Abstract
The mechanism of growth amelioration in salt-stressed pennyroyal (Mentha pulegium L.) was investigated by exogenous application of penconazole (PEN). Seven weeks after sowing, seedlings were treated with increasing NaCl concentrations (0, 25, 50, and 75 mM) with or without PEN (15 mg l−1) and were harvested randomly at different times. Results showed that some growth parameters and the relative water content (RWC) decreased under salt stress, while lipid peroxidation, H2O2 content, activities of superoxide dismutase (SOD; EC 1.15.1.1), peroxidase (POX; EC 1.11.1.7), polyphenol oxidase (PPO; EC 1.10.3.1), catalase (CAT; EC 1.11.1.6), and ascorbate peroxidase (APX; EC 1.11.1.1) remarkably increased. Exogenous application of PEN increased some growth parameters, RWC, antioxidant enzyme activities, and H2O2 content, but the effects of PEN were more significant under salt stress conditions. PEN treatment also decreased lipid peroxidation. These results suggest that PEN-induced tolerance to salt stress in M. pulegium plants may be related to regulation of antioxidative responses and H2O2 level.
Introduction
Salinity is an important environmental stress that in plants imposes both ionic toxicity and osmotic stress, leading to the reduction of plant growth and crop production (Munns et al. Citation2006; Bano et al. Citation2014). Pennyroyal (Mentha pulegium L.) is an aromatic and medicinal plants belonging to the Lamiaceae family present in the humid regions of Iran. It is widely used in traditional medicine, food processing, perfumery, and pharmaceutical products because of the high quality of its essential oil (Shirazi et al. Citation2004, Omran et al. Citation2012; Soares et al. Citation2012). Like most of the cultivated plants, growth and yield of this plant can be affected under salt stress.
One of the plant responses to environment stresses is the rapid and increased generation of reactive oxygen species (ROS), including hydrogen peroxide (H2O2), superoxide anion (O2−) and hydroxyl radicals (OH−), which can damage membrane lipids, proteins, and nucleic acids (Liang et al. Citation2003; Patade et al. Citation2011). Different plants develop several protection mechanisms to eliminate ROS and prevent oxidative damage. Among of them, enzymatic antioxidants such as superoxide dismutase (SOD; EC 1.15.1.1), catalase (CAT; EC 1.11.1.6), peroxidase (POX; EC 1.11.1.7), and ascorbate peroxidase (APX; EC 1.11.1.1) provide the first line of defense against ROS (Demiral & Turkan Citation2004; Jebara et al. Citation2005).
Manipulation of crop production with chemicals is one of the most important advancement achieved in agriculture. The medicinal plant yield under stress can be increased by application of plant growth regulators (PGRs). Penconazole (PEN) [1-(2,4-dichloro-β-propylphenethyl)-1 H-1,2,4-triazole] is a triazolic fungicide which has PGR properties (Fletcher et al. Citation2000). Triazole compounds induce a variety of morphological and biochemical responses in plants including stimulation of root growth, reduction in free-radical damage, and increase in antioxidant potential (Jaleel, Gopi, Manivannan & Panneerselvam Citation2007; Manivannan et al. Citation2007). These qualities make them ideal to increase resistance to salt stress in medicinal plants. Our previous work showed that PEN minimizes the negative effects of drought stress with lower membrane damage and could be used for partial amelioration of drought stress in M. pulegium plants (Hassanpour et al. Citation2012). The effect of salinity on antioxidative responses of M. pulegium plants was previously studied by Oueslati et al. (Citation2010). The aim of the present study was to investigate the effect of salt stress on some physiological and biochemical parameters and to assess the possibility of improving salt tolerance of M. pulegium by application of PEN.
Materials and methods
Plant material and treatments
Seeds of M. pulegium were collected during summer 2012 from Chalous (Province of Mazandaran, Iran). Seeds were sown in Tref peat in a greenhouse with a 16 h light/8 h dark photoperiod at 25/18°C day/night temperatures and 50% air humidity. Plastic pots were filled with perlite and seedlings were thinned to five per pot 7 weeks after sowing. Plants were irrigated with complete Hoagland solution. Pots were divided in eight groups and treated with different salinity concentration and the PEN treatment. Seedlings were treated with 0, 25, 50, and 75 mM NaCl with or without PEN (15 mg l−1). PEN was applied uniformly to the plants as a fine spray using an atomizer once a week and the optimum concentration was determined according to Hassanpour et al. (Citation2012). Seedlings were uprooted during four harvest times (10, 20, 30, and 40 days after treatments) and four plants per treatment were used for analyses in all the experiments.
Growth parameters
Plants were evaluated after four interval harvests of salt stress in terms of fresh weight (FW) and dry weight (DW). Relative water content (RWC) of leaves was estimated according to Wheatherley (Citation1973). Saturated weight (SW) of the plant was determined by keeping them in de-ionized water at 4°C in the dark for 24 h, and DW was obtained after oven drying at 45°C for 72 h, the time point at which a constant weight was reached.
Lipid peroxidation and H2O2 content
Lipid peroxidation was determined by measuring malondialdehyde (MDA) content (Heath & Packer Citation1968). Plant material (0.25 g) from leaf, shoot, and root samples was homogenized with 2.5 ml of 0.1% trichloroacetic acid (TCA) and the homogenate was centrifuged at 10,000×g for 10 min. For every 1 ml of the aliquot of the supernatant, 4 ml of 20% TCA containing 0.5% thiobarbituric acid (TBA) was supplemented. The mixture was heated at 95°C for 30 min and quickly chilled in an ice bath. Afterwards, the mixture was centrifuged at 10,000g for 15 min and the absorbance of the supernatant was evaluated at 532 and 600 nm. The concentration of MDA was estimated by using an extinction coefficient of 155 mM−1.
Hydrogen peroxide levels were assayed according to Velikova et al. (Citation2000). Plant materials (0.5 g) were homogenized with 5 ml of 0.1% (w/v) TCA in an ice bath. The homogenate was centrifuged at 12,000×g for 15 min, and 0.5 ml of the extract added to 0.5 ml of 10 mM potassium phosphate buffer (pH 7.0) and 1 ml of 1 M KI. Absorbance of the supernatant was estimated at 390 nm, and H2O2 content was calculated using a standard curve.
Extraction and estimation of antioxidant enzymes
For determination of enzyme activities, leaves, shoots, and roots of plants were homogenized at 4°C with a mortar in1 M Tris-HCl (pH 6.8) at 4°C with a mortar in 1 M Tris-HCl (pH 6.8) and 2% (w/v) polyvinyl polypyrrolidone to avoid phenol oxidative effects. The homogenate was centrifuged in a refrigerated centrifuge at 13,000×g for 30 min, and supernatant was utilized for total protein content assessment and enzyme assays (Bradford Citation1976).
SOD activity was estimated by measuring its ability to inhibit the photochemical reduction of nitroblue tetrazolium chloride (NBT) at 560 nm, as regarded by Giannopolitis and Ries (Citation1977) in a reaction mixture (1.5 mL) containing of 50 mM sodium phosphate buffer (pH 7.5), 0.1 µM EDTA, 13 mM L-methionine, 75 µM NBT, 75 µM riboflavin, and 30 µL enzyme extract. Riboflavin was added last and tubes were shaken and illuminated for 15 min, and absorbance of the reaction mixture was recorded at 560 nm against the non-illuminated blank. One unit of SOD activity was determined as the amount of enzyme that inhibits 50% NBT photoreduction.
POX activity was measured according to Abeles and Biles (Citation1991). The reaction mixture consisted 4 ml of 0.2 M acetate buffer (pH 4.8), 0.4 ml H2O2 (3%), 0.2 ml of 20 mM benzidine, and 0.03 ml enzyme extract. Alteration in absorbance of the reaction solution was measured at 530 nm. The POD activity was determined as 1 µM of benzidine oxidized per min per mg protein [unit mg−1 (protein)].
PPO activity was determined at 40°C by the method of Raymond et al. (Citation1993) based on the oxidization of pyrogallol. The reaction mixture contained 2.5 ml of 0.2 M sodium phosphate buffer (pH 6.8), 0.2 ml pyrogallol 20 mM, and 0.03 ml enzyme extract. The increase in absorbance was recorded at 430 nm. The PPO activity was determined as µM of pyrogallol oxidized per min per mg protein [unit mg−1 (protein)].
APX activity was measured according to Jebara et al. (Citation2005). The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.0), 0.5 mM ascorbic acid, 0.1 mM hydrogen peroxide, and 10 µl of enzyme extract in a total volume of 1 ml. The concentration of oxidized ascorbate was determined by the decrease in absorbance at 290 nm. The concentration of oxidized ascorbate was calculated by using extinction coefficient (ε = 2.8 mM−1 cm−1). One unit of APX was defined as 1 µM oxidized ascorbate per min per mg protein [unit mg−1 (protein)].
Total CAT activities were estimated according to Aebi (Citation1984), which measures the initial rate of disappearance of H2O2 at 240 nm. The reaction mixture consisted 0.625 ml of 50 mM sodium phosphate buffer (pH 7.0) with 75 µl H2O2 (3%) and 5 µl enzyme extract. The enzyme activity was defined as 1 µM of H2O2 oxidized per min per mg protein [unit mg−1 (protein)].
Statistical analysis
The experiments were carried out in completely randomized block design. Data were analyzed by one-way analysis of variance (ANOVA) using SPSS (version 19). The mean differences were compared by the lowest standard deviations (LSD) test. Each data point was the mean of four replicates (n = 4) in each group and P values ≤0.05 were considered as significant.
Results
Root and shoot growth was followed by measuring FW and DW on four interval harvest times. Under salt stress, growth parameters significantly decreased in M. pulegium with the increasing salinity concentration. There was a significant effect of harvest time on growth parameters ( and ). PEN treatment in salt-stressed and unstressed plants decreased the negative effect of salt stress on growth parameters, especially at 75 mM NaCl. PEN treatment significantly increased root growth (FW and DW) especially on long-term expose to salinity as compared to plants without PEN (P ≤ 0.05). At 50 and 75 mM NaCl with PEN, root FW increased 23.8% and 35.8%, and root DW increased 24% and 47.8% on day 40 as compared to without PEN, respectively (). It was found that PEN treatment had more effect on stress tolerance at severe stress conditions.
Table 1. Results of two-way analysis of variance (ANOVA) of PEN, NaCl, harvest time (Day) and their interaction (PEN × NaCl × Day) for growth parameters, RWC, H2O2 content, MDA level and SOD, POD, PPO, CAT, and APX activities in M. pulegium.
Table 2. Effects of NaCl and PEN treatments during four interval harvest times (10, 20, 30, and 40 days) on growth parameters and RWC content in M. pulegium.
The RWC content significantly decreased under different salinity conditions (). At 50 and 75 mM NaCl, RWC content decreased at all harvest times and the lower RWC content was observed with increasing harvest days. PEN treatment in salt-stressed and unstressed plants significantly increased RWC content, while the effects of PEN were more pronounced in salt-stressed plants. At 50 and 75 mM NaCl with PEN, RWC increased 18.9% and 20.9% on day 30, and 25.6% and 30.2% on day 40 with higher value than without PEN (). There was a significant interaction among salinity, PEN, and harvest time for RWC content ().
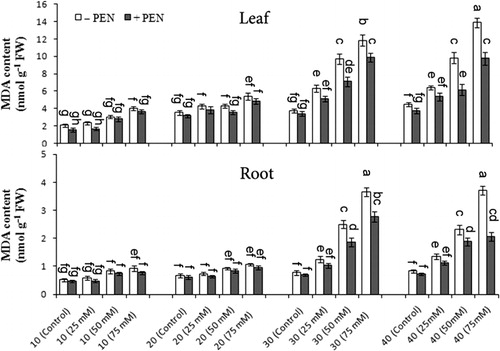
MDA content significantly increased under different salinity especially at 50 and 75 mM NaCl as compared to control, and the increase level was higher on day 40 than the other harvest days (). PEN treatment in salt-stressed and unstressed plants significantly decreased MDA content in both organs, and the effect of PEN was more pronounced in PEN-treated plants. At 75 mM NaCl and control with PEN, MDA content decreased 29.7% and 16.5% in leaves, and 44.3% and 12.04% in roots on day 40 as compared to without PEN, respectively. There was a significant interaction among salinity, PEN, and harvest time for MDA content in both the organs ().
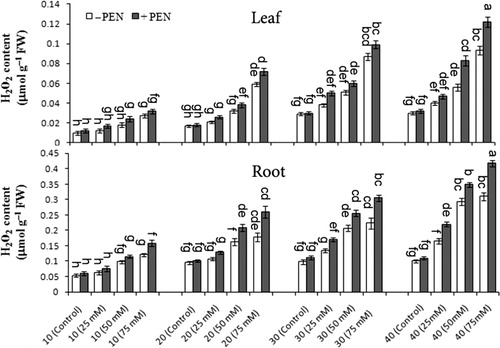
For determination of ROS scavenging capacity, H2O2 levels of leaf and root organs were estimated under stress conditions. The H2O2 level was higher in roots than leaves, and significantly increased at 50 and 75 mM NaCl as compared to controls especially on day 40 (). There was a significant effect of harvest time on H2O2 levels (). Under salt stress, H2O2 level significantly increased especially on day 40 at 75 mM NaCl in M. pulegium. PEN treatment to salt-stressed and unstressed plants increased the H2O2 level in leaves and roots organs, while the effect of PEN was only significant in leaves. At 75 mM NaCl and control with PEN, H2O2 level increased 31.2% and 6.7% in leaves and 34.8% and 8.9% in roots on day 40 with higher values than those obtained without PEN, respectively (). There was no significant interaction among salinity, PEN, and harvest time for H2O2 level in both organs of M. pulegium ().
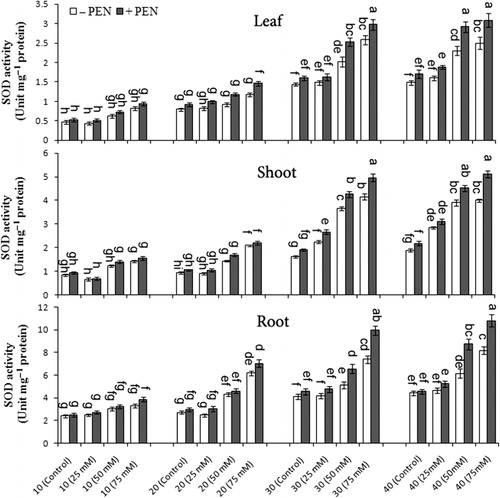
The differences in antioxidant enzyme (SOD, POX, APX, CAT, and PPO) activities are depicted in –. During the experimental period, PEN treatment caused more induction of these enzyme activities in all plant organs. Under different NaCl stress, SOD activity was significantly increased in all organs, especially in roots and activities increased with increasing harvest times. PEN treatment to salt-stressed plants caused a higher induction of SOD activity when compared to unstressed plants. At 75 mM NaCl with PEN, SOD activity increased 23.2% in leaves, 27.1% in shoots, and 34.64% in roots on day 40 as compared to without PEN (). There was significant interaction among salinity, PEN, and harvest time for SOD activity in roots and none significant in shoots ().
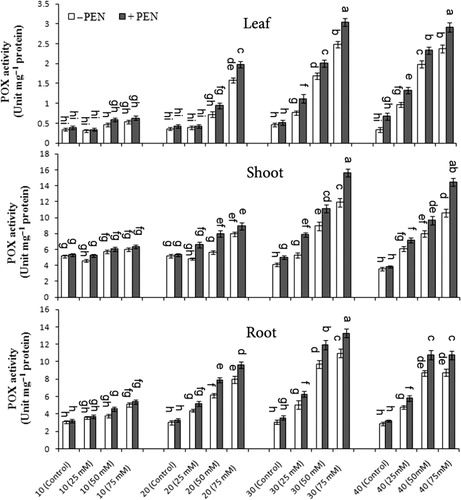
POX activity in M. pulegium significantly increased under salinity conditions (). The highest POX activity was observed at 50 and 75 mM NaCl on day 30, as compared to controls and then decreased slightly on day 40. POX activity was higher in shoots than in roots and was induced by the PEN treatment (). At 50 and 75 mM NaCl with PEN, POX activity increased 18.9% and 22.3.9% in leaves, 24.1% and 31.5% in shoots, and 24.9% and 21.1% in roots on day 30 with higher values than those obtained without PEN, respectively. In contrast to leaves, there was a significant interaction among salinity, PEN, and harvest time for SOD activity in shoots and roots.
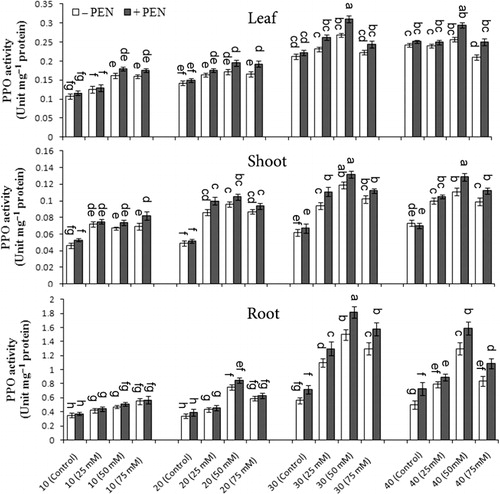
PPO activity significantly increased in all plant organs under salinity, PEN treatment, and harvest time, but there was no significant interaction between all of them (). PPO activity was higher in roots than in leaves and shoots, and more induced by the PEN treatment. The highest PPO activity was observed on day 30 as compared to other harvest times. Under salt stress, PPO activity increased from control to 50 mM NaCl and then decreased at higher NaCl concentration (75 mM NaCl) in all organs (). PEN treatment in salt-stressed and unstressed plants increased PPO activity in all organs and the increasing level was higher in salt-stressed plants. At 50 mM NaCl with PEN, PPO activity increased 15.67% in leaves, 10.9% in shoots, and 21.3% in roots on day 30 as compared to without PEN.
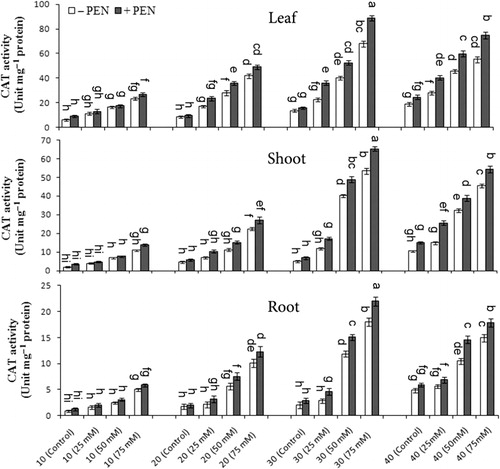
CAT activity significantly increased under different NaCl stress as compared to controls and was pronounced with PEN. The highest CAT activity was observed in leaves with respect to other organs, especially on day 30, and then decreased on day 40 (). At 75 mM NaCl with PEN, CAT activity increased 30.5% in leaves, 22.02% in shoots, and 22.2% in roots on day 30 as compared to without PEN, respectively. There was a significant interaction among salinity, PEN, and harvest time for CAT activity in leaves and roots, but it was not significant in shoots ().
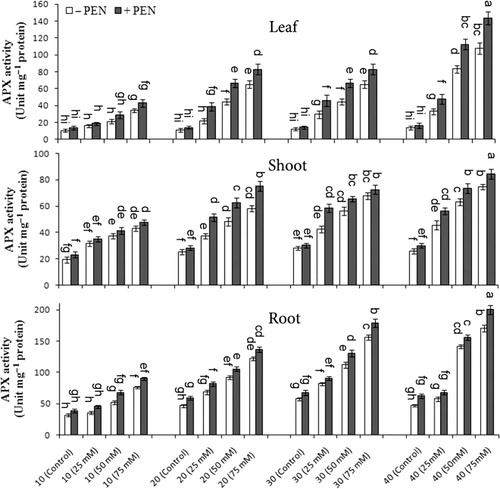
APX activity significantly increased under different salinity concentrations when compared to controls and was induced with PEN treatment. The highest APX activity in M. pulegium was observed in roots than the other organs and remarkably increased on day 40 as compared to other harvest days (). At 75 mM NaCl with PEN, APX activity increased 32.8% in leaves, 12.8% in shoots, and 17.8% in roots as compared to without PEN on day 40, respectively. There was a significant interaction among salinity, PEN, and harvest time for APX activity in leaves and roots, but it was not significant in shoots ().
Discussion
Plant adaptations to salt stress are complex and affected by internal constitutive salt tolerance mechanisms and external environmental factors. In our experiment, growth parameters and RWC content were remarkably inhibited under different salinity conditions in M. pulegium, especially on long-term NaCl exposure. Osmotic stress and ion toxicity are caused by salt stress, and can decrease cell chemical activity and turgor (Serrano et al. Citation1999). Since cell growth depends on turgor pressure which enlarges the cell walls, lack of turgor entails danger for cell survival. In this study, salt stress caused a growth inhibition and RWC reduction in M. pulegium plants (). Reduction of growth and water status have been previously observed in different studies (Jaleel, Gopi, Manivannan & Panneerselvam Citation2007; Shaheen et al. Citation2013). Indeed, in order to increase stress tolerance, M. pulegium plants with reduction growth could conduct energy to increase antioxidant defense system. Application of PEN ameliorated the adverse effects of salt stress by increasing root growth and RWC content in M. pulegium (). Similar results were reported in wheat (Hajihashemi et al. Citation2007) and Catharanthus roseus (Jaleel et al. Citation2008) with other triazoles. The increase of growth induced by PEN has been associated with the ability of triazoles to increase the endogenous cytokinin content (Grossman Citation1990) and cell division. Triazoles also induce the accumulation of osmolytes (Jaleel, Gopi, Manivannan & Panneerselvam Citation2007), which may function as a mechanism that maximizes the water potential gradient between soil and plant (Gomathinayagam et al. Citation2007). Hassanpour et al. (Citation2013) demonstrated that PEN induced the proline accumulation in drought-stressed M. pulegium plants.
Peroxidation of lipid membranes of higher plants reflects free radical-induced oxidative damage at the cellular level under oxidative stress conditions (Demiral & Turkan Citation2004). A significant increase in the MDA and H2O2 levels was observed under salinity stress especially at 75 mM NaCl on day 40 ( and ). PEN treatment in salt-stressed and unstressed plants caused an increase of H2O2 level and a decrease of MDA content. Our results are in agreement with the findings of Hassanpour et al. (Citation2012) and Berova et al. (Citation2002), who found that triazole treatment reduced MDA content in pennyroyal and wheat, respectively. It seems that triazoles are able to induce stress-like symptoms for stimulation of antioxidative system. This might be the reason for the general increase of H2O2 content in M. pulegium (). The increase in H2O2 was also reported previously in triazole-stressed C. roseous (Jaleel, Gopi & Panneerselvam Citation2007). Our study showed that H2O2 levels were higher in roots than those of leaves, in contrast to MDA. ( and ). Plants have evolved a complex antioxidant system to protect cellular membranes and organelles from damaging effects of ROS, which is composed of low-molecular mass antioxidants (glutathione, ascorbate, and carotenoids) as well as ROS-scavenging enzymes (Foyer et al. 1991; Salah et al. Citation2011; Bano et al. Citation2014). It seems that the high accumulation of H2O2 content in roots is able to induce antioxidant system and protect root cells.
Plants respond to abiotic stress by increasing their antioxidant capacity to support them to restore the normal cellular equilibrium between production and scavenging of ROS. SOD catalyzes the disproportion of superoxide radical to molecular oxygen and H2O2 (Scandalios Citation1993) and hence decreases the risk of hydroxyl radical formation from superoxide via the Haber–Weiss type reaction. SOD activity in all the organs of M. pulegium significantly increased under salinity condition (). Increased levels of SOD activity in roots reflect higher dismutating capacity as compared to shoots and leaves (). Similar results previously have been reported in M. pulegium under drought stress (Hassanpour et al. Citation2012) and in Zea mays under salt stress (Kaya et al. Citation2013). Salt stress induced NO and H2O2 productions in wheat (Triticum aestivum L.) leaves, and NO is known to affect O2− generation enzymes (Haihua et al. Citation2002). SOD activity remarkably increased on day 40 especially at 75 mM NaCl with PEN treatment. Similar findings were presented in higher plants such as paclobutrazol-treated salt-stressed C. roseus (Jaleel, Gopi, Manivannan & Panneerselvam Citation2007), propiconazole-treated Vigna unguiculata, and PEN-treated M. pulegium under drought stress (Manivannan et al. Citation2007; Hassanpour et al. Citation2012).
POXs are involved in removal of H2O2 from chloroplasts and cytosol, and many different physiological functions including the oxidation of toxic compounds, the biosynthesis of cell walls (lignin and suberin), growth and developmental processes, etc. (Jbir et al. Citation2001; Dicko et al. Citation2006). Activity of POX has been reported to increase under salt stress and relatively higher activity has been reported in sensitive rice cultivars than in tolerant ones (Demiral & Turkan Citation2004). Likewise, in this study, a decrease was found in POX activity of M. pulegium plants after 40 days exposure to different NaCl concentrations than that of day 30 (). In a similar way, Dani et al. (Citation2005) found decreased polypeptide levels of cell wall-associated peroxidases measured by 2D electrophoresis in the apoplastic fluid from leaves of tobacco plants under NaCl stress. Plants increase lignin synthesis during NaCl stress to prevent water loss through the cell walls (Garcia et al. Citation1997). The slight decrease of POX activity in M. pulegium after day 40 in salt-stressed and unstressed plants can prevent or reduce lignification processes, help to uptake water through the cell walls, or induce the activity of other antioxidant enzyme activities such as APX and CAT on day 40. PEN treatment significantly increased POX activity in salt-stressed and unstressed M. pulegium, while the effect of PEN was higher in salt-stressed plants.
PPO is the major enzyme responsible for oxidation of phenolic compounds. PPO activity considerably increased under salt stress in all organs of M. pulegium especially at 50 mM NaCl and then decreased slightly at 75 mM NaCl (). Increased PPO activity may reduce the phenol content thereby protecting the content of IAA (Fletcher et al. Citation2000), and this can enhance cell and cell wall growth. This result is consistent with the findings in V. unguiculata (Manivannan et al. Citation2007) under drought stress and propiconazole treatment. It seems that the inducing of root growth by PEN may be related to change of growth regulator levels and cell growth in the PEN-treated plants.
CAT and APX are two of the most important enzymes in removing toxic H2O2 from plant cells (Dewir et al. Citation2006). APX and CAT activities significantly increased under different NaCl-treated groups and more induced by PEN treatment ( and ). The highest APX activity was observed in PEN-treated plants on day 40 () and CAT activity was observed on day 30 in M. pulegium (). H2O2 is an integral component of cell signaling cascades and an indispensable second messenger in biotic and abiotic stress situations (Mittler Citation2002; Pastori & Foyer Citation2002). It seems that CAT and APX regulations serve to limit excessive H2O2 accumulation, and allow to occurring essential signaling functions.
In conclusion, salt stress caused a reduction in growth parameters, RWC, and an increase in MDA content, H2O2 level, and antioxidant enzyme activities of M. pulegium. Highest SOD and APX enzyme activities were observed on long-term stress conditions (day 40), whereas POX, PPO, and CAT activities were observed on day 30. There was a significant difference among different organs in responses to salinity and PEN treatments. PEN treatment in salt-stressed plants decreased the negative effect of salinity on growth, RWC, membrane damage, and induced H2O2 level and antioxidative defense responses. From these results, it can be concluded that the application of PEN alone or in combination with salt stress ameliorated salinity effects in M. pulegium plants by improving antioxidant systems and regulation of H2O2 level for signaling functions. Further work on the cellular signaling and non-enzymatic antioxidant compounds is required to gain more information about how PEN treatment ameliorates salinity effects in M. pulegium plants.
Acknowledgment
The authors wish to thank Tehran University for providing research facilities.
References
- Abeles FB, Biles CL. 1991. Characterization of peroxidases in lignifying peach fruit endocarp. J Plant Physiol. 95:269–273. 10.1104/pp.95.1.269
- Aebi H. 1984. Catalase in vitro. Methods Enzymol. 105:121–126.
- Bano S, Ashraf M, Akram NA. 2014. Salt stress regulates enzymatic and nonenzymatic antioxidative defense system in the edible part of carrot (Daucus carota L.). J Plant Interact. 9:324–329. 10.1080/17429145.2013.832426
- Berova M, Zlatev Z, Stoeva N. 2002. Effect of paclobutrazol on wheat seedlings under low-temperature stress. J Plant Physiol. 28:75–84.
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein-dye binding. J Anal Biochem. 72:248–254. 10.1016/0003-2697(76)90527-3
- Dani V, Simon WJ, Duranti M, Croy RRD. 2005. Changes in the tobacco leaf apoplast proteome in response to salt stress. J Proteomics. 5:737–745. 10.1002/pmic.200401119
- Demiral T, Turkan I. 2004. Does exogenous glycinebetaine affect antioxidative system of rice seedlings under NaCl treatment? J Plant Physiol. 161:1089–1100. 10.1016/j.jplph.2004.03.009
- Dewir YH, Chakrabarty D, Ali MB, Hahn EJ, Paek KY. 2006. Lipid peroxidation and antioxidant enzyme activities of Euphorbia millii hyperhydric shoots. J Environ Exp Bot. 58:93–99. 10.1016/j.envexpbot.2005.06.019
- Dicko MH, Gruppen H, Traore AS, Voragen AGJ, van Berkel WJH. 2006. Phenolic compounds and related enzymes as determinants of sorghum for food use. Biotechnol J Mol Biol Rev. 1:21–38.
- Fletcher RA, Gill A, Davis TD, Sankhla N. 2000. Triazoles as plant growth regulator and stress protectants. Rev Horticult. 24:55–138.
- Garcia AB, Engler JA, Iyer S, Gerats T, Van Montagu M, Caplan AB. 1997. Effects of osmoprotectants upon NaCl stress in rice. J Plant Physiol. 115:159–169.
- Giannopolitis CN, Ries SK. 1977. Superoxide dismutases: II. purification and quantitative relationship with water-soluble protein in seedlings. J Plant Physiol. 59:315–318. 10.1104/pp.59.2.315
- Gomathinayagam M, Jaleel CA, Lakshmanan GMA, Panneerselvam R. 2007. Changes in carbohydrate metabolism by triazole growth regulators in cassava (Manihot esculenta Crantz); effects on tuber production and quality. J Comptes Rendus Biol. 330:644–655. 10.1016/j.crvi.2007.06.002
- Grossman K. 1990. Plant growth retardants as tools in physiological research. Physiol Plant. 78:640–648. 10.1111/j.1399-3054.1990.tb05254.x
- Haihua R, Wenbiao S, Maobing YE, Langlai XU. 2002. Protective effects of nitric oxide on salt stress-induced oxida-tive damage to wheat (Triticum aestivum L.) leaves. Chin Sci Bull. 47:677–681. 10.1360/02tb9154
- Hajihashemi S, Kiarostami K, Saboora A, Enteshari S. 2007. Exogenously applied paclobutrazol modulates growth in salt-stressed wheat plants. J Plant Growth Regul. 53:117–128. 10.1007/s10725-007-9209-8
- Hassanpour H, Khavari-Nejad RA, Niknam V, Najafi F, Razavi K. 2012. Effects of penconazole and water deficit stress on physiological and antioxidative responses in pennyroyal (Mentha pulegium L.). Acta Physiol Plant. 34:1537–1549. 10.1007/s11738-012-0952-8
- Hassanpour H, Khavari-Nejad RA, Niknam V, Najafi F, Razavi K. 2013. Penconazole induced changes in photosynthesis, ion acquisition and protein profile of Mentha pulegium L. under drought stress. Physiol Mol Biol Plants. 19:489–498. 10.1007/s12298-013-0192-4
- Heath RL, Packer L. 1968. Photoperoxidation in isolated chloroplast I. kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 125:189–198. 10.1016/0003-9861(68)90654-1
- Jaleel CA, Gopi R, Kishorekumar A, Manivannan P, Sankar B, Panneerselvam R. 2008. Interactive effects of triadimefon and salt stress on antioxidative status and ajmalicine accumulation in Catharanthus roseus. Acta Physiol Plant. 30:287–292. 10.1007/s11738-007-0119-1
- Jaleel CA, Gopi R, Manivannan P, Panneerselvam R. 2007. Responses of antioxidant defense system of Catharanthus roseus (L.) G. Don. to paclobutrazol treatment under salinity. Acta Physiol Plant. 29:205–209. 10.1007/s11738-007-0025-6
- Jaleel CA, Gopi R, Panneerselvam R. 2007. Alterations in lipid peroxidation, electrolyte leakage, and proline metabolism in Catharanthus roseus under treatment with triadimefon, a systemic fungicide. J C R Biologies. 330:905–912. 10.1016/j.crvi.2007.10.001
- Jbir N, Chaibi W, Amar S, Jemmali A, Ayadi A. 2001. Root growth and lignification of two wheat species differing in their sensitivity to NaCl in response to salt stress. J C R Acad Sci Paris. 324:863–868. 10.1016/S0764-4469(01)01355-5
- Jebara S, Jebara M, Limam F, Elarbi Aouani M. 2005. Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. J Plant Physiol. 162:929–936. 10.1016/j.jplph.2004.10.005
- Kaya C, Sonmez O, Aydemir S, Ashraf M, Dikilitas M. 2013. Exogenous application of mannitol and thiourea regulates plant growth and oxidative stress responses in salt-stressed maize (Zea mays L.). J Plant Interact. 8:234–241. 10.1080/17429145.2012.725480
- Liang Y, Chen Q, Liu Q, Zhang W, Ding R. 2003. Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J Plant Physiol. 160:1157–1164. 10.1078/0176-1617-01065
- Manivannan P, Jaleel CA, Kishorekumar A, Sankar B, Somasundaram R, Sridharan R, Panneerselvam R. 2007. Changes in antioxidant metabolism of Vigna unguiculata (L.) Walp. By propiconazole under water deficit stress. Colloids Surf Biointerfaces. 57:69–74. 10.1016/j.colsurfb.2007.01.004
- Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7:405–410. 10.1016/S1360-1385(02)02312-9
- Munns R, James RA, Lauchli A. 2006. Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot. 57:1025–1043. 10.1093/jxb/erj100
- Omran AN, Porshamcian K, Hashemi J, Aliramaji S. 2012. Chemical composition and antifungal activity of of essential oils medicinal plants essential oils the northern Iran. Mycoses. 55:99. 10.1111/j.1439-0507.2011.02013.x
- Oueslati S, Karray-Bouraoui N, Attia H, Rabhi M, Ksouri R, Lachaal M. 2010. Physiological and antioxidant responses of Mentha pulegium (Pennyroyal) to salt stress. Acta Physiol Plant. 32:289–296. 10.1007/s11738-009-0406-0
- Pastori GM, Foyer CH. 2002. Common components, networks and pathways of cross-tolerance to stress. The central role of ‘redox’ and abscisic-acid-mediated controls. Plant Physiol. 129:460–468. 10.1104/pp.011021
- Patade VY, Bhargava S, Suprasanna P. 2011. Salt and drought tolerance of sugarcane under iso-osmotic salt and water stress: growth, osmolytes accumulation, and antioxidant defense. J Plant Interact. 6:275–282. 10.1080/17429145.2011.557513
- Raymond J, Rakariyatham N, Azanza JL. 1993. Purification and some properties of polyphenoloxidase from sunflower seeds. J Phytochem. 34:927–931. 10.1016/S0031-9422(00)90689-7
- Salah IB, Mahmoudi H, Gruber M, Slatni T, Boulaaba M, Gandour M, Messedi D, Hamed KB, Ksouri R, Hannoufa A, Abdelly C. 2011. Phenolic content and antioxidant activity in two contrasting Medicago ciliaris lines cultivated under salt stress. Biologia. 66:813–820. 10.2478/s11756-011-0102-6
- Scandalios JG. 1993. Oxygen stress and superoxide dismutases. J Plant Physiol. 101:7–12.
- Serrano R, Mulet JM, Rios G, Marquez JA, Larrinoa IF, Leube MP, Mendizabal I, Pascual-Ahuir A, Proft, M, Ros R, Montesinos C. 1999. A glimpse of the mechanisms of ion homeostasis during salt stress. J Exper Bot. 50:1023–1036. 10.1093/jxb/50.Special_Issue.1023
- Shaheen S, Naseer S, Ashraf M, Akram NA. 2013. Salt stress affects water relations, photosynthesis, and oxidative defense mechanisms in Solanum melongena L. J Plant Interact. 8:85–96. 10.1080/17429145.2012.718376
- Shirazi FH, Ahmadi N, Kamelinejad M. 2004. Evaluation of northern Iran Mentha pulegium cytotoxicity. Daru. 12:106–110.
- Soares PMG, de Freitas Pires A, de Souza EP, Assreuy AM, Criddle DN. 2012. Relaxant effects of the essential oil of Mentha pulegium L. in rat isolated trachea and urinary bladder. J Pharm Pharmacol. 64:1777–1784. 10.1111/j.2042-7158.2012.01558.x
- Velikova V, Yordanov I, Edreva A. 2000. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. J Plant Sci. 151:59–66. 10.1016/S0168-9452(99)00197-1
- Wheatherley PE. 1973. Studies in the water relations of cotton plants. The field measurement of water deficit in leaves. J New Phytol. 49:81–87. 10.1111/j.1469-8137.1950.tb05146.x
