants at 4°C.
Screening and selection of bacterial isolates
The rhizosphere and endophytic bacteria grown on nutrient broth with constant shaking on rotary shaker at 150 rpm for 48 h at room temperature (28 ± 2°C) were harvested by centrifugation at 6000 rpm for 15 min and bacterial cells were resuspended in phosphate buffer (PB) (0.01 M, pH 7.0). The concentration was adjusted using a spectrophotometer to approximately 108 colony forming unit (CFU) (OD595 = 0.3) and used as inoculums for treating rice seeds (Thompson et al. Citation1996). Plant-growth-promoting activities of bacterial strains were assessed based on the seedling vigor index by pot culture studies in saline sodic soils of pH 9.35 and electrical conductivity 4.4 dS/m2, sodium (Na+) 18.65 meq/l, and potassium (K+) 0.126 meq/l. The vigor index was calculated by using the formula as described by Abdul-Baki and Anderson (Citation1973):
Further, the promising strains were characterized and selected for microbial inoculation in gladiolus corms grown in sodic soils.
Soil analysis
The soil samples collected were air dried and ground to pass through 2-mm sieve, and a saturated extract of them was prepared. Various physicochemical parameters were determined in the extracts by adopting standard methods. The pH of the soil extract was determined potentiometrically by an ORION ion analyzer (5 star series; Thermo Orion, USA) using a pH electrode calibrated with a pH buffer of 7.0 and 10.0. Carbonate (CO3) and bicarbonate (HCO3) were determined by titrimetric method (acid–base titration) (Richards Citation1954). Calcium (Ca) and magnesium (Mg) were determined by versenate method (EDTA titration) (Cheng & Bray Citation1951). Sodium (Na) was determined by flame photometer (Richards Citation1954), while sodium adsorption ratio (SAR) was determined by the following generic equation:
Characterization of bacteria-based polymerase chain reaction amplification, restriction, and analysis of bacterial 16S rDNA
Bacterial DNA of best-performing strains were extracted according to the method given by Araujo et al. (Citation2002), with amplification of 16S rDNA being performed in a 25-µl reaction mixture, which consisted of 25 ng genomic DNA, 10× reaction buffer with 15 mM MgCl2, 2.5 mM each of dNTPs, 0.2 mM bacterial universal primer (27F-AGAGTTTGATCMTGGCTCAG) (1492R-GGYTACCTTGTTACGACTT), and 1 unit of Taq DNA polymerase (Bangalore Genei Pvt Ltd, India). The reaction was carried out in a thermal cycler (PTC-200, MJ Research Inc, USA). The DNA amplification program was set to a 5-minute initial denaturation at 94°C followed by 30 cycles of 1 min at 94°C for denaturation, 1 min at 54°C for annealing, 2 min at 72°C for extension, and ended with a final 10-min extension at 72°C. The reaction products were separated by running 5 µl of the polymerase chain reaction mixture in 1.2% (w/v) agarose gel and staining the bands with ethidium bromide (Sambrook et al. Citation1989). For amplified rDNA restriction analysis, 1 µg of amplified 16S rDNA fragment (1350 bp) was digested with Alu I, Hae III, EcoR I, and BamH I restriction enzyme (Invitrogen, USA) according to the manufacturer's recommendations and the products run in 2.5% (w/v) agarose gel and stained with ethidium bromide. The strains were identified by sequencing of partial 16S rDNA and comparing the sequence by BLAST.
Preparation of formulation and inoculation of microbial isolates
A loopful of bacterium was inoculated into the CSR patent-protected culture media (Rai et al. Citation2012) and incubated in a rotary shaker at 150 rpm for 48 h at room temperature (28 ± 2°C). After 48 h of incubation, the broth containing 6 × 108 CFU/ml was used for treating the gladiolus corms at 0.2% for 2 h and for foliar application during four and eight leaved stages of the crop (Nandakumar et al. Citation2001).
Field study
The field experiment was conducted at the sodic soil experimental research farm of Central Soil Salinity Research Institute, Regional Research Station, Lucknow, India. The experiment was laid out in randomized block design (RBD) with a plot size 2 × 2 m, and each treatment consisted of 80 plants grown during two successive rabi seasons (September–December) of the years 2011 and 2012, where all agricultural practices were followed. The bulbs of gladiolus var. Nova Lux were planted in the first week of September in rows of 50 cm apart, 10 cm between, and 15 cm in depth. During the flowering period of each season, plant height (cm), number of leaves/plant, spike length (cm), number of florets/spike, and number of cormlets were recorded. The pre-experimental and post-experimental soil parameters were recorded.
Assay of stress-related enzymes and compounds
The activities of stress-related enzymes were assessed in leaves of the gladiolus plants at harvest. Peroxidase (PO) enzyme assay was carried out using the reaction mixture (3 ml) consisting of (0.25%) v/v guaicol in 10 mM potassium PB (pH 6.9) containing 10 mM hydrogen peroxide (Hammerschmidt et al. Citation1982). Polyphenol oxidase (PPO) was assayed using the modified method of Mayer et al. (Citation1966) using 0.1 M PB (pH 6.5) and 0.01 N catechol along with enzyme extract. The phenyl alanine lyase (PAL) assay was conducted using 400 ml of 50 mM Tris–HCl (pH 8.8) and 600 ml of 1 mM L-phenylalanine along with enzyme extract as reaction mixture (Whetten & Sederoff Citation1992).
Enzyme extract was stored in a deep freezer (−20°C) until used for biochemical analysis. Superoxide dismutase (SOD) activity (EC 1.15.1.1) was determined as described by El-moshaty et al. (Citation1993) and was expressed in units/g tissue. Total soluble phenols were estimated calorimetrically by using Folin ciocalteu reagent (AOAC Citation1985). Proline was estimated using sulpho salicylic acid extraction method (Bates et al. Citation1973).
Statistical analysis
The experiment was conducted in RBD and the data were analyzed using SAS 9.2 version. Prior to analysis of variance, the percentage values of germination were arcsine transformed. The pooled data for two seasons were subjected to significance at P < 0.05 and were compared by Duncan's multiple-range test.
Results
Plant growth promotion by bacterial strains
Sixteen rhizospheric and endophytic strains were isolated from the soil, roots, culms, and leaves of phyto-ameliorant grasses grown in barren sodic soils of pH from 9.7 to 10.37 and assessed for their efficacy as plant-growth promoters in pot culture experiment with rice crop. Among the 16 strains tested for their efficacy to improve the vigor index of rice seedlings under sodic soils of pH 9.35, 6 showed plant-growth-enhancing activity, and interestingly CSR-B-2 (Bacillus pumilus) strain showed 95% germination with a vigor index of 4566.5, while the vigor index of CSR-G-1 and CSR-B-3 strains were on par and showed 90% germination. Apart from this, the strains CSR-G-5, CSR-B-1, and CSR-G-4 also showed higher germination percentage of>70% and vigor index between 2355.2 and 3655.1. Conspicuously, some of the strains inhibited the growth of rice seedlings and those bacterial strains were discarded () along with others which did not show any significant increase in the growth. Further, characterization using 16S rDNA for identification using partial sequences and microbial inoculation (bio-primed) in gladiolus corms was carried out with the effective bacterial strains (CSR-B-1, CSR-B-2, CSR-B-3, CSR-G-1, CSR-G-4, and CSR-G-5).
Table 1. Plant promotion activity of rhizospheric and endophytic bacteria in rice.
Identification of promising bacterial strains
Bacterial strains isolated from the rhizosphere zone of sodic soils were found to be gram-negative type. Six strains with higher PGPR potential under sodic conditions were partially characterized by ARDA technique with Alu I, Hae III, EcoR I, and BamH I restriction enzymes, resulting in two groups. The strains were identified using 16S rDNA sequencing and validated with BLAST (ncbi.nlm.gov.blast/Blast.cgi) database. The results showed that all the strains belonged to Bacillus group where the strain CSR-B-1 was identified as Bacillus cereus, CSR-B-2 as Bacillus pumilus, CSR-B-3 as Bacillus thuringensis, CSR-G-1 as Bacillus subtilis, CSR-G-4 as Bacillus marisflavi, and CSR-G-5 as Bacillus saffensis, with accession numbers JQ768235, JQ768236, KF383226, KCA33669, KC433668, and JQ768237, respectively.
Effect of plant bio-priming
As observed, the presence of sodicity in soil reduced the growth characters of plant significantly in untreated control, while the treatments of promising bacterial strains relieved the plant rhizosphere from salt stress, resulting in improvement of growth characters (). Significant differences were observed between treatments in plant height and spike length. Plant height was maximum in CSR-G-1 (82.22 cm)-treated plants followed by CSR-B-3 (75.77 cm). Longer spikes were obtained in treatments involving CSR-B-3 (50.83 cm) followed by CSR-B-2 (48.66 cm), while the smallest (14.16 cm) being witnessed in untreated control. Application of rhizobacteria and endophytic strains increased the number of florets in treatments than the untreated control. Treatments involving the strains CSR-G-1 and CSR-B-3 recorded higher number of florets (8.111 and 8.057, respectively) than other strains, while the lowest number of florets (3.967) was observed in the untreated control. In general, a rapid and significant increase in the corm weight and corm diameter was witnessed in all the treatments compared to control. Among the treatments, plants treated with CSR-G-1 and CSR-B-3 strains recorded significantly higher corm weights of 30.220 and 29.590 g, respectively. Significantly higher corm diameter was observed in corms treated with CSR-B-3 (5.733 cm) followed by CSR-B-2 (3.200 cm).
Table 2. Efficacy of bacterial strains on morphological characters of gladiolus in sodic soils.
Stress-related enzymes
The activities of the stress-related enzymes, SOD, PAL, catalase, PO, PPO, phenol, and proline, assessed in various treatments showed highly significant differences between treatments (). Invariably, the microbial inoculated plants showed nearly 2–3 times higher activity than the control. Among the six strains, CSR-G-1, CSR-B-2, and CSR-B-3 were found to be effective in inducing higher activities of these enzymes.
Table 3. Efficacy of stress-related enzymes' activity in gladiolus leaves at flowering.
Effect of microbial inoculation against sodicity in the field
Microbial inoculation of gladiolus corms before planting followed by foliar sprays during the critical growth periods revealed that treatments involving the strains CSR-G-1, CSR-B-2, and CSR-B-3 recorded the least symptoms of sodicity in their rhizospheric soil. A significant decrease in pH, total carbonate (CO3 + HCO3), and SAR between pre- and post-treatment was found. The maximum decrease in pH was noticed in CSR-B-3 (0.26) followed by CSR-B-2 (0.24) (). The highest reduction in total carbonate was observed in CSR-B-3 (3.04)-treated rhizosphere soil followed by CSR-G-1 (1.66) (). The decrease in SAR was the maximum in CSR-G-1 (1.46)-treated soil followed by CSR-B-3 (1.28) (). The lowest change in the pH, total carbonate, and SAR was recorded in the rhizosphere soil of untreated control. The Na+/K+ ratio () in the leaves of gladiolus was significantly higher in the untreated control (1.221) compared to the treated ones. Among the six strains, the lowest Na+/K+ ratio was recorded in the strain CSR-B-2 (0.437) followed by CSR-B-3 (0.507). Higher potassium contents were observed in plants treated with CSR-G-1 (2.246 meq/l) followed by CSR-B-3 (2.187).
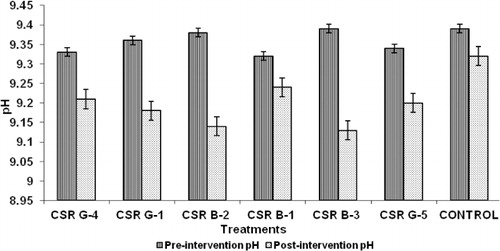
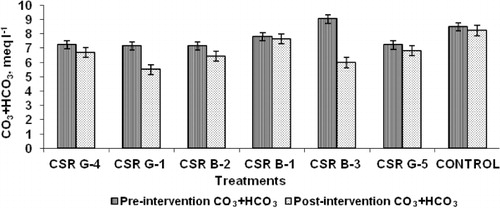
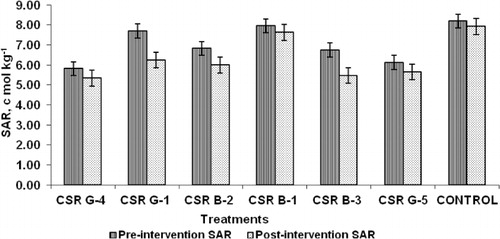
Table 4. Effect of microbial inoculation on the Na/K ratio in the gladiolus leaves.
Discussion
Gladiolus, a commercial cut flower, has been grown traditionally for years in the river basins of perennial rivers, and it suffered the salt injury due to the development of sodicity in its rhizosphere. The approaches of using gypsum and pyrite in reclamation of these sodic soils have not been able to change the physical and biological properties of the soil, which limited its cultivation. Furthermore, majority of the bio-products developed using microbes were restricted to function at the pH range of 7.0–8.5, which suggest the requirement of microbes to function at high pH (>8.5). Therefore, alternative biological approach of isolation of PGPR tolerant to high pH was attempted in the current study to induce salt tolerance in gladiolus grown in sodic soils. The use of endophytic bacteria for increasing the growth of plants in neutral soils (Kloepper et al. Citation1992; Joseph et al. Citation2007) and saline soils under pot experiment (Egamberdieva Citation2012) in tomato and cucumber has been earlier established in various studies. Yasmin and Hasnain (Citation2001) and Chakraborty et al. (Citation2011) also isolated salt-tolerant bacteria and tested for germination under laboratory conditions. As observed, the presence of salinity reduced the plant growth under pot culture studies in rice for vigor index, but the treatment with strains CSR-B-2, CSR-B-3, CSR-G-1, CSR-G-4, and CSR-G-5 relieved the rice plants from stress and made them to exhibit higher vigor index compared to the plants treated with other strains and untreated control. Similar results were also reported by Munns (Citation2002), mentioning suppression of plant growth under saline stress, which may be due to water congestion in the surface soil and also the effect of sodium (Na+) and carbonates in the rhizosphere. Significant decrease in shoot and root length was also observed in the presence of high NaCl concentration in the root zone by Kashyap and Sharma (Citation2006) and Nakbanpote et al. (Citation2013).
The knowledge about the genetic relationship and its identity may be useful in determining further use of the promising strains in gladiolus for induction of salt tolerance. The identification was carried out using 16S rDNA sequencing and comparing them by BLAST database. Similar process was adopted in characterization by many earlier workers (Sturz et al. Citation2000; Kuklinsky-Sobral et al. Citation2004; Tank & Saraf Citation2010).
In the current study, the promising strains (CSR-B-2, CSR-B-3, CSR-G-1, CSR-G-4, and CSR-G-5) were tested for their interaction with their plant and soil in inducing salt tolerance in gladiolus, a salt-sensitive commercial crop grown in sodic conditions. High soil pH and water congestion result in poor development of underground corms and their germination. In this study, microbial inoculation of the corms enabled a favorable soil rhizospheric environment through inducing developmental and physiological changes in plants and their rhizospheric soils. Bio-priming of banana suckers of var. Pisang Awak with CSR-B-3 strain increased the tolerance of banana to sodicity (Damodaran et al. Citation2013). Similar result was also observed by Kavino et al. (Citation2007) in roots of primary hardened-tissue-cultured banana plantlets for inducing tolerance to banana bunchy top virus.
Microbial inoculated gladiolus plants showed enhanced growth and yield characters like plant height, spike length, and number of florets than the untreated control. Similar influence on the growth and development was reported in horticultural crops like strawberry (Kokalis-Burelle Citation2003) and Prunus sp. (Bonaterra et al. Citation2003) and stevia (Vafadar et al. Citation2013). In the present study, other than floral characters, higher corm weight and corm diameter were observed in the bio-primed corms, whereas a decreased corm weight and corm diameter were found in untreated control. An overall reduction in control under salt stress was earlier observed by Soussi et al. (Citation1998). This reduction in weight of plants under stress may be due to the inhibition or hydrolysis of reserved food and its translocation to growing shoot portion (Singh et al. Citation2008). The increase in root or corm character in microbial inoculated plants was witnessed earlier by Vivas et al. (Citation2003) in lettuce inoculated with Bacillus spp. under salt stress. It may be postulated that effective rhizobacterial strain on inoculation decreases the endogenous ethylene levels because of aminocyclopropane carboxylate (ACC) deaminase activity, thereby resulting in the formation of longer roots (Nadeem et al. Citation2006).
The salt induced adverse effects on plants are primarily due to specific ion toxicity, production of oxidants, and so on (Ashraf Citation2009). Plants produce low-molecular-weight organic solutes such as proline or enzymes like SOD and PO to tolerate the effects of salt stress (Ali & Ashraf Citation2011). However, most plant species do not produce sufficient antioxidants to fulfill their growth requirements under salt stress. Therefore, bio-inoculation induces salt tolerance by increasing the activity of antioxidant enzymes (SOD, PO, and catalase) and organic solutes like proline (Kaya et al. Citation2013). The bio-inoculation also enhanced the defense mechanism of the plants due to the elevated level of the enzyme activity in the host plants (Ashraf & Foolad Citation2007). Inoculation of wheat seeds with Pseudomonas putida strain AKMP7 counteracted the adverse effect of heat on leaf antioxidant enzymes by lowering the reactive oxygen scavenger (ROS) generation compared to uninoculated plants (Ali et al. Citation2011). The microbial inoculated gladiolus plants produced more defense enzymes and proline than the untreated controls. The higher activities of PO, PPO, PAL, SOD, and proline content increased the ability of the plants to tolerate salt stress and formulate Na+ exclusion mechanism. Antioxidant enzymes in roots show inverse relationship with sodicity of the soil (Myrene & Devaraj Citation2010). Higher activity of antioxidant has been reported in mycorrhiza-treated plants of tomato in saline soils (Hajiboland et al. Citation2010).
The treatment with bacterial strains increased the uptake of potassium (K+) as compared to sodium (Na+), resulting in lower Na/K ratio. The treatment with bio-inoculum reduced the soil pH, total carbonates, and soil SAR in the rhizosphere soils, facilitating favorable plant growth and nutrient mobilization. The endophyte enhances the uptake of nutrients through biological processes (Handafy et al. Citation1995). The higher K accumulation by mycorrhizal plants in saline soils was reported to be beneficial in maintaining high K/Na ratio (Giri et al. Citation2007). Earlier studies on use of phyto-ameliorants attributed the secretion of higher organic acids in the rhizosphere due to the decomposition of the biomass, which helps in diluting the carbonates and also decreasing the pH of the soil (Treeby et al. Citation1989; Ghaly Citation2002).
Taken together, corm treatment and foliar application during the critical stage of growth using native rhizobacterial strains of CSR-B-3, CSR-B-2, and CSR-G-1 were significantly effective in reducing the adverse effect of salt stress (sodicity) in gladiolus under sodic soil conditions, resulting in improved production levels. These bio-inoculums may be easily incorporated into existing production systems as an eco-friendly management strategy and as a bio-growth enhancer to restore the production of commercial crops in salt-affected soils.
Acknowledgments
The authors thank National Agricultural Innovation Project, ICAR, India, and World Bank for generously funding the project under which the study was undertaken.
References
- Abdul-Baki AA, Anderson JD. 1973. Vigour determination in soybean seed by multiple criteria. Crop Sci. 13:630–633. 10.2135/cropsci1973.0011183X001300060013x
- Ali Q, Ashraf M. 2011. Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: growth, photosynthesis, water relations and oxidative defense mechanism. J Agron Crop Sci. 197:258–271. 10.1111/j.1439-037X.2010.00463.x
- Ali SZ, Sandhya V, Grover M, Linga VR, Bandi V. 2011. Effect of inoculation with a thermotolerant plant growth promoting Pseudomonas putida strain AKMP7 on growth of wheat (Triticum spp.) under heat stress. J Plant Interact. 6:239–246. 10.1080/17429145.2010.545147
- AOAC. 1985. Official of analysis of the Association of Agriculture Chemist. 13th ed. Washington (DC): AOAC.
- Araujo WL, Marcon J, Maccheroni W, Van Elsas JD, Van Vuurde JW, Azevedo JL. 2002. Diversity of endophytic bacterial populations and their interaction with Xylella fastidiosa in citrus plants. Appl Environ Microbiol. 68:4906–4914. 10.1128/AEM.68.10.4906-4914.2002
- Ashraf M. 2009. Biotechnological approach of improving plant salt tolerance using antioxidant as markers. Biotechnol Adv. 27:84–93. 10.1016/j.biotechadv.2008.09.003
- Ashraf M, Foolad MR. 2007. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot. 59:206–216. 10.1016/j.envexpbot.2005.12.006
- Bates LS, Waldren RP, Teare ID. 1973. Rapid determination of free proline for water stress studies. Plant Soil. 39:205–207. 10.1007/BF00018060
- Bonaterra A, Ruz L, Badosa E, Pinochet J, Montesinos E. 2003. Growth promotion of Prunus rootstocks by root treatment with specific bacterial strains. Plant Soil. 255:555–569. 10.1023/A:1026033115984
- Bouwer H. 2000. Integrated water management: emerging issues and challenges. Agric Water Manage. 45:217–228. 10.1016/S0378-3774(00)00092-5
- Cattelan AJ, Hartel PG, Fuhrmann JJ. 1999. Screening for plant growth-promoting rhizobacteria to promote early soybean growth. Soil Sci Soc Am J. 63:1670–1680. 10.2136/sssaj1999.6361670x
- Chakraborty AP, Dey P, Chakraborty B, Chakraborty U, Roy S. 2011. Plant growth promotion and amelioration of salinity stress in crop plants by a salt-tolerant bacterium. Recent Res Sci Technol. 3:61–70.
- Cheng KL, Bray RH. 1951. Determination of calcium and magnesium in soil and plant material. Soil Sci. 72:449–458. 10.1097/00010694-195112000-00005
- Damodaran T, Mishra VK, Sharma DK, Jha SK, Verma CL, Rai RB, Kannan R, Nayak AK, Dhama K. 2013. Management of sub-soil sodicity for sustainable banana production in sodic soil – an approach. Int J Curr Res. 5:1930–1934.
- Damodaran T, Rai RB, Mishra VK, Sharma DK, Ram RA, Rai S, Kumar H. 2011. Integrated farming system and livelihood security: an approach. Karnal (India): Central Soil Salinity Research Institute (CSSRI); p. 1–108.
- Egamberdieva D. 2012. Pseudomonas chlororaphis: a salt-tolerant bacterial inoculant for plant growth stimulation under saline soil conditions. Acta Physiol Plant. 34:751–756. 10.1007/s11738-011-0875-9
- El-moshaty FIB, Pike SM, Novacky AJ, Sehgal OP. 1993. Lipid peroxidation and superoxide production in cowpea (Vigna unguiculata) leaves infected with tobacco ringspot virus or southern bean mosaic virus. Physiol Mol Plant Pathol. 43:109–119. 10.1006/pmpp.1993.1044
- Ghaly FM. 2002. Role of natural vegetation in improving salt affected soil in northern Egypt. Soil Till Res. 64:173–178. 10.1016/S0167-1987(01)00240-9
- Giri B, Kapoor R, Mukerji KG. 2007. Improved tolerance of Acacia niloticato salt stress by arbuscular mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues. Microb Ecol. 54:753–760. 10.1007/s00248-007-9239-9
- Gupta RK, Abrol IP. 2000. Salinity build-up and changes in the rice-wheat system of the Indo-Gangetic plains. Exp Agric. 36:273–284. 10.1017/S0014479700002076
- Hajiboland R, Aliasgharzadeh N, Laiegh SF, Poschenrieder C. 2010. Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil. 331:313–327. 10.1007/s11104-009-0255-z
- Hallmann J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW. 1997. Bacterial endophytes in agricultural crops. Can J Microbiol. 43:895–914. 10.1139/m97-131
- Hammerschmidt R, Nuckles EM, Kuc J. 1982. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol Plant Pathol. 20:73–82. 10.1016/0048-4059(82)90025-X
- Handafy AH, Kheir NF, Abdel-Latif EA, Amin MAC. 1995. Effect of NPK fertilizers and foliar application of some chemicals on growth, yield and chemical composition of faba bean and wheat. Egypt J Appl Sci. 10:652–676.
- Joseph B, Patra RR, Lawrence R. 2007. Characterization of plant growth promoting rhizobacteria associated with chickpea (Cicer arietinum L.). Int J Plant Prod. 2:141–152.
- Kashyap S, Sharma S. 2006. In vitro selection of salt tolerant Morus alba and its field performance with bioinoculants. Hort Sci. 33:77–86.
- Kavino M, Harish S, Kumar N, Saravanakumar D, Damodaran T, Soorianathasundaram K, Samiyappan R. 2007. Rhizosphere and endophytic bacteria for induction of systemic resistance of banana plantlets against bunchy top virus. Soil Biol Biochem. 39:1087–1098. 10.1016/j.soilbio.2006.11.020
- Kaya K, sonmez O, Aydemir S, Ashraf M, Dikilitas M. 2013. Exogenous application of mannitol and thiourea regulates plant growth and oxidative stress responses in salt-stressed maize (Zea mays L.). J Plant Interact. 8:234–241. 10.1080/17429145.2012.725480
- Kloepper JW, Wei G, Tuzun S. 1992. Rhizosphere population dynamics and internal colonization of cucumber by plant growth-promoting rhizobacteria which induce systemic resistance to Colletotrichum orbiculare. In: Tjamos ES, editor. Biological control of plant diseases. New York (NY): Plenum Press; p. 185–191.
- Kokalis-Burelle N. 2003. Effects of transplant type, plant growth-promoting rhizobacteria, and soil treatment on growth and yield of strawberry in Florida. Plant Soil. 256:273–280. 10.1023/A:1026124828038
- Kuklinsky-Sobral J, Araujo WL, Mendes R, Geraldi IO, Pizzirani-Kleiner AA, Azevedo JL. 2004. Isolation and characterization of soybean-associated bacteria and their potential for plant growth promotion. Environ Microbiol. 6:1244–1251. 10.1111/j.1462-2920.2004.00658.x
- Madigan MT, Martinko JM, Parker J. 1998. Brock's biology of microorganisms. 8th ed (international edition), XVIII. Hemel Hempstead (UK): Prentice Hall; p. 342.
- Mayer AM, Harel F, Ben-Shaul R. 1966. Assay of catechol oxidase—a critical comparison of methods. Phytochemistry5:783–789. 10.1016/S0031-9422(00)83660-2
- Munns R. 2002. Comparative physiology of salt and water stress. Plant Cell Environ. 25:239–250. 10.1046/j.0016-8025.2001.00808.x
- Myrene RD, Devaraj VR. 2010. Biochemical responses of Hyacinth bean (Lablab purpureus) to salinity stress. Acta Physiol Plant. 32:341–353. 10.1007/s11738-009-0412-2
- Nadeem SM, Hussain I, Naveed M, Asghar HN, Zahir ZA, Arshad M. 2006. Performance of plant growth promoting rhizobacteria containing ACC-deaminase activity for improving growth of maize under salt stressed conditions. Pak J Agric Sci. 43:114–121.
- Nakbanpote W, Panitlurtumpai N, Sangdee A, Sakulpone N, Sirisom P, Pimthong A. 2013. Salt tolerant and plant growth promoting bacteria isolated from Zn/Cd contaminated soil: identification and effect on rice under saline conditions. J Plant Interact. doi:10.1080/17429145.2013.842000
- Nandakumar R, Viswanathan R, Babu S, Sheela J, Raguchander T, Samiyappan R. 2001. A new bio-formulation containing plant growth promoting rhizobacterial mixture for the management of sheath blight and enhanced grain yield in rice. Biocontrol. 46:1–18. 10.1023/A:1014131131808
- Qadir M, Schubert S. 2002. Degradation processes and nutrient constraints in sodic soils. Land Degrad Dev. 13:275–294. 10.1002/ldr.504
- Quadt-Hallmann A, Hallmann J, Kloepper JW. 1997. Bacterial endophytes in cotton: location and interaction with other plant-associated bacteria. Can J Microbiol. 43:254–259. 10.1139/m97-035
- Rai RB, Damodaran T, Kannan R, Rathore RS, Srivastava AP, Sharma DK, Mishra VK, Jha SK, Sah V. 2012. Low cost multiplication technology of salt tolerant bio-growth enhancers (Bacillus, Pseudomonads & Trichoderma) for increasing productivity of agri-horti crops in normal and sodic soils. Patent No. 3857/DEL/2012.
- Richards LA. 1954. Diagnosis and improvement of saline and alkali soils. Washington (DC): United States Department of Agriculture; p. 160.
- Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning. In: Ashburner M, editor. Drosophila: a laboratory manual. 2nd ed. New York (NY): Cold Spring Harbor Laboratory; p. 1626.
- Singh N, Pandey P, Dubey RC, Maheshwari DK. 2008. Biological control of root rot fungus Macrophomina phaseolina and growth enhancement of Pinus roxburghii (sarg.) by rhizosphere competent Bacillus subtilis BN1. World J Microbiol Biotechnol. 24:1669–1679. 10.1007/s11274-008-9680-z
- Soussi M, Ocana A, Lluch C. 1998. Effect of salt stress on growth, photosynthesis and nitrogen fixation in chickpea (Cicer arietinum L.). J Exp Bot. 49:1329–1337.
- Sturz AV, Christie BR, Nowak J. 2000. Bacterial endophytes: potential role in developing sustainable systems of crop production. Crit Rev Plant Sci. 19:1–30. 10.1016/S0735-2689(01)80001-0
- Tank N, Saraf M. 2010. Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. J Plant Interact. 5:51–58. 10.1080/17429140903125848
- Thompson DC, Clarke BB, Kobayashi DY. 1996. Evaluation of bacterial antagonist for reduction of summer patch symptoms in Kentucky blue grass. Plant Dis. 80:856–862. 10.1094/PD-80-0856
- Treeby M, Marschner H, Römheld V. 1989. Mobilization of iron and other micronutrient cations from a calcareous soil by plant-borne, microbial and synthetic metal chelators. Plant Soil. 114:217–226. 10.1007/BF02220801
- Vafadar F, Amooaghaie R, Otroshy M. 2013. Effects of plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungus on plant growth, stevioside, NPK, and chlorophyll content of Stevia rebaudiana. J Plant Interact. doi:10.1080/17429145.2013.779035
- Vivas A, Marulanda A, Ruiz-Lozano JM, Barea JM, Azcón R. 2003. Influence of Bacillus spp on physiological activities of two arbuscular mycorrhizal fungi and plant responses to PEG-induced drought stress. Mycorrhiza 13:249–256. 10.1007/s00572-003-0223-z
- Whetten RW, Sederoff RR. 1992. Phenylalanine ammonia-lyase from loblolly pine. Purification of the enzyme and isolation of complementary DNA clones. Plant Physiol. 98:380–386. 10.1104/pp.98.1.380
- Wilfret GJ. 1992. Gladiolus. In: Larson RA, ed. Introduction to floriculture. New York (NY): Academic Press; p. 114–157.
- Yasmin A, Hasnain S. 2001. Bacterial diversity in athalassic saline habitats. Pak J Microbiol. 1:67–76.
- Zahir ZA, Munir A, Asghar HN, Shaharoona B, Arshad M. 2008. Effectiveness of rhizobacteria containing ACC deaminase for growth promotion of peas (Pisum sativum) under drought conditions. J Microbiol Biotechnol. 18:958–963.
- Zhender GW, Yao C, Murphy JF, Sikora ER, Kloepper JW, Schuster DJ, Polston JE. 1999. Microbe-induced resistance against pathogens and herbivores: evidence of effectiveness in agriculture. In: Agarwal AA, Tuzun S, Bent E, editors. Induced plant defences against pathogens and herbivores: biochemistry, ecology and agriculture. St Paul (MN): APS Press; p. 33.
