Abstract
The sea buckthorn, Hippophae rhamnoides L., is a thorny, nitrogen-fixing, dioecious, and deciduous shrub which has been attacked by a catastrophic outbreak of Holcocerus hippophaecolus in the ‘Three North Areas’ of China recently. The behavioral responses of female individuals to their dioecious host sea buckthorn, H. rhamnoides ssp. sinensis, were tested by Y-tube bioassay, and intraspecific emission variations and the circadian rhythm of male and female sea buckthorn plants were compared, together with the electrophysiological responses of sea buckthorn carpenter moths to these parameters. Y-tube olfactometry indicated that mated female H. hippophaecolus individuals did not display a significant preference for either sex of sea buckthorns. Additionally, no unique chemical compound was found. Female antennae significantly responded to 1-octene, methyl salicylate, and (Z)-3-Hexen-1-ol acetate, among which methyl salicylate was more abundant in females than in males. In addition, the circadian variation of (Z)-3-Hexen-1-ol acetate suggested that it was an effective compound for host location.
Keywords:
Introduction
The sea buckthorn, Hippophae rhamnoides L., is a thorny, nitrogen-fixing, dioecious deciduous, shrub native to northwestern Europe and Asia including western and northern China, the Altai Mountains, and the northern Himalayas (Rousi Citation1971; Mao et al. Citation2010). H. rhamnoides is divided into nine subspecies based on genetic variations (Cao Citation1999), among which H. rhamnoides ssp. sinensis Rousi and H. rhamnoides ssp. yunnanensis Rousi are distributed only in China (Tian et al. Citation2004).
The sea buckthorn plants have many multipurpose benefits including economic and environmental benefits. For its economic aspects, all parts of Hippophae are a good source of bioactive substances (Stobdan et al. Citation2013). Its berries have been used as a traditional medicine, mainly in China and middle Asia (Yang et al. Citation2000). The seed oil or extract is rich in unsaturated fatty acids, phytosterols, carotenoids, and flavonoids that have significant anti-atherogenic and anti-cardio activity (Geetha et al. Citation2002, Citation2003; Raffo et al. Citation2004; Zeb Citation2004; Negi et al. Citation2005; Parimelazhagan et al. Citation2005; Basu et al. Citation2007; Dhyani et al. Citation2011). Extracts from the leaves of the sea buckthorn have highly effective antioxidant, anti-inflammatory, and hepatoprotective activities (Geetha et al. Citation2003; Ganju et al. Citation2005; Padwad et al. Citation2006). In its ecological aspect, benefits of planted sea buckthorn forests on soil-and-water conservation and enhancement of soil nitrogen are well documented (Wei et al. Citation2004; Chen et al. Citation2005; Li, Wang, et al. Citation2005; Zhang et al. Citation2010; Liu et al. Citation2011).
More than 1.4 million ha of pure sea buckthorn shrublands have been planted in China under threat from the sea buckthorn carpenter moth Holcocerus hippophaecolus Hua, Chou, Fang, and Chen (Lepidoptera: Cossidae; Fang et al. Citation2005). In recent decades, the northern population, H. rhamnoides ssp. sinensis, has been attacked by a catastrophic outbreak of the carpenter moth in the ‘Three North Areas’ of China. Although some other factors play a role in losses of sea buckthorn plantings, damage from the moth appears to be the primary reason. The carpenter moth has destroyed more than 10,000 ha of H. rhamnoides ssp. sinensis monoculture plantations. In Liaoning province, China, the carpenter moth infested 85% of the sea buckthorn (Luo et al. Citation2003).
Plant volatiles play a significant role in guiding female herbivores to locate their host plants for oviposition (Angioy et al. Citation2003; Webster et al. Citation2008; Lu et al. Citation2013; Yoneya & Takabayashi Citation2013). The chemical content of Hippophae has also attracted wide considerable attention because of its therapeutic value (Zeb Citation2004; Stobdan et al. Citation2013). There is an increasing interest in using chromatography methods to analyze the chemical compounds of H. rhamnoides (Guliyev et al. Citation2004; Tiitinen et al. Citation2006). Compounds from H. rhamnoides dried leaves have been analyzed to investigate the variability between interpopulations of H. rhamnoides ssp. yunnanensis and H. rhamnoides ssp. sinensis (Tian et al. Citation2004). For the northern population, H. rhamnoides ssp. sinensis, volatile compounds of healthy and insect-damaged stems have been analyzed by Zong et al. (Citation2012). In addition, sea buckthorn is divided into female and male plants. The female plants have berries, and therefore are considered to have greater value than male plants. Many studies have suggested that male and female plants differ in morphology (Correia & Diaz Barradas Citation2000; Li et al. Citation2007), physiological response to the environment (Li et al. Citation2004, Li, Yang, et al. Citation2005), and life history traits (Letts et al. Citation2008). In particular, it has been suggested that the pest susceptibility was significantly correlated with the female plant (Krischik & Denno Citation1990; Cepeda-Cornejo & Dirzo Citation2010). Furthermore, change in the rhythm of volatile production is an important element associated with pest infestations. For instance, several volatiles were released exclusively at night and were highly repellent to female moths (De Moraes et al. Citation2001). With another Lepidoptera, Mythimna separata (Walker), host volatiles rather than light determine the moth's daily rhythms (Shiojiri et al. Citation2006). The oviposition preference of adult H. hippophaecolus is primarily influenced by the volatile secondary metabolites and bark structure (Zong et al. Citation2013). However, up to now, neither the intraspecific emission variation of H. rhamnoides ssp. sinensis nor its daily rhythm's effect on the sea buckthorn carpenter moth damage has been elucidated.
The objectives of the present study were: (1) to test behavioral responses of the female H. hippophaecolus to male and female H. rhamnoides ssp. sinensis in a laboratory with a Y-tube bioassay; (2) to identify headspace collected volatiles derived from male or female sea buckthorn and analyze the intraspecific emission variations and circadian rhythms of male and female plants; and (3) to evaluate the electrophysiology response of sea buckthorn carpenter moths to crucial components.
Material and methods
Insects and plants
Sea buckthorn roots damaged by mature larvae were obtained from sea buckthorn shrubs in March 2011, and stored outdoors in a man-made pit and covered with a soil layer to simulate its natural conditions, at an experimental base in Pengyang city, Ningxia, China (35°50′N, 106°41′E). The H. hippophaecolus pupae were collected ca. two months later from split sea buckthorn roots. A portion of the pupae was then transferred to the Beijing Forestry University laboratory, Beijing (39°92′N, 116°46′E), China, for use in the electrophysiological experiments, and the others were kept at an experimental base in Pengyang city for bioassays. At both locations, the pupae and emerging adults were maintained at 24 ± 1°C with 60 ± 10% relative humidity, and a photoperiod of 16 L: 8 D. All emerging adults were placed in a small cage (30 × 30 × 30 cm). Mated females were obtained by placing ca. 20 newly emerged females together with 30 males in the same cage for two scotophases to ensure mating. The status of female moths was checked by dissection of the bursa copulatrix for the presence of spermatophore at the end of the test. All females used had successfully mated, and each was used only once and had not been exposed to odor sources before the bioassay test. Furthermore, 2- to 4-day-old mated females were chosen without a conscious bias from the cages for the electrophysiological experiments and bioassays.
Chemical standards
Chemicals standards used in the test were purchased from various companies. The standard curve, resource, and purity are shown in .
Table 1. The source, purity, and standard curve of compounds.
Field investigation
The field investigation was carried out in a 12-year-old, planted, circular, sea buckthorn field at Pengyang city. Four sample plots (30 × 30 m) were set in four cardinal directions. Five equal sample points (10 × 10 m) were set in each plot. For each point, five adjacent-paired sea buckthorn plants of different sex but similar age were evaluated. The roots were dissected and larvae numbers were used to determine the damage to male and female plants.
Laboratory investigation
Female response to volatiles of sea buckthorn branches in a Y-tube
Twelve sea buckthorn plants (six males and six females) were transferred to the research base from the wild. Branches were picked within 20 min before an assay. The main chamber of the Y-tube olfactometer () was a bottomless cylindrical glass bottle (30 cm length, 20 cm diam.) with two test chamber bottomless cylindrical glass bottles (20 cm length, 4.5 cm diam.) as a unit. Two hollow glass balls (10 cm diam.) containing the branches were connected to the end of each test chamber by a short cylindrical glass tunnel (5 cm length, 4.7 cm diam.) with a ground glass joint at the end, where the branches were put in. Humidified air was pumped into the glass balls at a rate of 500 ml/min.
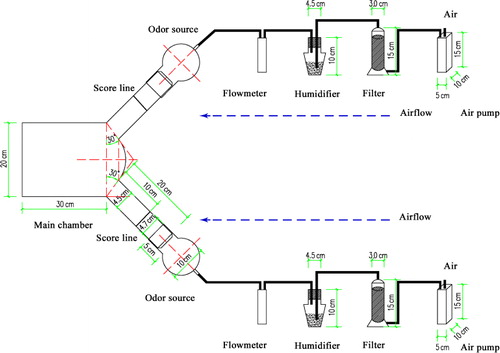
Healthy male and female sea buckthorn branches were used as odor sources to investigate a possible preference of mated females. Three treatments: (1) female sea buckthorn branches versus male branches; (2) female sea buckthorn branches versus a blank control; and (3) male branches versus a blank. Bioassays were conducted at 25 ± 3°C and 60 ± 15% relative humidity between 18:00 and 21:00 hours, which was determined as the mating and egg laying peak in the field (Luo et al. Citation2003). A total of 90 females were released into the main chamber from the smaller end of the arm, and allowed to move upwind to choose an odor source. Thirty females were used per treatment and were released into the arm one by one. For each replicate, the position of the odor chambers was altered to prevent positional bias. Branches were exchanged every trial session (10 individuals tested), and the arena was then cleaned with Hexane (purity > 98%) and alcohol (purity > 98%), and dried with an electric blower. A positive response was recorded when a moth crossed a score line of the two odor arms (10 cm from the point where these arms joined) within 5 min after release and stayed behind the score line for at least 2 min. No response was recorded when the moth remained in the arm for more than 5 min.
Volatile analysis
Volatile collection
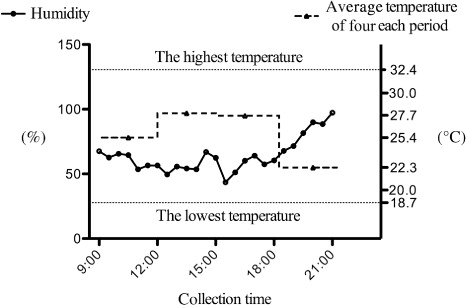
Dynamic headspace was used for collection of the shrub volatiles (Vercammen et al. Citation2000). Branches of healthy sea buckthorn were enclosed in polyethylene oven bags (406 × 444 mm, Reynolds, Richmond, VA), which were connected to adsorption tubes (TENAX-TA 60/80 mesh, Alltech, Deerfield, IL). Clean air was filtered through activated charcoal, pumped into a bag at 500 ml/min, and then absorbed in Tenax-TA tubes. The experiment was carrying out in July, when the mating peak began. Volatiles were collected for 3 hours (Liu et al. Citation2010) between 18:00 and 21:00 hours based on peak field activity (Luo et al. Citation2003). To evaluate the circadian rhythm of volatile release from male and female plants, additional collections were also set up at 9:00–12:00, 12:00–15:00, and 15:00–18:00 hours. After collection, the branches in the airtight bags were cut off. The wet weights of the branches were recorded and used to quantify the emission rate. Three replications were performed per collection period, and a blank control of a hollow bag was also set up to eliminate bias.
Identification with coupled gas chromatography-mass spectrometry
The quality and quantity of volatiles derived from sea buckthorn branches were analyzed by using a Turbo Matrix 650 automated thermal desorption combined with the Clarus 600 gas chromatography-mass spectrometry (ATD-GC/MS; PerkinElmer, Waltham, MA). The ATD was used to desorb collected volatiles from the Tenax tube into the GC injector at a 5.0% injection rate. The Clarus 600 GC was fitted with a DB-5 capillary column (30 m length, 0.25 mm internal diam., 0.15 µm film thickness; Agilent Technologies, Santa Clara, CA).
Helium carrier gas flow rate was 1.5 ml/min. The injection temperature was kept at 260°C. The initial oven temperature was 40°C maintained for 3 min; the temperature was then increased to 180°C at a rate of 5°C/min, held for 2 min, and then increased to 270°C at a rate of 10°C/min, followed by a final holding for 2 min. The total running time was 44 min. The split ratio was 2:1. The electron ionization voltage was 70 eV. A full scan was run with a mass range of 20–350 m/z and a scanning rate of 2.5 times per second.
Volatile compounds were identified by comparison of both chromatographic retention time with commercially available authentic standards, and mass spectra data from the National Institute of Standards and Technology Mass Spectral (NIST) used with Turbo Mass 5.4.2 GC-MS software (PerkinElmer, Waltham, MA). In addition, the Kovats indices of all compounds were estimated by using the dwell time of C5–C27 straight-chain alkanes at the same chromatographic column and temperature program. Kovats indices were rechecked with the data of a DB-5 capillary column from the Pherobase (http://www.pherobase.com).
Female response to fractions (EAG test)
A micromanipulator assembly (MP-15, Syntech, NJ) was connected to a stimulus controller (CS-55, Syntech), which provided continuous clean airflow or stimulus airpulse. The signal sources were connected to a serial data acquisition interface (IDAC-4, Syntech). Antennae from H. hippophaecolus females were excised using a micro-scissors. A few segments of the antennae were clipped off and then mounted on two metal electrodes on the antenna holder using a conductive gel (Spectra 360, Parker Lab, NJ). The electrode holder was then connected to the EAG probe. Only one antenna was used at a time, and a relatively stable baseline was visible before the testing started. Each sample in the was tested six times with six antennae from different females. Ten mg of each compound was added into 1 ml paraffin, and then diluted proportionally into 100, 10−1, 10−2, 10−3, and 10−4. All stimuli compounds were formulated into six concentrations: 10−3, 10−2, 10−1, 100, 101, and 102 µg (10 µl injected/concentration). A random sequence of tested compounds was used, and a solvent blank (10 µl paraffin) and a standard control stimulus (1 µg Linalool) were interspersed with the test concentrations. Each stimulus lasted for 0.1 s and was followed by a minimum of 60 s for recovery. The EAG responses were normalized to the response (%) of the control.
Statistical analysis
A Shapiro–Wilk test was used to make sure that the collected data fit normal distribution. Then paired t-tests (p < 0.05) were used to show damage similarity between male and female plants. Statistical comparisons of volatiles difference between male and female plants were performed using a nonparametric Mann–Whitney U test (p < 0.05). The quantification of volatile compounds was based on peak area (PA) relative to that of the external standard curve, whereas other quantifications used the relative PA, which was integrated for the total ion current signal (chromatograms).
Student–Newman–Keuls and Dunnett (p < 0.05) tests were used to show significant differences between volatile emissions of individual plants at different collecting time, as well as the difference between male and female plants. Positive responses of female moths from the Y-tube olfactometer were analyzed with a χ2 test. EAG responses for the same compound at different concentrations and under the same concentration of different chemicals were analyzed by two-way ANOVA followed by Turkey's multiple comparison test (p < 0.05). All the analyses were performed using the statistical program SPSS 17.0 (IBM Corp., Armonk, NY).
Results
Field investigation
Evaluations of 86 paired samples indicated that sex of plants were not significantly correlated with plant damage (two-paired-samples test, σ (two-tailed) = 0.366). The age of male and female plants in each paired sample was determined to be the same (N = 86, correlation = 0.642, σ = 0.000). The result of the Shapiro–Wilk (P = 1.26 > 0.05) evaluation showed that the data fit a normal distribution.
Laboratory investigation
Female response to volatiles of sea buckthorn branches in a Y-tube
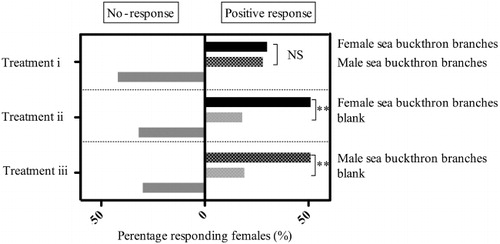
Mated female H. hippophaecolus showed a significant preference to either male or female sea buckthorn branches over the blank control (Treatments ii and iii; ). However, there was no significant difference between attractions of the moths to male or female branches (Treatment i; ).
Volatiles compounds of intact male and female sea buckthorn during 18:00–21:00 hours
Forty-two compounds were identified from sea buckthorn branches by GC-MS, among which 22 were verified by their standards. No chemical compound was unique in plants of both sexes. (Z)-3-Hexen-1-ol acetate was the most abundant component (21.14 ± 2.63%) of female sea buckthorn, followed by 3-Carene (9.89 ± 2.58%), 2-ehtylhexan-1-ol (8.10 ± 0.75%), 1-Nonanal (7.74 ± 3.49%), and 1-Hexadecene (6.25 ± 1.28%). The percentages of 1-octene (2.17 ± 0.53%), naphthalene (3.66 ± 0.42%), decanal (4.33 ± 1.88%), and longifolene (4.78 ± 1.44%) were all under 5%. The proportion of all the above-mentioned chemical compounds was more than 50% of the total content ( and ).
Table 2. Comparison between intact male and female sea buckthorns for volatile compounds tentatively identified released from 18:00 to 21:00 hours (N = 3).
Table 3. Comparison between intact male and female sea buckthorns for volatile compounds confirmed and calibrated by authentic standards released from 18:00 to 21:00 hours (N = 3).
The male and female sea buckthorn had the same types of compounds but in differing amounts. The Mann–Whitney U-test results indicated that 10 components, including methyl 3-methylbutanoate, 3-heptanone, henzaldehyde, 1-heptanol, 6-methyl-5-hepene-2-one, naphthalene, dodecane, isobutyl 2-hydroxydecanoate, and 4-methylnonanoic acid, were significantly more abundant in the female than the male plants.
Circadian rhythms of main volatiles of male and female sea buckthorn branches
All components were divided into three groups based on the circadian rhythm of volatiles released during the four time periods (9:00–12:00, 12:00–15:00, 15:00–18:00, and 18:00–21:00 hours). For components in Group 1, no significant difference was found during the four periods for both sexes of the plants. For components in Group 2, a tendency toward decrease was found from morning to night. For components in Group 3, a different release trend was observed at the end of collection.
Group 1 () was composed of decanal, benzothiazole, 1-octene, 6-methyl-5-hepene-2-one, benzaldehyde, and g-terpinene. All six compounds constantly released at the same level during the different collection times from both male and female plants. Benzothiazole was significantly higher in abundance in female than male plants from 12:00 to 18:00 hours. 6-methyl-5-hepene-2-one and benzaldehyde were significantly more abundant in female than male branches at the beginning and end of the test (09:00–12:00 hours and 18:00–21:00 hours). g-Terpinene was significantly more abundant in males than females from12:00 to 15:00 hours.
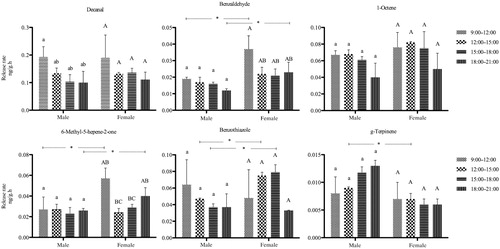
In Group 2, all seven compounds () were released more abundantly in an earlier period than in the latter period, expect for Heneicosane, which had the peak at the second period (12:00–15:00 hours). 1-Nonanal was the significantly abundant component derived from female than male branches at the second and third collecting periods. 3-Carene was a typical compound in the group with more abundant emission in females than males at the third period. Meanwhile, methyl salicylate and 2-ehtylhexan-1-ol were also significantly more abundant in both sexes at the first and fourth period.
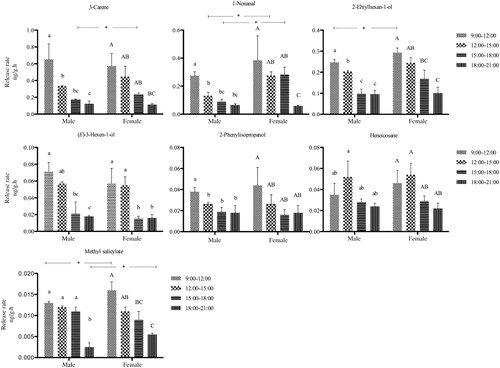
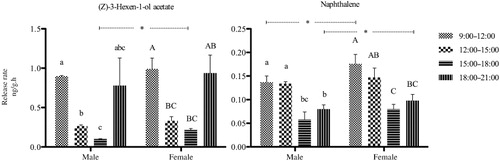
In Group 3, (Z)-3-Hexen-1-ol acetate was the main sea buckthorn volatile, and was variable during the four periods from both male and female plants (), where its emission decreased from the first to third period, and then increased in the fourth period. Similarly, naphthalene presented a valley at the third collection period, but reached a peak average value at the last period when it approached the average value. (Z)-3-Hexen-1-ol and naphthalene were more abundant from female than male branches at the third and the first period, respectively.
EAG response to different components in three groups
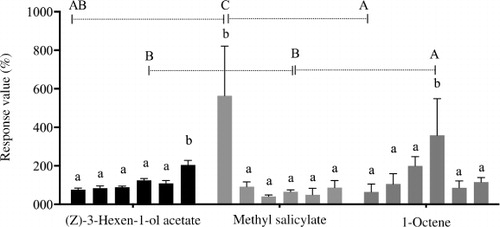
The female antennae responded to all compounds of Group 1, Group 2, and Group 3 at the six dosages (not shown). One µg 1-octene elicited significantly higher antennal response. 10−3 µg methyl salicylate elicited a significantly higher response than other dosages. 102 µg (Z)-3-Hexen-1-ol acetate elicited the most significant response among the six dosages. Comparisons made between 1-octene, methyl salicylate, and (Z)-3-Hexen-1-ol acetate at the six dosages () indicated that 10−3 µg methyl salicylate and 1 µg 1-octene were the two most active chemicals for female sea buckthorn carpenter worm adults.
Discussion
In natural forest, the sex ratios of sea buckthorn were significantly male-biased at 2000–3600 m above sea level in the Balang Mountains within the Wolong Nature Reserve (Li et al. Citation2007). It has been indicated that male sea buckthorn could resist drought; however, the female sea buckthorn is more sensitive to water stress, and female sea buckthorn grew worse in dry season (Li et al. Citation2004). On the other hand, sea buckthorn carpenter moth occurred more serious in a ‘dry year’ than ‘rainy year’ (Luo et al. Citation2003). So, we post a hypothesis that (1) the sea buckthorn carpenter moth may damage the female sea buckthorn more seriously during the dry season, and (2) the response of sea buckthorn carpenter moth to male and female plants volatiles of sea buckthorn was different. However, field investigation indicated that the sex of plants was not significantly correlated with plant damage and mated female H. hippophaecolus individuals did not display a significant preference for either sex of sea buckthorns in Y-tube olfactometry.
However, methyl 3-methylbutanoate, 3-heptanone, benzaldehyde, 1-heptanol, 6-methyl-5-hepene-2-one, naphthalene, methyl salicylate, dodecane, isobutyl-2-hydroxydecanoate, and 4-methylnonanoic acid were more abundant in the female than in the male plants ( and ). Maffei (Citation2010) indicated that benzaldehyde, methyl salicylate, and 6-methyl-5-hepten-2-one are the most common single compounds in floral scent, which provide important guides to pollinator (Knudsen et al. Citation2006). It is unclear whether these mentioned compounds play a role to guide the pollinator, and further research should be carried out to evaluate the response of pollinator to these compounds.
EAG response to different components in three groups showed that 1 µg 1-octene, 10−3 µg methyl salicylate, and 102 µg (Z)-3-Hexen-1-ol acetate elicited significantly higher antennal response (). 1-octene (a minor component of sea buckthorn) was not significantly different between the male and female sea buckthorn, with consistent release during the day time (). It has been indicated that isoprene is positively active in thermoprotection, and thermotolerance of alkenes (e.g. 1-octene) is similar to isoprene (Velikova Citation2008). During the collection, the highest temperature was 32.4°C (), and the plant of sea buckthorn was heat-threatened.
(Z)-3-Hexen-1-ol acetate (the most abundant content) elicited a significant female antennae response at a dose of 102 µg. Dai et al. (Citation2008) indicated that (Z)-3-Hexen-1-ol significantly enhanced Plutella xylostella male catches when combining the synthetic female sex pheromone and (Z)-3-Hexen-1-ol acetate compared with traps baited with the sex pheromone alone in the field trapping experiments. Abundant (Z)-3-Hexen-1-ol acetate was released from 18:00 to 21:00 hours in sea buckthorn (), when mated H. hippophaecolus reached peak (Zong et al. Citation2006). Host plant volatiles combined with sex pheromones may provide more information for the reproduction of insects. Plant volatiles can be a mediatory effluence in host finding and may synergize sexual attraction (Dickens et al. Citation1993; Light et al. Citation1993; Landolt & Phillips Citation1997; Yang et al. Citation2004; Dickens Citation2006; Pope et al. Citation2007; Namiki et al. Citation2008). In addition, female antennae responded most strongly to its highest concentration; accordingly, the release amount of (Z)-3-Hexen-1-ol acetate was abundant, which should be a kind of adaptive strategy.
Methyl salicylate (a micro-content of sea buckthorn) is a volatile derivative of the plant hormone salicylic acid (Lee et al. Citation1995), and was an active compound with the female antennae in our study (). Dickens (Citation2006) indicated that 1-nonanal and a blend of three compounds of (Z)-3-Hexen-1-ol acetate, (±)-linalool, and methyl salicylate enhanced attraction of Colorado potato beetle to its aggregation pheromone. In addition, Erigonidium graminicolum (Sund), a natural enemy of that beetle, is also attracted to the blend of 1-nonanal, (Z)-3-Hexen-1-ol acetate, and methyl salicylate (Yu et al. Citation2008). In this study, methyl salicylate declined drastically from day to night, and the lowest dose of the compound was the most effective to the female antennae.
Yue et al. (Citation2001) indicated that the most abundant volatile of fescue Festuca arundinacea Schreb was (Z)-3-Hexen-1-ol acetate at 25°C, and changed into 1-nonanal at 32°C. In our study, the volatile emission of (Z)-3-Hexen-1-ol acetate was consistent between day (9:00–12:00 hours) and night (18:00–21:00 hours), although the temperature changed from 32.4°C to 18.7°C (). Vallat et al. (Citation2005) indicated that the emission of (Z)-3-Hexen-1-ol was affected by both temperature and humidity in the apple tree and was more associated with humidity. Loughrin et al. (Citation1994) indicated that terpenoids released by intact plants associated with herbivores are light-dependent. On the other hand, Pseudaletia unipuncta female calling behavior (associated with the release of the long-distance sex pheromone) has an endogenous circadian rhythm (Turgeon & McNeil Citation1982), but the periodicity of calling is very markedly affected by the temperature, with female calling much later in the scotophases under warm rather than cool conditions (Delisle & Mcneil Citation1987). So all the humidity, light, and the temperature may affect the emission of volatiles and the interaction between insect and plant.
Larvae of H. hippophaecolus mainly infested of nitrogen-fixing the roots of sea buckthorn plants, which help plants to absorb water and nutrients in the soil by mycorrhizal fungi and rhizobacteria (Andrea Citation2013). It is important to compare volatile emissions from branches of sea buckthorn plants whose roots were damaged by sea buckthorn carpenter moths with healthy branches (Rasmann & Turlings Citation2007; Matthias et al. Citation2011).
Acknowledgment
We are also indebted to two anonymous reviewers for their valuable comments on the manuscript.
Funding
This work was supported by ‘Twelfth Five-year’ National Science and Technology Support Program of China under Grant [grant number 2012BAD19B07]; and Beijing Natural Science Foundation under Grant [grant number 6142015]; and National Natural Science Foundation of China under Grants [grant number 31270693], [grant number 81102747].
Additional information
Funding
References
- Andrea O. 2013. Plant coevolution: evidences and new challenges. J Plant Interact. 3:188–196.
- Angioy AM, Desogus A, Barbarossa IT, Anderson P, Hansson BS. 2003. Extreme sensitivity in an olfactory system. Chem Senses. 28:279–284. 10.1093/chemse/28.4.279
- Basu M, Prasad R, Jayamurthy P, Pal K, Arumughan C, Sawhney RC. 2007. Anti-atherogenic effects of seabuckthorn (Hippophaea rhamnoides) seed oil. Phytomedicine. 14:770–777. 10.1016/j.phymed.2007.03.018
- Cao Y. 1999. Juice from Hippophae fruit and reservation techniques. Prod Agr Herd. 9:116–117.
- Cepeda-Cornejo V, Dirzo R. 2010. Sex-related differences in reproductive allocation, growth, defense and herbivory in three dioecious neotropical palms. PLoS One. 5:e9824. 10.1371/journal.pone.0009824
- Chen Y, Liu G, Xu B. 2005. Effects of artificial seabuckthorn forest on soil and water conservation in loess hilly region. Chin J App Ecol. 16:595–599.
- Correia O, Diaz Barradas M. 2000. Ecophysiological differences between male and female plants of Pistacia lentiscus L. J Pant Ecol. 149:131–142.
- Dai J, Deng J, Du J. 2008. Development of bisexual attractants for diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) based on sex pheromone and host volatiles. Appl Entomol Zool. 43:631–638. 10.1303/aez.2008.631
- De Moraes C, Mescher MC, Tumlinson JH. 2001. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature. 410:577–580. 10.1038/35069058
- Delisle J, Mcneil JN. 1987. The combined effect of photoperiod and temperature on the calling behaviour of the true armyworm, Pseudaletia unipuncta. Physiol Entomol. 12:157–164.
- Dhyani D, Maikhuri RK, Dhyani S. 2011. Seabuckthorn: an underutilized resource for the nutritional security and livelihood improvement of rural communities in Uttarakhand Himalaya. Ecol Food Nutr. 50:168–180. 10.1080/03670244.2011.552375
- Dickens J. 2006. Plant volatiles moderate response to aggregation pheromone in Colorado potato beetle. J Appl Entomol. 130:26–31. 10.1111/j.1439-0418.2005.01014.x
- Dickens JC, Smith JW, Light DM. 1993. Green leaf volatiles enhance sex attractant pheromone of the tobacco budworm, Heliothis virescens (Lep.: Noctuidae). Chemoecology. 4:175–177. 10.1007/BF01256553
- Fang YL, Sun JH, Zhao CH, Zhang ZN. 2005. Sex pheromone components of the sandthorn carpenterworm, Holcocerus hippophaecolus. J Chem Ecol. 31:39–48. 10.1007/s10886-005-0972-6
- Ganju L, Padwad Y, Singh R, Karan D, Chanda S, Chopra MK, Bhatnagar P, Kashyap R, Sawhney RC. 2005. Anti-inflammatory activity of seabuckthorn (Hippophae rhamnoides) leaves. Int Immunopharmacol. 5:1675–1684. 10.1016/j.intimp.2005.03.017
- Geetha S, Ram MS, Mongia SS, Singh V, Ilavazhagan G, Sawhney RC. 2003. Evaluation of antioxidant activity of leaf extract of seabuckthorn (Hippophae rhamnoides L.) on chromium (VI) induced oxidative stress in albino rats. J Ethnopharmacol. 87:247–251. 10.1016/S0378-8741(03)00154-5
- Geetha S, Sai Ram M, Singh V, Ilavazhagan G, Sawhney RC. 2002. Anti-oxidant and immunomodulatory properties of seabuckthorn (Hippophae rhamnoides) – an in vitro study. J Ethnopharmacol. 79:373–378. 10.1016/S0378-8741(01)00406-8
- Guliyev VB, Gul M, Yildirim A. 2004. Hippophae rhamnoides L.: chromatographic methods to determine chemical composition, use in traditional medicine and pharmacological effects. J Chromatogr B. 812:291–307. 10.1016/j.jchromb.2004.08.047
- Knudsen JT, Eriksson R, Gershenzon J, Stahl B. 2006. Diversity and distribution of floral scent. Bot Rev. 72:1–120. 10.1663/0006-8101(2006)72[1:DADOFS]2.0.CO;2
- Krischik VA, Denno RF. 1990. Patterns of growth, reproduction, defense, and herbivory in the dioecious shrub Baccharis halimifolia (Compositae). Oecologia. 83:182–190. 10.1007/BF00317750
- Landolt PJ, Phillips TW. 1997. Host plant influences on sex pheromone behavior of phytophagous insect 1. Annu Rev Entomol. 42:371–391. 10.1146/annurev.ento.42.1.371
- Lee HI, Leon J, Raskin I. 1995. Biosynthesis and metabolism of salicylic acid. Proc Natl Acad Sci USA. 92:4076–4079. 10.1073/pnas.92.10.4076
- Letts MG, Phelan CA, Johnson DRE, Rood SB. 2008. Seasonal photosynthetic gas exchange and leaf reflectance characteristics of male and female cottonwoods in a riparian woodland. Tree Physiol. 28:1037–1048. 10.1093/treephys/28.7.1037
- Li C, Ren J, Luo J, Lu R. 2004. Sex-specific physiological and growth responses to water stress in Hippophae rhamnoides L. populations. Acta Physiol Plant. 26:123–129. 10.1007/s11738-004-0001-3
- Li C, Xu G, Zang R, Korpelainen H, Berninger F. 2007. Sex-related differences in leaf morphological and physiological responses in Hippophae rhamnoides along an altitudinal gradient. Tree Physiol. 27:399–406. 10.1093/treephys/27.3.399
- Li C, Yang Y, Junttila O, Palva ET. 2005. Sexual differences in cold acclimation and freezing tolerance development in sea buckthorn (Hippophae rhamnoides L.) ecotypes. Plant Sci. 168:1365–1370. 10.1016/j.plantsci.2005.02.001
- Li PJ, Wang ZJ, Sun TH, Tai PD, Chang SJ, Xiong XZ, Li YM. 2005. Application of wastewater land treatment technique to the construction of ecological engineering in sand land. China Environ Sci. 26:73–77.
- Light DM, Flath RA, Buttery RG, Zalom FG, Rice RE, Dickens JC, Jang EB. 1993. Host-plant green-leaf volatiles synergize the synthetic sex pheromones of the corn earworm and codling moth (Lepidoptera). Chemoecol. 4:145–152. 10.1007/BF01256549
- Liu SJ, Jing YJ, Zong SX, Luo YQ, Yao GL, Li Y. 2010. Study on collection method and component analysis of trace volatiles from Hippophae rhamnoides. Chin Agric Sci Bull. 26:77–81. 10.1016/S1671-2927(09)60070-5
- Liu X, Li FM, Liu DQ, Sun GJ. 2011. Soil organic carbon, carbon fractions and nutrients as affected by land use in semi-arid region of Loess Plateau of China. Pedosphere. 20:146–152. 10.1016/S1002-0160(10)60002-1
- Loughrin JH, Manukian A, Heath RR, Turlings TCJ, Tumlinson JH. 1994. Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plants. Proc Natl Acad Sci USA. 91:11836–11840.
- Lu PF, Qiao HL, Xu ZC, Cheng J, Zong SX, Luo YQ. 2013. Comparative analysis of peach and pear fruit volatiles attractive to the oriental fruit moth, Cydia molesta. J Plan Interact. 9:388–395.
- Luo YQ, Lu CK, Xu ZC. 2003. Control strategies on a new serious forest pest insect–seabuckthorn carpenterworm, Holcocerus hippophaecolus. Forest Pest Dis. 22:25–28.
- Maffei M. 2010. Sites of synthesis, biochemistry and functional role of plant volatiles. S Afr J Bot. 76:612–631. 10.1016/j.sajb.2010.03.003
- Mao R, Zeng DH, Ai GY, Yang D, Li LJ, Liu YX. 2010. Soil microbiological and chemical effects of a nitrogen-fixing shrub in poplar plantations in semi-arid region of northeast China. Eur J Soil Biol. 46:325–329. 10.1016/j.ejsobi.2010.05.005
- Matthias E, Christelle AM, Robert CJT, Ted CJT. 2011. Induction of root-resistance by leaf-herbivory follows a vertical gradient. J Plant Interact. 6:133–136 10.1080/17429145.2010.545958
- Namiki S, Iwabuchi S, Kanzaki R. 2008. Representation of a mixture of pheromone and host plant odor by antennal lobe projection neurons of the silkmoth Bombyx mori. J Comp Physiol A. 194:501–515. 10.1007/s00359-008-0325-3
- Negi PS, Chauhan AS, Sadia GA, Rohinishree YS, Ramteke RS. 2005. Antioxidant and antibacterial activities of various seabuckthorn (Hippophae rhamnoides L.) seed extracts. Food Chem. 92:119–124. 10.1016/j.foodchem.2004.07.009
- Padwad Y, Ganju L, Jain M, Chanda S, Karan D, Banerjee PK, Sawhney RC. 2006. Effect of leaf extract of seabuckthorn on lipopolysaccharide induced inflammatory response in murine macrophages. Int Immunopharmacol. 6:46–52. 10.1016/j.intimp.2005.07.015
- Parimelazhagan T, Chaurasia OP, Ahmed Z. 2005. Seabuckthorn: oil with promising medicinal value. Curr Sci. 88:8–9.
- Pope TW, Campbell CAM, Hardie J, Pickett JA, Wadhams LJ. 2007. Interactions between host-plant volatiles and the sex pheromones of the bird cherry-oat aphid, Rhopalosiphum padi and the damson-hop aphid, Phorodon humuli. J Chem Ecol. 33:157–165. 10.1007/s10886-006-9199-4
- Raffo A, Paoletti F, Antonelli M. 2004. Changes in sugar, organic acid, flavonol and carotenoid composition during ripening of berries of three seabuckthorn (Hippophae rhamnoides L.) cultivars. Eur Food Res Technol. 219:360–368. 10.1007/s00217-004-0984-4
- Rasmann S, Turlings TCJ. 2007. Simultaneous feeding by aboveground and belowground herbivores attenuates plant-mediated attraction of their respective natural enemies. Ecol Lett. 10:926–936. 10.1111/j.1461-0248.2007.01084.x
- Rousi A. 1971. The genus Hippophae L.: a taxonomic study. Ann Bot Fenn. 8:177–227.
- Shiojiri K, Ozawa R, Takabayashi J. 2006. Plant volatiles, rather than light, determine the nocturnal behavior of a caterpillar. PLoS Biol. 4:e164. 10.1371/journal.pbio.0040164
- Stobdan T, Korekar G, Srivastava RB. 2013. Nutritional attributes and health application of seabuckthorn (Hippophae rhamnoides L.) a review. Current Nutr Food Sci. 9:151–165. 10.2174/1573401311309020008
- Tian C, Nan P, Chen J, Zhong Y. 2004. Volatile composition of Chinese Hippophae rhamnoides and its chemotaxonomic implications. Biocheml Syst Ecol. 32:431–441. 10.1016/j.bse.2003.08.007
- Tiitinen K, Hakala M, Kallio H. 2006. Headspace volatiles from frozen berries of sea buckthorn (Hippophae rhamnoides L.) varieties. Eur Food Res Technol. 223:455–460. 10.1007/s00217-005-0224-6
- Turgeon JJ, McNeil JN. 1982. Calling behaviour of the armyworm, Pseudaletia unipuncta. Entomol Exp Appl. 31:402–408.
- Vallat A, Gu H, Dorn S. 2005. How rainfall, relative humidity and temperature influence volatile emissions from apple trees in situ. Phytochem. 66:1540–1550. 10.1016/j.phytochem.2005.04.038
- Velikova VB. 2008. Isoprene as a tool for plant protection against abiotic stresses. J Plant Interact. 3:1–15. 10.1080/17429140701858327
- Vercammen J, Baltussen E, Sandra T, David F. 2000. Considerations on static and dynamic sorptive and adsorptive sampling to monitor volatiles emitted by living plants. J High Res Chromatogra. 23:547–553. 10.1002/1521-4168(20000901)23:9%3C547::AID-JHRC547%3E3.0.CO;2-7
- Webster B, Bruce T, Dufour S, Birkemeyer C, Birkett M, Hardie J, Pickett J. 2008. Identification of volatile compounds used in host location by the black bean aphid, Aphis fabae. J Chem Ecol. 34:1153–1161. 10.1007/s10886-008-9510-7
- Wei Y, Liang Z, Cui L, Han R. 2004. Relationships between water and productivity of seabuckthorn (Hippophae) in different habitats of the Loess Plateau, China. Chin J Appl Ecol. 15:195–200.
- Yang B, Kalimo KO, Tahvonen RL, Mattila LM, Katajisto JK, Kallio HP. 2000. Effect of dietary supplementation with sea buckthorn (Hippophae rhamnoides) seed and pulp oils on the fatty acid composition of skin glycerophospholipids of patients with atopic dermatitis. J Nutr Biochem. 11:338–340. 10.1016/S0955-2863(00)00088-7
- Yang Z, Bengtsson M, Witzgall P. 2004. Host plant volatiles synergize response to sex pheromone in codling moth, Cydia pomonella. J Chem Ecol. 30:619–629. 10.1023/B:JOEC.0000018633.94002.af
- Yoneya K, Takabayashi J. 2013. Interaction, an information networks mediated by plant volatiles: a case study on willow trees. J Plant Interact. 8:197–202. 10.1080/17429145.2013.782514
- Yu H, Zhang Y, Wu K, Gao XW, Guo YY. 2008. Field-testing of synthetic herbivore-induced plant volatiles as attractants for beneficial insects. Environ Entomol. 37:1410–1415. 10.1603/0046-225X-37.6.1410
- Yue Q, Wang C, Gianfagna TJ, Meyer WA. 2001. Volatile compounds of endophyte-free and infected tall fescue (Festuca arundinacea Schreb). Phytochemistry. 58:935–941.
- Zeb A. 2004. Important therapeutic uses of sea buckthorn (Hippophae): a review. J Biol Sci. 4:687–693. 10.3923/jbs.2004.687.693
- Zhang Y, Liu J, Wang LH, Yao XJ, Zhang X, Yao L. 2010. Study on Hippophae rhamnoides Linn. Roots in improving the shear characteristics of soil. Proceedings of the 4th International Conference on Bioinformatics and Biomedical Engineering (iCBBE), June 18–20; Chengdu, China.
- Zong SX, Luo YQ, Xu ZC, Yao GL, Wang Y. 2006. Harm characteristics and population dynamics of two important borers in Hippophae rhamnoidea. For Pest Dis. 25:7–10.
- Zong SX, Luo YQ, Zhou J, Liu SJ. 2012. Volatile compounds of healthy and insect-damaged Hippophae rhamnoidessinensis in natural and planted forests. Z Naturforsch C. 67:244. 10.5560/ZNC.2012.67c0244
- Zong SX, Zhao YX, Wang ZZ, Luo YQ, Wen JB. 2013. Oviposition choice and mechanisms of Holcocerus hippophaecolus on different sea buckthorn trees. Deut Entomol Z. 60:235–240.