Abstract
Storage insect pests cause serious losses to all legume crops in both quality and quantity. This study was conducted to study some morphological characters and biochemical components in eight genotypes of faba bean to determine biochemical and molecular markers for resistance and susceptibility to infestation with stored insects. The results showed that chlorophyll content in leaves, phenol, tannin, peroxidase (POD), polyphenol oxidase (PPO), and protein content in leaves and seeds of faba bean plants either significantly or non significantly increased the effect of insect infestation in the resistance genotypes (L.551, L.512, L.153, NA112, and L.1) as compared with the susceptible genotypes (L.16 and T.W). Protein electrophoresis showed a wide variation between genotypes and determined some biochemical markers (sodium dodecyl sulfate poly acrylamide gel electrophoresis, SDS-PAGE). In addition, molecular genetic markers for stored insects' resistance were obtained using inter-simple sequence repeat polymerase chain reaction (ISSR-PCR) analyses.
| Abbreviations | ||
| SDS-PAGE | = | sodium dodecyl sulfate poly acrylamide gel electrophoresis |
| ISSR-PCR | = | inter-simple sequence repeat polymerase chain reaction |
| POD | = | peroxidase |
| PPO | = | polyphenol oxidase |
Introduction
Storage insect pests cause serious losses to all legume crops in both quality and quantity, particularly in the tropics and subtropics where temperatures and relative humidity are high. The most important species of storage insect pests of food legumes include: Callosobruchus chinensis, Callosobruchus maculatus, Callosobruchus analis, Acanthoscelides obtectus, and Bruchus incarnatus (Kashiwaba et al. Citation2003). In addition, Bruchus rufimanus, Bruchus dentipes, Bruchus quinqueguttatus, Bruchus emarginatus, Bruchus ervi, Bruchus lentis, and Bruchus pisorum may also cause significant losses in some legumes (Desroches et al. Citation1995). The seeds of legumes, once damaged by storage insects, are no longer fit for planting (due to poor germination) or for food or feed (due to spoilage and bad smell; Haile Citation2006).
Several environmental manipulations can be attained by employing a number of control measures like the use of chemical insecticides and cultural and physical control methods. Chemical pesticides are effectively used against storage insect pests but are inseparably associated with a number of drawbacks including high costs and concerns about environmental pollution and food safety. An effective and environment-friendly management option against storage insect pest in different legume crops could be achieved by improving the genetic resistance of the host plant (Somta et al. Citation2008).
Molecular tools give us an opportunity to develop genotypes that carry resistance traits (Ranjekar et al. Citation2003), and these tools have been utilized in DNA fingerprinting for identification of cultivars, marker-assisted selections and, to a limited extent, for genetic modification in breeding for insect resistance (Acosta-Gallegos et al. Citation2008).
Plant–insect interaction is a dynamic system, subjected to continual variation and change. In order to reduce insect attack, plants developed different defense mechanisms including chemical and physical barriers such as the induction of defensive proteins (Haruta et al. Citation2001), volatiles that attract predators of the insect herbivores (Birkett et al. Citation2000), secondary metabolites (Baldwin Citation2001), and trichome density (Fordyce & Agrawal Citation2001).
Chlorophyll content is one of the most important parameters in the relationships between plants and herbivores. Chlorophyll levels change during plant development (Costa et al. Citation2001). Chlorosis is the most obvious plant injury symptom on cabbage leaves after aphid feeding and is indicative of chlorophyll loss (Khattab Citation2007). Among the secondary metabolites, plant phenols constitute one of the most common and widespread group of defensive compounds, which play a major role in host plant resistance (HPR) against herbivores, including insects (Sharma et al. Citation2009). Tannins have a strong deleterious effect on phytophagous insects and affect the insect growth and development by binding to the proteins, reduce nutrient absorption efficiency, and cause midgut lesions (Barbehenn & Constabel Citation2011).
Oxidative state of the host plants has been associated with HPR to insects (He et al. Citation2011) which results in the production of reactive oxygen species (ROS) that are subsequently eliminated by antioxidative enzymes. Peroxidase (POD) constitutes one such group of enzymes which scavenges the ROS besides having other defensive roles. PODs are an important component of the immediate response of plants to insect damage (Gulsen et al. Citation2010; War et al. Citation2012). The polyphenol oxidase (PPOs) are also important enzymes in plants that regulate feeding, growth, and development of insect pests and play a leading role in plant defense against the biotic and abiotic stresses (He et al. Citation2011).
Vicia faba is the most important legume crop for human and livestock in Egypt. It makes an important contribution to the diet of people in many countries. It represents a very interesting class of food crops due to its high protein content (30%; Gaber et al. Citation2000). It can grow successfully in different soil types and it increases soil fertility.
The present study aimed to study the effect of stored insects on morphological, physiological, and molecular changes in eight genotypes of Vicia faba and to obtain molecular and biochemical markers for resistance and susceptibility to stored insects.
Materials and methods
The present investigation was carried out during two growing seasons (2010–2012) at Bhtem Research Station, Egypt. Eight faba bean genotypes were utilized in this study ().
Table 1. The name of faba bean genotypes and its origin.
Preliminary study
Tannin contents in seeds, the preference of stored insects to host seeds, the ability to complete development and the susceptibility index in host seeds were used to determine the resistance of faba bean genotypes to stored insects.
Preference experiment: Three cylinders were divided into 48 equal compartments to represent three replicates. Each genotype was represented by 2 g of seeds which were placed on each compartment. Each experiment cylinder was provided with 75 pairs of newly emerged adults. Preference percentage was measured as the following equation:
Mean development period (MDP): Number of days from time of egg laying up to adult emergence from the seeds was counted.
Field experiments: Seeds of eight faba bean genotypes were planted after screening in a randomized block design with three replications. The experimental plant consisted of three ridges 3.0-m long, 60-cm apart with single-seeded hills, 20-cm apart on both sides of ridges. Three plants of each genotype were collected after 45 days (vegetative stage) to determine chlorophyll content, phenol, tannin, POD, PPO, total protein, protein electrophoresis, and DNA isolation. At the end of growing season, the plants were collected to measure morphological criteria and yield.
Determination of photosynthetic pigments
Chlorophyll a, Chlorophyll b, and carotenoids were determined in faba bean leaves. The spectrophotometric method recommended by Vernon and Seely (Citation1966) was used. The pigment contents were calculated as mg g–1 fresh weight of leaves.
Determination of total phenols
Levels of soluble phenols in faba bean leaves were determined in accordance with Dihazi et al. (Citation2003). The absorbance of the developed blue color was read at 725 nm. Tannic acid was used as the standard and the amount of soluble phenols was expressed as mg tannic acid g–1 dry weight.
Determination of tannin content
Tannin was determined using vanillin hydrochloric acid method as described by Burn (Citation1971). The absorbance of samples was measured at 500 nm using spectrophotometer. Pure catechin was used for standard curve.
Extraction of enzymes and total soluble protein
Two grams of fresh sample were homogenized in cold phosphate buffer (0.05 M at pH 6.5). The homogenate was centrifuged at 10,000g for 10 minutes. The pigments were removed from the supernatant by adsorbing on activated charcoal and filtered. The filtrate was completed to a known volume and used to determine enzymes and total soluble protein.
Assay of enzymes activity
POD (EC 1.11.1.7) and PPO (EC 1.14.18.1) was assayed following the method of Kar and Mishra (Citation1976). The sample was read at 430 nm and the enzyme activity was expressed as enzyme activity/gram fresh weight/h.
Estimation of total soluble protein
The total soluble protein content in the supernatant was determined according to Lowry et al. (Citation1951). The quantity of total soluble protein was calculated according to the standard curve of Bovine Serum Albumin and expressed as mg g−1 fresh weight.
Protein electrophoresis
Gel electrophoresis sodium dodecyl sulfate poly acrylamide gel electrophoresis (SDS-PAGE) was carried out with gel slabs according to the method of Laemmli (Citation1970). Polypeptide pattern was analyzed on 12% SDS polyacrylamide gels. Protein subunit bands were stained with Coomassie blue R-250 by standard techniques. The gel was scanned using the Gel-Pro analyzer. The molecular weights were calculated by Gel-Pro analyzer program according to the molecular weight marker.
ISSR-PCR of genomic DNA
Seeds of faba bean plants (100 mg) were ground under liquid nitrogen to a fine powder to extract DNA as described by Dellaporta et al. (Citation1983). Inter-simple sequence repeat polymerase chain reaction (ISSR-PCR) was conducted using seven 10-mer arbitrary primers with the sequences shown in . The DNA amplifications were performed in an automated thermal cycle (model Techno 512) programmed for one cycle at 94°C for 4 min followed by 45 cycles of 1 min at 94°C, 1 min at 57°C, and 2 min at 72°C. The reaction was finally stored at 72°C for 10 min. PCR products were run at 100 V for 1 hour on 1.4% agarose gels to detect polymorphism between various genotypes under study. After electrophoresis, the ISSR patterns were visualized with UV transilluminator. Gels were photographed using a Polaroid camera.
Table 2. List of primer names and their nucleotide sequences used in the study for ISSR procedure.
Statistical analysis
All data were subjected to statistical analysis and means were compared by Duncan's multiple range test using Mstat C computer package.
Results and discussion
Effect on morphological criteria
Nine traits were measured on eight genotypes of faba bean: plant height, number of branches, first fertile nodes, number of seeds per pod, number of pods per plant, number of seeds per plant, seed weight per plant, 100 seeds’ weight, and pod length. Mean comparison for the yield and seed quality traits were done according to Duncan's multiple range test as shown in . These mean comparisons revealed highly significant and nonsignificant differences between the investigated genotypes. Number of pods per plant and number of seeds per plant significantly increased in the tolerant genotypes. These results are in accordance with El-Sayed (Citation2006).
Table 3. Effect of infestation with stored insects on morphological criteria in susceptible and tolerant genotypes of Vicia faba plants.
Effect on photosynthetic pigments and biochemical components
Chlorosis of Vicia faba leaves which results from stored insects attack may be explained by the significant reduction in total photosynthetic pigments content observed in the infested leaves of the susceptible genotypes (L.16 and T.W) as compared with the other genotypes and the resistance genotypes (L.551, L.512, L.153, NA112, and L.1; ). These results are in accordance with El-Khawas (Citation2012) who found that significant reduction in chlorophyll a and b levels in Pisum sativum leaves was attacked by leaf miners as well as the significant increase in carotenoids was observed in the infested leaves as compared with the healthy ones. The decrease in the photosynthetic pigments may be due to the inhibition of pigment biosynthesis due to Mg deficiency which is a constituent of chlorophyll or due to damage of palisade tissue which contains the chloroplast or lack of assimilates which drain toward the insect or to the effect of ROS on these pigments (Stacey & Keen Citation1996; Khattab Citation2007).
Table 4. Effect of infestation with stored insects on photosynthetic pigments and biochemical components in susceptible and tolerant genotypes of Vicia faba leaves and seeds.
The obtained results in revealed that total phenols content was significantly accumulated in leaves and seeds of all Vicia faba genotypes as compared with the susceptible genotypes (L.16 and T.W). These results are in harmony with El-Khawas (Citation2012) who found that the total phenols in Pisum sativum leaves attacked by leaf miners showed highly significant increments of infested host plants as compared with the healthy ones. In addition, Perveen et al. (Citation2001) reported that resistant and semi-resistant varieties of cotton attacked by insects possessed significantly greater phenolic content than susceptible varieties. The greater phenol concentration in resistant genotypes may contribute to the inhibited herbivory due to the toxic or phagodeterrent effect of phenolics toward insects. The elevation of phenols can be explained as a mechanism of defense that acts as a barrier to insect feeding. These phenolic compounds are known to inhibit the larval development and growth by acting as feeding deterrents. Therefore, the activity of phenols is specific to the plant and pest interaction. The feeding deterrence can be due to the generation of the ROS in the insect's digestive tract, particularly in the midgut. They aid in the oxidative damage of the midgut lipids and proteins, resulting in death of the insect. As such, it may be the main reason for the enhanced phenolic acid levels in the plant as a defense strategy.
Data obtained in demonstrate significant increment in the tannin content in seeds of all Vicia faba genotypes as being compared with the susceptible genotypes (L.16 and T.W). The tolerant genotypes recorded the maximum accumulation in tannin content. In addition, tannin content increased significantly in leaves of two resistant genotypes (L.551 and L.512) but showed nonsignificant effects in the other genotypes as compared with the susceptible genotypes. These results are in accordance with Panda and Khush (Citation1995) who found that the biosynthesis of secondary metabolites (like lignins, tannins, etc.) plays an important role in the seed defense against insects such as repellents, feeding inhibitors, and anti-nutritional factors. The increase in tannin content in tolerant genotypes may be due to the toxic effect on insects because they bind to salivary proteins and digestive enzymes including trypsin and chymotrypsin resulting in protein inactivation. Insect herbivores that ingest high amounts of tannins fail to gain weight and may eventually die. In addition, the tannin content in seeds of all Vicia faba genotypes showed significant increase as compared with susceptible genotypes except triple white genotype.
The activity of POD in leaves and seeds of all Vicia faba genotypes was found to be significantly increased as compared with susceptible genotypes (). These results are in accordance with He et al. (Citation2011) who found that POD activity in three chrysanthemum cultivars ‘Keiun’, ‘Han6’, and ‘Jinba’ were enhanced by aphid herbivory. POD is one such enzyme of prime importance in plant defense that eliminates the ROS besides its other defensive roles. It has been implicated as an important component of the immediate response of plants to insect damage (Rani & Jyothsna Citation2010). Production of phenoxy and other oxidative radicals by the PODs in association with phenols directly deter the feeding by insects and/or produces toxins that reduce the plant digestibility, which in turn leads to nutrient deficiency in insects with drastic effects on their growth and development (Zhang et al. Citation2008). In addition, PODs have been reported to have direct toxicity in guts of herbivores (Zhu-Salzman et al. Citation2008).
Infestation with stored insects caused either significant increase in some resistant genotypes or nonsignificant effect in the others resistance genotypes in PPO in leaves and seeds of Vicia faba genotypes as compared with the susceptible one ().These results are similar to Rani and Pratyusha (Citation2013) who reported that pest infestation in cotton plants elevated the PPO levels, which may be due to the reduced nutritional quality of plant and indigestibility to the insect. In addition, He et al. (Citation2011) found that PPO activity in three chrysanthemum cultivars, ‘Keiun’, ‘Han6’, and ‘Jinba,’ were enhanced by aphid herbivory. In several studies, higher levels of PPO activity have been associated with the resistance of plants to insects (Chen et al. Citation2006). The PPO's role in reduction of nutrient quality, digestibility, and palatability of plant tissues to insects, and the production of quinones by catalyzing phenolic oxidation is highly appreciating defensive response against herbivory (Bhonwong et al. Citation2009).
The results in indicated that the total soluble protein in leaves and seeds of the resistance genotypes of Vicia faba (L.551, L.512, L.153, L.1, and NA112) significantly increased as compared with susceptible genotypes (L.16 and T.W). These results are similar to Rani and Pratyusha (Citation2013) who found that infested cotton plant expressed higher levels of proteins than normal plant. Also, War et al. Citation2012 found that proteins content were increased in insect damaged three groundnut genotypes as compared to uninfested control plants. In general, the induction was greater in the insect-resistant genotypes than in the susceptible one. Increase in the protein concentration may be due to the generation of defense-related proteins after stored insect's infestation. Plants defend themselves by producing these defense-related proteins at high concentrations (Lawrence & Koundal Citation2002).
Protein electrophoresis
Variation in SDS-PAGE banding patterns of protein extracted from Vicia faba leaves in response to infestation with stored insects are shown in and . Three types of modifications are observed in the protein patterns of Vicia faba leaves: some protein bands disappeared, other proteins were selectively increased; and synthesis of a new set of protein was induced. These responses were observed under infestation with stored seeds. It is clearly shown that the total number of protein bands in all faba bean genotypes under infestation with stored insects ranged between 27 and 34 bands. The protein banding patterns of seedlings in all genotypes comprise 12 major bands. These bands were recorded at the molecular weights of 111.9, 105.3, 68.2, 63.1, 35.5, 31.2, 27.8, 22.4, 16.8, 14.9, 14.3, and 13 kDa.
Note: 1 – L.551, 2 – L.512, 3 – L.153, 4 – L.1, 5 – T.W, 6 – NA112, 7 – Giza3, 8 – L.16.
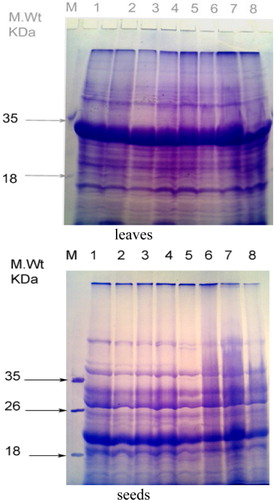
Table 5. Molecular weight and intensities (%) of protein banding patterns extracted from leaves of eight Vicia faba genotypes infested with stored insects.
Infestation with stored insects induced a considerable variation in the protein patterns of all genotypes of faba bean leaves. These results are similar to Khattab (Citation2007) who found that the infested and the control healthy cabbage leaves exhibited similar protein profiles but quantitative differences in some polypeptide chains were observed. The electrophoretic patterns of infested cabbage leaves showed an increase in polypeptide chains and a decrease in the amount of other polypeptide chains compared with their respective control. Changes in protein synthesis under infestation of stored insects may be due to changes in the efficiency of mRNA translation or the regulation of RNA transcription transport and stability. Also, biotic stress (insects) leads to difference in gene expressions where alterations in protein could be due to alteration in regulation of transcription, mRNA processing, or due to altered rates of protein degradation.
The total number of bands in leaves of sensitive genotypes (L. 16 and T.W) was decreased as being compared with the tolerance genotypes, but the intensity of protein of these genotypes increased. These results indicated that the decrease in the protein level in biotic stressed plants might be attributed to a decrease in protein synthesis, the decrease availability of amino acids and the denaturation of enzymes involved in amino acid and protein synthesis. These results, in accordance with Rafie et al. (Citation1996) and Jerez (Citation1998), demonstrated changes in protein profiles in resistant plants after insect feeding. It was suggested that synthesis or increased expression of specific plant proteins may serve to enhance the plant resistance to stresses (Ni et al. Citation2001).
Four bands can be considered as negative markers associated with tolerance to stored insects infestation. These bands were found in all tolerance genotypes (L.551, L.512, L.153, L.1, and NA112) and disappeared in the resistance genotypes (L.16 and T.W). These bands have molecular weights of 80.6, 44.4, 18.1, and 15.3 kDa.
The results of SDS-PAGE of proteins extracted from seeds of eight faba bean revealed a total number of 17 bands with molecular weights ranging from about 76.3 to 16.5 kDa which were not necessarily present in all genotypes. Data showed three common bands which have molecular weights 34.5, 28.0, and 20.1 kDA ( and ). The genotypes exhibited a different pattern in the presence of the bands. The maximum number of bands (17 bands) was recorded in the tolerant genotypes (NA112). The band which has a molecular weight 43.2 kDa can be considered as a negative molecular marker for tolerance to stored insects. These results are similar to the results obtained by El-Sayed (Citation2006) who found that protein electrophoresis of two genotypes of faba bean infested with bruchid detected distinct bands which differentiated the most susceptible from the most resistant.
Table 6. Molecular weight and intensities (%) of protein banding patterns extracted from seeds of eight Vicia faba genotypes infested with stored insects.
Molecular markers
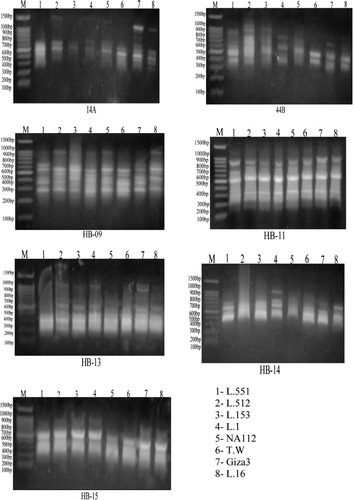
ISSR markers are useful in gene tagging and can be used for detecting markers linked to the gene of interest. In the present study, seven primers for ISSR were used to identify the molecular markers for resistance and susceptibility of Vicia faba genotypes to infestation with stored insects. All selected primers developed PCR products with a variable number of bands ().
Table 7. DNA polymorphism of eight genotypes of Vicia faba infested with stored insects.
The data showed that 71 DNA bands were detected among all genotypes of Vicia faba leaves, of which 62 bands were polymorphic (87.3%) ( and ). The highest number of ISSR bands was detected for primer HB-11 (13 bands), while the lowest was scored for HB-15 (6 bands). shows the polymorphic bands were generated from each primer. Three primers gave three molecular markers (negative markers) associated with tolerance to stored insects infestation (). The results of ISSR analysis using the seven primers indicated the appearance and disappearance of DNA polymorphic bands at all genotypes.
Table 8. Number and types of the amplified DNA bands as well as the percentage of the total polymorphism generated by seven ISSR primers in eight genotypes of Vicia faba.
Table 9. ISSR markers for the tolerance assessment to infestation with stored insects.
At the level of ISSR molecular markers, four primers showed specific markers for the studied traits. These results confirmed the useful application of ISSR-PCR analysis to detect the genetic variability between genotypes, similar to with Joshi et al. (Citation2000) who demonstrated that ISSR markers are a valuable method for detecting genetic variability among rice varieties and for rapidly identifying cultivars. Also, Abo El-kheir et al. (Citation2010) detected some specific ISSR molecular markers associated with Orobanche infestation tolerance in some faba bean cultivars.
Conclusion
The present data suggest that five faba bean genotypes may be resistant genotypes and can be used through breeding programs. In general, the eventual incorporation of yield traits, the biochemical and the molecular genetic markers for the selected faba bean genotypes are efficient tools to be applied as marker-assisted selection closely linked to important traits which greatly contribute to practical crop improvement programs.
References
- Acosta-Gallegos JA, Kelly JD, Gepts P. 2008. Prebreeding in common bean and use of genetic diversity from wild germplasm. Crop Sci. 48:3–16.
- Abo El-kheir ZA, Abdel-Hady MS, El-Naggar HMH, Abd El-Hamed AR. 2010. Molecular and biochemical markers of some Vicia faba L. cultivars in response to broomrape infestation. Nat Sci. 8:252–260.
- Baldwin IT. 2001. An ecologically motivated analysis of plant-herbivore interactions in native tobacco. Plant Physiol. 127:1449–1458. 10.1104/pp.010762
- Barbehenn RV, Peter Constabel C. 2011. Tannins in plant herbivore interactions. Phytochemistry. 72:1551–1565. 10.1016/j.phytochem.2011.01.040
- Bhonwong A, Stout MJ, Attajarusit J, Tantasawat P. 2009. Defensive role of tomato Polyphenol oxidase against cotton bollworm (Helicoverpa armigera) and Beet armyworm (Spodoptera exigua). J Chem Ecol. 35:28–38. 10.1007/s10886-008-9571-7
- Birkett MA, Campbell CAM, Chamberlain K, Guerrieri E, Hick AJ, Martin JL, Matthes M, Napier JA, Pettersson J, Pickett JA, et al. 2000. New roles for cis-jasmone as an insect semiochemical and in plant defense. Proc Natl Acad Sci USA. 97:9329–9334. 10.1073/pnas.160241697
- Burn ER. 1971. Methods foe estimation of tannin in grain sorghum. Agron J. 69:511. 10.2134/agronj1971.00021962006300030050x
- Chen W, Zhou Q, Li X, He GF. 2006. Physiological responses of different rice cultivars under herbivore stress. Acta Ecologica Sinica. 26:2161–2166. Chinese.
- Costa C, Dwyer LM, Dutilleul P, Stewart DW, Ma LB, Smith DL. 2001. Inter-relationships of applied nitrogen, SPAD, and yield of leafy and non-leafy maize genotypes. J Plant Nutr. 24:1173–1194. 10.1081/PLN-100106974
- Dellaporta SL, Wood J, Hicks JB. 1983. A plant DNA minipreparation: version II. Plant Mol Biol Rep 1:19–21. 10.1007/BF02712670
- Desroches P, Elshazly E, Mandon N, Duc G, Huignard J. 1995. Development of Callosobruchus chinensis (L.) and C. maculatus (F.) (Coleoptera: Bruchidae) L. in seeds of Vicia faba differing in tannin, convicine and vicine Contents. J Stored Prod Res. 31:83–89. 10.1016/0022-474X(94)00028-R
- Dihazi AD, Jaitt F, Zouine J, Hassni ME, Hardami IE. 2003. Effect of salicylic acid on phenolic compounds related to data palm resistance to Fusarium oxysporum sp. Albedimis Phytopath Medit. 423:9–16.
- El-Khawas SA. 2012. Priming Pisum sativum with salicylic acid against the leafminer Liriomyza trifolii. Afri J Agri Res. 7:4731–4737.
- El-Sayed ZG. 2006. Genetic markers for some important traits in faba bean (Vicia faba L.) [PhD Thesis]. Department of Genetics, Faculty of Agriculture, Ain Shams University.
- Fordyce JA, Agrawal AA. 2001. The role of plant trichomes and caterpillar group size on growth and defence of the pipevine swallowtail Battus philenor. J Anim Ecol. 70:997–1005. 10.1046/j.0021-8790.2001.00568.x
- Gaber AM, Mostafa HAM, Ramadan AA. 2000. Effect of gamma irradiation of faba beans (Vica faba) plant on its chemical composition, favism causative agent and hormonal level. Egypt J Physiol Sci. 24:1–16.
- Gulsen O, Eickhoff T, Heng-Moss T, Shearman R, Baxendale F, Sarath G, Lee D. 2010. Characterization of peroxidase changes in resistant and susceptible warm-season turfgrasses challenged by Blissus occiduus. Arthropod Plant Interact. 4:45–55. 10.1007/s11829-010-9086-3
- Haile A. 2006. On-farm storage studies on sorghum and chickpea in Eritrea. Afr J Biotechnol. 5:1537–1544.
- Haruta M, Major IT, Christopher ME, Patton JJ, Constabel CP. 2001. A Kunitz trypsin inhibitor gene family from trembling aspen (Populus tremuloides Michx.): cloning, functional expression, and induction by wounding and herbivory. Plant Mol Biol. 46:347–359. 10.1023/A:1010654711619
- He J, Chen F, Chen S, Lv G, Deng Y, Fang W, Liu Z, Guan Z, He C. 2011. Chrysanthemum leaf epidermal surface morphology and antioxidant and defense enzyme activity in response to aphid infestation. J Plant Physiol. 168:687–93. 10.1016/j.jplph.2010.10.009
- Jerez MI. 1998. Response to two-maize inbred lines to chinch bug feeding [MS Thesis]. Department of Plant and Soil Sciences, Mississippi State University.
- Joshi SP, Gupta VS, Aggarwal RK, Ranjekar PK, Brar DS. 2000. Genetic diversity and phylogenetic relationship as revealed by inter simple sequence repeat (ISSR) polymorphism in the genus Oryza. Theor Appl Genet. 100:1311–1320. 10.1007/s001220051440
- Kar M, Mishra D. 1976. Catalase, peroxidase, and polyphenoloxidase activity during leaf senescence. Plant Physiol. 57:315–319. 10.1104/pp.57.2.315
- Kashiwaba K, Tomooka N, Kaga A, Han OK, Vaughan DA. 2003. Characterization of resistance to three bruchid species (Callosobruchus spp., Coleoptera, Bruchidae) in cultivated rice bean (Vigna umbellata). J Econ Entomol. 96:207–213. 10.1603/0022-0493-96.1.207
- Khattab H. 2007. The defense mechanism of cabbage plant against phloem-sucking aphid (Brevicoryne brassicae L.). Aust J Basic Appl Sci. 1:56–62.
- Laemmli UK. 1970 Cleavage of structural proteins during assembly of head bacteriophage T4. Nature. 227:680–685. 10.1038/227680a0
- Lawrence PK, Koundal KR. 2002. Plant protease inhibitors in control of phytophagous insects. Electron J Biotechnol. 5:93–109. 10.2225/vol5-issue1-fulltext-3
- Lowry OH, Rosembrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J Bio Chem. 193:267–275.
- Ni X, Quisenberry SS, Hegn-Moss J, Markwell J, Sarath G, Klucas R, Baxendale F. 2001. Oxidative responses of resistant and susceptible cereal leaves to symptomatic and nonsymptomatic cereal aphid (Hemiptera: Aphididae) feeding. J Econ Entomol. 94:743–751. 10.1603/0022-0493-94.3.743
- Panda N, Khush GS. 1995. Host plant resistance to insects. Guildford (UK): CAB International in association with International Rice Research Institute (IRRI), Biddles Ltd.
- Perveen SS, Qaisrani TM, Amin S, Perveen R, Nagvi SHM. 2001. Biochemical basis of insect resistance in cotton. Online J Biol Sci. 1:496–500.
- Rafie MM, Zemetra RS, Quisenberry SS. 1996. Interaction between Russian wheat aphid (Homoptera: Aphididae) and resistant and susceptible genotypes of wheat. J Econ Entomol. 89:239–246.
- Rani UP, Jyothsna Y. 2010. Biochemical and enzymatic changes in rice as a mechanism of defense. Acta Physiol Plant. 32:695–701. 10.1007/s11738-009-0449-2
- Rani PU, Pratyusha S. 2013. Defensive role of Gossypium hirsutum L. anti-oxidative enzymes and phenolic acids in response to Spodoptera litura F. feeding. J Asia-Pac Entomol. 16:131–136. 10.1016/j.aspen.2013.01.001
- Ranjekar PK, Patankar A, Gupta V, Bhatnagar R, Bentur J, Kumar PA. 2003. Genetic engineering of crop plants for insect resistance. Curr Sci. 84:321–329.
- Sharma HC, Sujana G, Rao DM. 2009. Morphological and chemical components of resistance to pod borer, Helicoverpa armigera in wild relatives of pigeonpea. Arthropod-Plant Interact. 3:151–161. 10.1007/s11829-009-9068-5
- Somta P, Kaga A, Tomooka N, Isemura T, Vaughan DA, Srivines P. 2008. Mapping of quantitative trait loci for a new source of resistance to bruchids in the wild species Vigna nepalens Tateishi & Maxted (Vigna subgenus Ceratotropis). TAG Theor Appl Genet. 117:621–628. 10.1007/s00122-008-0806-3
- Stacey G, Keen NT. 1996. Plant–microbe interactions. St. Paul, Minnesota, MN: Aps Press.
- Vernon LP, Seely GR. 1966. The chlorophylls. New York, NY: Academic Press.
- War AR, Paulraj MG, War MY, Ignacimuthu S. 2012 Differential defensive response of groundnut germplasms to Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). J Plant Interact. 7:45–55. 10.1080/17429145.2011.587898
- Zhang SZ, Hau BZ, Zhang F. 2008. Induction of the activities of antioxidative enzymes and the levels of malondialdehyde in cucumber seedlings as a consequence of Bemisia tabaci (Hemiptera: Aleyrodidae) infestation. Arthropod-Plant Interact. 2:209–213. 10.1007/s11829-008-9044-5
- Zhu-Salzman K, Luthe DS, Felton GW. 2008. Arthropodinducible proteins: broad spectrum defenses against multiple herbivores. Plant Physiol. 146:852–858. 10.1104/pp.107.112177