Abstract
To investigate the responses of castor bean to repeated drying–wetting cycles (RDWC), morpho-physiological parameters of two cultivars (Jiaxiang 2 and Hangbi 8) were determined by a pot experiment under well-watered control and RDWC. RDWC inhibited plant growth and leaf development, decreased water loss rate (WLR), and enhanced leaf mass per area (LMA) and chlorophyll content as indicated by spectral reflectance indices for both cultivars. Photosynthesis was inhibited by progressive drought stress but quickly recovered after rewatering for each cycle. Both cultivars exhibit a similar pattern of acclimation to RDWC: (1) higher LMA and lower WLR, (2) increased photosynthetic capacity under drought stress with increasing cycle numbers, (3) quick recovery and over-compensation for photosynthesis after rewatering, and (4) increased chlorophyll content. Jiaxiang 2 shows a high capacity for water preservation under drought stress and an over-compensation for photosynthesis after rewatering compared with Hangbi 8.
Introduction
The morpho-physiological responses of crops to persistent water deficit and their recovery after rewatering have been extensively reported (Lauriano et al. Citation2004; Oukarroum et al. Citation2007; Kang et al. Citation2011; Zegada-Lizarazu & Monti Citation2013). However, little information is available on the responses of plants to repeated dry periods with intermediate watering or repeated drying–wetting cycles (RDWC). In comparison with the continuous drought, RDWC is the more realistic soil water condition that crops face either in rain-fed or in irrigation agricultural areas (Galle et al. Citation2011). Understanding the morpho-physiological responses of crops to RDWC provides scientific basis and useful guidelines to farmers on how to optimize water-saving irrigation technology for sustainable crop production.
Castor bean (Ricinus communis L.) is an indeterminate, non-edible oil seed crop grown throughout the world. Castor oil is the only commercial source of ricinoleic acid (over 85% of oil) that is used for manufacturing of surfactants, coatings, greases, fungistats, pharmaceuticals, cosmetics, and a variety of other products (Pinheiro et al. Citation2008; Babita et al. Citation2010). Castor oil enjoys tremendous demand worldwide at an estimated 220,000 tons per annum, of which only 60% can be met with the current production (Babita et al. Citation2010). To satisfy the industrial demand for seeds, plantations of castor bean require expansion of marginal lands, where the plants often suffer from drought stress. Even in the major production regions (i.e. India, China, and Brazil), castor bean is almost entirely grown in arid and semi-arid tracts.
Castor bean has long been recognized to have a drought hardy nature and exhibits a high developmental and physiological plasticity to drought stress (Vijaya Kumar et al. Citation1996; Heckenberger et al. Citation1998; Schurr et al. Citation2000). With regard to photosynthetic responses, Dai et al. (Citation1992) reported that under increased vapor pressure deficits, stomatal limitation may be responsible for the inhibitory effect of drought on photosynthesis in castor bean plants. Sausen and Rosa (Citation2010) suggested that drought resistance of the castor bean could be related to a pronounced early growth response, an efficient stomatal control, and a capacity to keep high net CO2 fixation. However, little is known about the physiological responses of castor bean to RDWC. These responses are important in assessing its ability to adapt to varying low-water conditions.
Plastic responses include both inevitable effects of environmental limits on growth and physiology and adaptive adjustments that enable a given genetic individual to withstand sudden environmental changes (Sultan Citation2000). Plants respond to drought stress by altering a range of leaf characteristics, such as stomatal closure, leaf area reduction, and osmotic adjustment (Marron et al. Citation2002). The stomatal closure and the decreased leaf area limit water loss but inevitably inhibit photosynthesis. These responses may not be considered as adaptive plasticity for plants. Therefore, the question arises whether there is some compensatory mechanism in drought-tolerant plants that enables them to maintain a high photosynthesis while limiting transpiration under RDWC.
To answer this question, a pot experiment was performed on two cultivars of castor bean under two water regimes: well-watered conditions and repeated drought and wet cycles. The cultivar difference in the morpho-physiological responses of castor bean plants to RDWC and the potential of RDWC for enhancement of water productivity were also investigated.
Materials and methods
Plant materials and experimental setup
The pot experiment was conducted in a growth chamber at Huaibei Normal University, Huaibei, China. The average temperature throughout the test period was 27.2°C (daytime)/21.7°C (nighttime), and the average relative humidity was 44.3% (daytime) and 66.1% (nighttime). A blackland soil (sand:silt:clay ratio of 23.8%:20.3%:55.9%) used in this study was collected from a farmland in Huaibei, which was characterized as follows: pH, 7.08; total N, 1.02 g kg−1; available P, 14.5 mg kg−1; available K, 121.7 mg kg−1; organic matter, 28.6 g kg−1; CaCO3, 65.6 g kg−1; and electrical conductivity, 23.6 µs cm−1. The soil was air-dried and passed through a 2-mm sieve and then fertilized with 5 g kg−1 of NH4NO3 and 1 g kg−1 of KH2PO4. Measured 4.0 kg soil was placed in each pots (16 cm × 18 cm).
Two cultivars of castor bean, cv. Jiaxiang 2 and cv. Hangbi 8, were used for this study. Jiaxiang 2 has been demonstrated to be a drought-resistant cultivar (Bi Citation2009). However, the behavior of the cv. Hangbi 8 toward drought is unknown. Seeds of similar weight per cultivar were submerged in a water bath for about 24 h at room temperature and then directly sown in pots. The moisture content in the soil was maintained at 75% of water-holding capacity (WHC) using tap water. After emergence, the seedlings were thinned, and two uniform seedlings were retained at uniform spacing. When the seedlings were grown at the three-leaf stage, they were subjected to RDWC. For each cultivar, there were two water supply treatments: (1) the well-watered conditions (control) and (2) RDWC (three cycles). In total, there were four treatments (2 cultivars × 2 water supplies) in three replicates arranged in a completely randomized design. For control, the soil was maintained at 75% of WHC (17.8%) and watered daily at 6 pm to compensate for the full evapotranspiration water loss during the previous day. For RDWC, water was withheld until the leaf stomatal conductance (Gs) during mid-morning dropped below 0.05 mol m−2 s−1 (Flexas & Medrano Citation2002; Galle et al. Citation2011) and rewatered to the initial water content (75% of WHC). During the experiment, pots were randomly moved daily to minimize position effects.
Plant growth measurement
At the end of each drying–wetting cycle (DWC), leaf length and width were recorded. Seedlings were harvested at the end of experiment, and then, they were separated into roots and shoots. After oven-dried for 30 min at 105°C, plant materials were dried at 65°C to a constant weight and weighed. The root/shoot ratio was calculated as the ratio of dry root weight to dry shoot weight.
Determination of leaf area, leaf mass per area (LMA), and water loss of excised leaves
The water loss rate (WLR) of excised leaves was determined by using the method of Araghi and Assad (Citation1998) with some modification. The first fully expanded leaves from the tip were excised, and their areas and fresh weight (W0) were immediately taken. Subsequently, they were hung in a controlled environment room at 25°C and 50% relative humidity for 6 h. The leaves were weighed at 2, 4, and 6 h (W2, W4, and W6, respectively), dried in an oven at 70°C (until a constant weight was achieved), and then weighed (Wd). WLR was calculated as follows:
Leaf gas exchange and chlorophyll fluorescence
During the experimental period, net photosynthetic rate (Pn), Gs, intercellular CO2 concentrations (Ci), and transpiration rate (E) were measured daily on the first fully expanded upper canopy leaves from 10 am to 12 pm with the LiCor-6400 photosynthesis system (LiCor Inc., Lincoln, NE, USA) and a LED light source (6400-02). The light intensity, leaf temperature, and CO2 concentration inside the leaf chamber were maintained at 1000 µmol m−2 s−1, 28 ± 0.8°C, and 380 ± 5 µmol CO2 mol−1, respectively.
The chlorophyll fluorescence parameters of the first fully expanded leaves from the tip were measured every day using the Mini PAM (Walz, Effeltrich, Germany). After dark-adapted for 30 min, the leaves were used to record the minimum (F0) and maximum fluorescence (Fm), the variable to maximum fluorescence ratio (Fv/Fm) were calculated. The effective quantum yield of PSII (ΦPSII) was monitored under ambient irradiation (500 µmol quanta m−2 s−1). The intensity of the light pulse for Fm and ′ measurements was 5000 µmol quanta m−2 s−1 (Liu et al. Citation2011).
Spectral reflectance determination
The spectral features of the adaxial leaf surface were measured every day using a UNIspec spectral analysis system (PP Systems, Haverhill, MA, USA) according to the method described by Poulos et al. (Citation2007). Three indices based on the spectral reflectance (R) at a particular wavelength were calculated as follows: (1) the structure independent pigment index (SIPI) was calculated as SIPI = (R800 − R445)/(R800 − R680) (Penuelas et al. Citation1995), (2) the normalized difference vegetation index (chlNDI) as chlNDI = (R705 − R445)/(R750 + R445) (Datt Citation1999), and (3) the modified red edge simple ratio index (mSR705) as mSR705 = (R705 − R445)/(R750 − R445) (Datt Citation1999).
Statistical analysis
Analyses were carried out in SPSS for Windows (Version 13.0; SPSS, Inc., Chicago, IL, USA). The one-time measured data were subject to analysis of variance (ANOVA). For the repeated measured data, we first conducted a repeated-measures ANOVA, with the days after treatment as the within-subjects factor and cultivars and water supply as the between-subjects factors. Statistical significance of the means was compared using Duncan's multiple range test at the 5% probability level.
Results and discussion
Effects of RDWC on plant growth and leaf morphology in two cultivars of castor bean
Results revealed that, morphologically, the castor bean showed a high plasticity to RDWC in both plant growth and leaf development ( and ; ). At the whole-plant level, RDWC inhibited plant growth as indicated by a decrease in root and shoot biomass (). At the leaf level, RDWC decreased leaf length (), leaf width (), and leaf area but enhanced LMA (). Less and smaller leaves with high LMA decreased evaporative water losses, thus enhancing water availability.
Table 1. Biomass of roots and shoots, and root/shoot ratio in two cultivars of castor bean under well-watered condition (control) and RDWC.
Table 2. Leaf area (LA), LMA, and WLR of excised leaves in two cultivars of castor bean under well-watered condition (control) and RDWC.
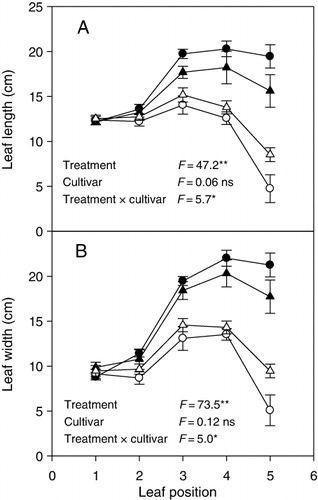
Although the responses of most morphological traits to RDWC were similar to those results obtained from continuous drought experiments (Jaleel et al. Citation2009), the root/shoot ratio was not affected by RDWC for both cultivars (). The result disagrees with the common knowledge that drought induces an increase in root/shoot ratio in various plant species (Xu et al. Citation2010).
In the case of cultivars, no significant difference was observed for root and shoot biomass, root/shoot ratio, leaf area and LMA between Hangbi 8 and Jiaxiang 2 ( and ). However, there were significant interactive effects of treatment and cultivar on leaf length (p < 0.05) and width (p < 0.05), indicating RDWC-induced inhibition in leaf development was more pronounced in Hangbi 8 than in Jiaxiang 2 ().
Effects of RDWC on WLR in two cultivars of castor bean
Low WLR is indicative of drought resistance, and its assessment has shown promise for characterizing the drought resistance of wheat genotypes (Araghi & Assad Citation1998; Dhanda & Sethi Citation1998). In the present study, although the final WLR decreased by RDWC for both cultivars, the reduction was more notable in Jiaxiang 2 than in Hangbi 8 (). This observation suggests that RDWC may induce physiological acclimation in plants of Jiaxiang 2, enabling them to prevent water loss under drought stress.
Effects of RDWC on gas exchange in two cultivars of castor bean
The data of Pn, Gs, Ci, and E in stressed plants were expressed as a percentage of the corresponding control values to reveal the patterns and motifs of gas exchange responses to RDWC (). All parameters of gas exchange exhibited large plasticity under RDWC treatment, showing a gradual decrease under progressive drought stress and then a quick recovery after rewatering for each cycle (–). The simultaneous change in Pn, Gs, and Ci, along with RDWC, may indicate that stomatal closure was the main factor that limited the photosynthetic activity under water deficit. However, under drought stress, the minimal Pn was averaged at 38% and 25% of the corresponding control for Hangbi 8 and Jiaxiang 2, respectively, and the minimal Ci was averaged at 84% and 86% of the control for Hangbi 8 and Jiaxiang 2, respectively ( and ). These results suggest that both stomatal and non-stomatal limitation are involved in the response of castor bean to RDWC. Previous studies have demonstrated that stomatal limitations could largely decrease photosynthesis under mild to moderate drought stress, whereas non-stomatal limitation could account for a larger part under more severe drought (Chaves et al. Citation2003; Flexas et al. Citation2004; Grassi & Magnani Citation2005; Flexas et al. Citation2009; Signarbieux & Feller Citation2011).
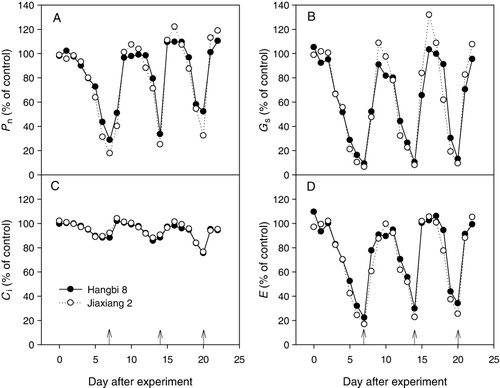
Interestingly, the minimal Pn under drought stress for each cycle increased when the number of RDWC increased, and this was accompanied by a decline in Ci ( and ). The reverse changes in Pn and Ci suggest that the increased Pn under drought stress may result from the elevated CO2 fixation, which may be a mechanism for castor plants to compensate for the inhibition in photosynthesis induced by stomatal closure. Cramer et al. (Citation2007) found that during the acclimation to water stress, some genes encoding Calvin cycle enzymes, including Rubisco activase, and PSI- and PSII-related genes are conversely up-regulated.
In general, plants subjected to mild stress recover fast (within 1 or 2 days) after stress is alleviated (Chaves et al. Citation2009). However, under severe stress, only 40–60% of the maximum photosynthesis rate was recovered during the day after rewatering, and although recovery continued during the next days, the maximum photosynthesis rates never recovered (Chaves et al. Citation2009). In the present study, Pn was recovered to 51% and 40% of the control for Hangbi 8 and Jiaxiang 2, respectively, during the day after rewatering in the first cycle. The recovery continued to 96% and 101% of the control for Hangbi 8 and Jiaxiang 2, respectively, during the next days (). Furthermore, during the last two cycles, Pn recovered immediately to the control values for both cultivars during the day after rewatering (). These results disagree with the previous observations (Chaves et al. Citation2009), as a quick and complete photosynthetic recovery was observed after rewatering. This recovery may be another acclimation mechanism to RDWC in castor bean.
The over-compensation for plant growth upon rewatering after the drought has been confirmed by many experimental investigations (Liu et al. Citation2001; Siopongco et al. Citation2006). In the present study, Pn was higher in the RDWC treatment than in the control for both cultivars after rewatering. The increasing DWCs increased the maximal values of Pn after rewatering, whereas the maximal values of Ci decreased ( and ). These data indicate that over-compensation for photosynthesis occurs upon rewatering after drought. Despite this phenomenon, the final biomass and leaf size in stressed plants for both cultivars were considerably lower than those of the control ( and ), which could be caused by the short time for plant recovery compared with that in other studies (Liu et al. Citation2001; Siopongco et al. Citation2006). Similarly, Xu and Zhou (Citation2007) and Xu et al. (Citation2009) found that the final biomass or leaf area in plants subjected to long-term or severe drought could not reach the level of the control treatment. Whether or not plant growth completely recovers following rewatering may depend on the pre-drought intensity or duration, the frequency of DWCs, and the time for recovery.
Results of the repeated measures ANOVA revealed that there were generally significant difference in Pn (F = 9.807, p < 0.05) and Gs (F = 5.955, p < 0.05) between the cultivars of castor bean, whereas no significant difference was observed in Ci (F = 1.483, p = 0.258) and E (F = 4.407, p = 0.069). Interactions of cultivar and water regimes on gas exchange were not significant (p > 0.05). Overall, Hangbi 8 showed a higher Pn and Gs than Jiaxiang 2. However, Jiaxiang 2 exhibited a slightly greater plasticity in Pn and Gs than Hangbi 8. Under drought stress, the minimal Pn and E were lower in Jiaxiang 2 than those in Hangbi 8, implying that Jiaxiang 2 has a higher capacity for water preservation under drought stress at the cost of loss of photosynthesis. However, after rewatering, Jiaxiang 2 showed a higher Pn, Gs, and E than Hangbi 8 in terms of the percentage of control values, suggesting that photosynthetic compensation after rewatering was more efficient in Jiaxiang 2.
Effects of RDWC on chlorophyll a fluorescence in two cultivars of castor bean
Chlorophyll a fluorescence as a non-invasive measurement of photosynthesis reflects plant performance in response to drought. Results reported in previous studies demonstrate that chlorophyll fluorescence parameters, such as Fv/Fm and ΦPSII, exhibit a smaller plasticity in response to water deficit. In C3 plants, both Fv/Fm and ΦPSII are usually unaffected by mild drought stress (Dias & Brüggemann Citation2010). Moreover, some species have been reported to die under intense drought with unaltered Fv/Fm (Morales et al. Citation2006; Peguero-Pina et al. Citation2009; Vilagrosa et al. Citation2010). The results of repeated measures ANOVA indicated that Fv/Fm was similar between the two cultivars (F = 0.602, p = 0.447), while it shows a slight but significant increase (F = 11.2, p < 0.01) in RDWC treatment compared with the control ( and ). The Fv/Fm ratio indicates the potential photochemical efficiency of PSII, and the increased Fv/Fm in RDWC treatment with respect to control suggests that the photosynthetic electron transport was not blocked by drought.
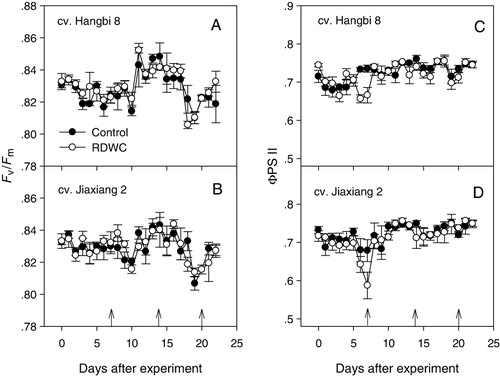
By contrast, ΦPSII decreased under severe drought stress during the first DWC for both cultivars ( and ). The reduction in ΦPSII indicates a decrease in capacity for carbon metabolism or a low utilization of ATP and NADPH in a dark phase of photosynthesis. The stronger decrease in ΦPSII than in Fv/Fm indicates the presence of a second inhibition target besides PSII, such as the inhibition of electron transfer after PSII or the inhibition of the water-splitting complex, as postulated earlier (Liu et al. Citation2011). Nevertheless, during the second and third DWCs, no significant difference was observed in ΦPSII between the RDWC and the control treatment for both cultivars. Unaltered ΦPSII between treatment (F = 2.304, p = 0.145) was also demonstrated by the result of repeated measures ANOVA.
Effects of RDWC on spectral reflectance in two cultivars of castor bean
Photosynthetic pigments provide valuable information about the physiological response to environmental stress, including water deficit. The reflectance spectra technique has been extensively used to estimate pigment contents in many plants (Carter & Knapp Citation2001; Thorhaug et al. Citation2006; Poulos et al. Citation2007; Liu et al. Citation2011) because it is an accurate, quick, and non-destructive tool that can measure in situ. In the present study, the results of repeated measures ANOVA showed that RDWC significantly increased the mSR705 (F = 438.2, p < 0.001) and chlNDI (F = 403.6, p < 0.001) for both cultivars of the castor bean, but SIPI (F = 0.769, p = 0.384) remained unaffected (). The two cultivars differed from each other in mSR705 (F = 9.133, p < 0.01) and SIPI (F = 40.1, p < 0.001), while in chlNDI (F = 3.453, p = 0.067), no significant difference was detected (). Given that the mSR705 is generally considered an effective index in describing changes in chlorophyll content and chlNDI is usually correlated with chlorophyll a concentration (Sims & Gamon Citation2002; Su et al. Citation2013), these results indicate that RDWC can enhance the leaf chlorophyll content of castor bean plants. Considering that SIPI is positively correlated with the carotenoid/chlorophyll a ratio (Penuelas et al. Citation1995), the unchanged SIPI indicates that the pigment composition never changed by RDWC, even if the chlorophyll increased.
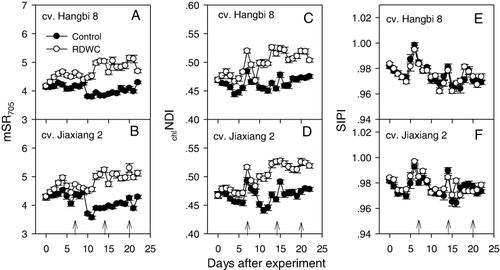
The effects of water stress on the pigment contents are highly species dependent (Morales et al. Citation2006). Most previous observations indicate that drought induces the reduction in pigment contents and the alteration of their composition (Jaleel et al. Citation2009; França et al. Citation2012; Batra et al. Citation2014; Maksup et al. Citation2014). However, some crop species, such as barley, coffee, grapevine, and others, maintain a high leaf chlorophyll content under water stress, despite having a decreased photosynthetic capacity (Morales et al. Citation2006). Although reduced photosynthetic pigments may decrease the light harvesting, thus preventing the accumulation of potentially harmful photosynthetic products that may be harmful to PSII (França et al. Citation2012). Therefore, increased chlorophyll contents, as indicated by mSR705 and chlNDI, seem to suggest that additional photoprotective mechanisms may exist in castor bean plants under RDWC.
Conclusion
Castor beans show various responses to RDWC and develop a wide range of acclimation mechanisms from the morphological to the physiological aspects. RDWC considerably suppressed plant growth and leaf development, decreased WLR, and enhanced LMA, chlorophyll content (mSR705 and chlNDI), and Fv/Fm. The root/shoot ratio, SIPI, and ΦPSII were not affected by RDWC. Photosynthesis was inhibited by progressive drought stress but quickly recovered after rewatering for each cycle. The acclimation of castor bean to RDWC has the following features: (1) higher LMA and lower WLR, (2) increased minimal Pn (drought period) when cycle numbers increased, (3) quick recovery and over-compensation for photosynthesis after rewatering, and (4) increased chlorophyll content as indicated by mSR705 and chlNDI. Two cultivars differ from each other in their response to RDWC. Jiaxiang 2 shows a high capacity for water preservation under drought stress and an over-compensation for photosynthesis after rewatering compared with Hangbi 8.
Acknowledgments
The authors are thankful to the Opening Foundation of the State Key Laboratory of Soil Erosion and Dryland Farming on the Loess Plateau for providing financial assistance to the work through the research group project No. 10501-277.
References
- Araghi SG, Assad MT. 1998. Evaluation of four screening techniques for drought resistance and their relationship to yield reduction ratio in wheat. Euphytica. 103:293–299. 10.1023/A:1018307111569
- Babita M, Maheswari M, Rao LM, Shanker AK, Rao DG. 2010. Osmotic adjustment, drought tolerance and yield in castor (Ricinus communis L.) hybrids. Environ Exp Bot. 69:243–249. 10.1016/j.envexpbot.2010.05.006
- Batra NG, Sharma V, Kumari N. 2014. Drought-induced changes in chlorophyll fluorescence, photosynthetic pigments and thylakoid membrane proteins of Vigna radiata. J Plant Interact. 9:712–721. 10.1080/17429145.2014.905801
- Bi T. 2009. RAPD analysis of the different castor plants and characteristics of their anti-drought capacity [dissertation]. Tianjin: Tianjin University of Science and Technology.
- Carter GA, Knapp AK. 2001. Leaf optical properties in higher plants: linking spectral characteristics to stress and chlorophyll concentration. Am J Bot. 88:677–684. 10.2307/2657068
- Chaves MM, Flexas J, Pinheiro C. 2009. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot. 103:551–560. 10.1093/aob/mcn125
- Chaves MM, Maroco JP, Pereira JS. 2003. Understanding plant responses to drought-from genes to the whole plant. Funct Plant Biol. 30:239–264. 10.1071/FP02076
- Cramer GR, Ergül A, Grimplet J, Tillett RL, Tattersall EA, Bohlman MC, Vincent D, Sonderegger J, Evans J, Osborne C. 2007. Water and salinity stress in grapevines: early and late changes in transcript and metabolite profiles. Funct Integr Genomics. 7:111–134. 10.1007/s10142-006-0039-y
- Dai Z, Edwards GE, Ku MSB. 1992. Control of photosynthesis and stomatal conductance in Ricinus communis L. (castor bean) by leaf to air vapor pressure deficit. Plant Physiol. 99:1426–1434. 10.1104/pp.99.4.1426
- Datt B. 1999. A new reflectance index for remote sensing of chlorophyll content in higher plants: tests using Eucalyptus leaves. J Plant Physiol. 154:30–36. 10.1016/S0176-1617(99)80314-9
- Dhanda SS, Sethi GS. 1998. Inheritance of excised-leaf water loss and relative water content in bread wheat (Triticum aestivum). Euphytica. 104:39–47. 10.1023/A:1018644113378
- Dias MC, Brüggemann W. 2010. Limitations of photosynthesis in Phaseolus vulgaris under drought stress: gas exchange, chlorophyll fluorescence and Calvin cycle enzymes. Photosynthetica. 48:96–102. 10.1007/s11099-010-0013-8
- Flexas J, Barón M, Bota J, Ducruet JM, Gallé A, Galmés J, Jiménez M, Pou A, Ribas-Carbó M, Sajnani C. 2009. Photosynthesis limitations during water stress acclimation and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri × V. rupestris). J Exp Bot. 60:2361–2377. 10.1093/jxb/erp069
- Flexas J, Bota J, Loreto F, Cornic G, Sharkey TD. 2004. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 6:269–279. 10.1055/s-2004-820867
- Flexas J, Medrano H. 2002. Drought-inhibition of photosynthesis in C3 plants: stomatal and non-stomatal limitations revisited. Ann Bot. 89:183–189. 10.1093/aob/mcf027
- França MGC, Prados LMZ, de Lemos-Filho JP, Ranieri BD, Vale FHA. 2012. Morphophysiological differences in leaves of lavoisiera campos-portoana (Melastomataceae) enhance higher drought tolerance in water shortage events. J Plant Res. 125:85–92. 10.1007/s10265-011-0416-z
- Galle A, Florez-Sarasa I, El Aououad H, Flexas J. 2011. The mediterranean evergreen quercus ilex and the semi-deciduous Cistus albidus differ in their leaf gas exchange regulation and acclimation to repeated drought and re-watering cycles. J Exp Bot. 62:5207–5216. 10.1093/jxb/err233
- Grassi G, Magnani F. 2005. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ. 28:834–849. 10.1111/j.1365-3040.2005.01333.x
- Heckenberger U, Roggatz U, Schurr U. 1998. Effect of drought stress on the cytological status in Ricinus communis. J Exp Bot. 49:181–189. 10.1093/jxb/49.319.181
- Jaleel CA, Manivannan P, Wahid A, Farooq M, Al-Juburi HJ, Somasundaram R, Panneerselvam R. 2009. Drought stress in plants: a review on morphological characteristics and pigments composition. Int J Agric Biol. 11:100–105.
- Kang Y, Han Y, Torres-Jerez I, Wang M, Tang Y, Monteros M, Udvardi M. 2011. System responses to long-term drought and re-watering of two contrasting alfalfa varieties. Plant J. 68:871–889. 10.1111/j.1365-313X.2011.04738.x
- Lauriano JA, Ramalho JC, Lidon FC, do Céu Matos M. 2004. Peanut photosynthesis under drought and re-watering. Photosynthetica. 42:37–41. 10.1023/B:PHOT.0000040567.42444.c2
- Liu C, Guo J, Cui Y, Lü T, Zhang X, Shi G. 2011. Effects of cadmium and salicylic acid on growth, spectral reflectance and photosynthesis of castor bean seedlings. Plant Soil. 344:131–141. 10.1007/s11104-011-0733-y
- Liu XY, Luo YP, Shi YC. 2001. The stimulating effects of rewatering in subjecting to water stress on leaf area of winter wheat. Sci Agric Sin. 34:422–428.
- Maksup S, Roytrakul S, Supaibulwatana K. 2014. Physiological and comparative proteomic analyses of Thai jasmine rice and two check cultivars in response to drought stress. J Plant Interact. 9:43–55. 10.1080/17429145.2012.752042
- Marron N, Delay D, Petit J, Dreyer E, Kahlem G, Delmotte FM, Brignolas F. 2002. Physiological traits of two Populus × euramericana clones, Luisa Avanzo and Dorskamp, during a water stress and re-watering cycle. Tree Physiol. 22:849–858. 10.1093/treephys/22.12.849
- Morales F, Abadía A, Abadía J. 2006. Photoinhibition and photoprotection under nutrient deficiencies, drought and salinity. In: Demmig-Adams B, Adams WW III, Mattoo AK, editors. Photoprotection, photoinhibition, gene regulation, and environment. Dordrecht: Springer; p. 65–85.
- Oukarroum A, Madidi SE, Schansker G, Strasser RJ. 2007. Probing the responses of barley cultivars (Hordeum vulgare L.) by chlorophyll a fluorescence OLKJIP under drought stress and re-watering. Environ Exp Bot. 60:438–446. 10.1016/j.envexpbot.2007.01.002
- Peguero-Pina JJ, Sancho-Knapik D, Morales F, Flexas J, Gil-Pelegrín E. 2009. Differential photosynthetic performance and photoprotection mechanisms of three mediterranean evergreen oaks under severe drought stress. Funct Plant Biol. 36:453–462. 10.1071/FP08297
- Penuelas J, Baret F, Filella I. 1995. Semi-empirical indices to assess carotenoids/chlorophyll a ratio from leaf spectral reflectance. Photosynthetica. 31:221–230.
- Pinheiro HA, Silva JV, Endres L, Ferreira VM, Câmara CA, Cabral FF, Oliveira JF, Carvalho LWT, Santos JM. 2008. Leaf gas exchange, chloroplastic pigments and dry matter accumulation in castor bean (Ricinus communis L) seedlings subjected to salt stress conditions. Indust Crops Prod. 27:385–392. 10.1016/j.indcrop.2007.10.003
- Poulos HM, Goodale UM, Berlyn GP. 2007. Drought response of two Mexican oak species, Quercus laceyi and Q. sideroxyla (Fagaceae), in relation to elevational position. Am J Bot. 94:809–818. 10.3732/ajb.94.5.809
- Sausen TL, Rosa LMG. 2010. Growth and limitations to carbon assimilation in Ricinus communis (Euphorbiaceae) under soil water stress conditions. Acta Bot Brasilica. 24:648–654. 10.1590/S0102-33062010000300008
- Schurr U, Heckenberger U, Herdel K, Walter A, Feil R. 2000. Leaf development in Ricinus communis during drought stress: dynamics of growth processes, of cellular structure and of sink-source transition. J Exp Bot. 51:1515–1529. 10.1093/jexbot/51.350.1515
- Signarbieux C, Feller U. 2011. Non-stomatal limitations of photosynthesis in grassland species under artificial drought in the field. Environ Exp Bot. 71:192–197. 10.1016/j.envexpbot.2010.12.003
- Sims DA, Gamon JA. 2002. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens Environ. 81:337–354. 10.1016/S0034-4257(02)00010-X
- Siopongco JDLC, Yamauchi A, Salekdeh H, Bennett J, Wade LJ. 2006. Growth and water use response of doubled-haploid rice lines to drought and rewatering during the vegetative stage. Plant Prod Sci. 9:141–151. 10.1626/pps.9.141
- Su Y, Wang X, Liu C, Shi G. 2013. Variation in cadmium accumulation and translocation among peanut cultivars as affected by iron deficiency. Plant Soil. 363:201–213. 10.1007/s11104-012-1310-8
- Sultan SE. 2000. Phenotypic plasticity for plant development, function and life history. Trends Plant Sci. 5:537–542. 10.1016/S1360-1385(00)01797-0
- Thorhaug A, Richardson AD, Berlyn GP. 2006. Spectral reflectance of Thalassia testudinum (Hydrocharitaceae) seagrass: low salinity effects. Am J Bot. 93:110–117. 10.3732/ajb.93.1.110
- Vijaya Kumar P, Srivastava NN, Victor US, Gangadhar Rao D, Subba Rao AVM, Ramakrishna YS, Ramana Rao BV. 1996. Radiation and water use efficiencies of rainfed castor beans (Ricinus communis L.) in relation to different weather parameters. Agric For Meteor. 81:241–253. 10.1016/0168-1923(95)02309-7
- Vilagrosa A, Morales F, Abadía A, Bellot J, Cochard H, Gil-Pelegrín E. 2010. Are symplast tolerance to intense drought conditions and xylem vulnerability to cavitation coordinated? An integrated analysis of photosynthetic, hydraulic and leaf level processes in two Mediterranean drought-resistant species. Environ Exp Bot. 69:233–242. 10.1016/j.envexpbot.2010.04.013
- Xu ZZ, Zhou GS. 2007. Photosynthetic recovery of a perennial grass Leymus chinensis after different periods of soil drought. Plant Prod Sci. 10:277–285. 10.1626/pps.10.277
- Xu Z, Zhou G, Shimizu H. 2009. Are plant growth and photosynthesis limited by pre-drought following rewatering in grass? J Exp Bot. 60:3737–3749. 10.1093/jxb/erp216
- Xu Z, Zhou G, Shimizu H. 2010. Plant responses to drought and rewatering. Plant Signal Behav. 5:649–654. 10.4161/psb.5.6.11398
- Zegada-Lizarazu W, Monti A. 2013. Photosynthetic response of sweet sorghum to drought and re-watering at different growth stages. Physiol Plant. 149:56–66. 10.1111/ppl.12016
