Abstract
Changes in the activity of lysine decarboxylase (LDC), tyrosine decarboxylase (TyDC), and ornithine decarboxylase (ODC) within orchid (Phalaenopsis × hybridum ‘Innocence’) leaves, infested by two mealybug species: Pseudococcus longispinus (Targ. Tozz.) and Pseudococcus maritimus (Ehrh.) were quantified. The pattern of changes was dependent on the insect species and duration of infestation. P. longispinus feeding increased LDC and TyDC activity after one week during the total period of observations. This species inhibited ODC activity after one week but increased later. P. maritimus decreased LDC activity in orchid leaves at all studied terms. TyDC action also went up during the first week of the infestation and was reduced after two weeks, while ODC was decreased after one day and induced later. The mechanism for the participation of analysed amino acid decarboxylases in local and/or systemic steps of orchid responses to mealybug infestation is discussed.
Introduction
Mealybugs are serious pests of orchids and probably the most difficult to control pests of Phalaenopsis. Some individuals from the Pseudococcidae family, especially young instar larvae, are very active, crawling from one plant to another, pot to pot, across benches and they can be found on all plant parts. As all scale insects, they have piercing-sucking mouthparts that remove sap from plants. They feed in leaf, stem axils and cause stunting, leaf yellowing as well as distortion. The damage done to plants by these scale insects is considerable, causing a loss of vigor, weakening, loss of leaves, buds, and flowers through their feeding. In addition, mealybugs excrete copious amounts of honeydew, which make plant parts sticky, attract ants, and provide a substrate for sooty mold. Although there are some mealybugs vector plant viruses, apparently no orchid viruses are known to be transmitted by these insects. Mealybugs are not particular about their host and probably all species of orchids are susceptible to mealybugs, especially when cultivated (Ben-Dov et al. Citation2013). Pseudococcus longispinus (Targ. Tozz.) called the long-tailed mealybug is widely distributed in the nature, on a large range of plants, over most territories of the tropical and subtropical regions, in addition to greenhouses in temperate zones. It is found throughout North America and in Africa, Europe, the Middle East, Asia, New Zealand, and Australia. Pseudococcus maritimus (Ehrh.) so-called the grape mealybug has been encountered in the Nearctic, Neotropical Oriental, and Palearctic regions. The species is a polyphage, inhabiting plants which represent 37 botanical families (Ben-Dov et al. Citation2013). These two species have a relatively narrow temperature tolerance but a wide host range. For this reason, mealybugs are very important pests of ornamentals in glasshouse and indoor plantscapes as well as being the most frequently and burdensome pests on orchids. Although a great number of studies on Sternorrhyncha have been carried out on main host plants, the majority of the information concerns aphids, whereas the interactions between plants and scale insects are poorly described (Bogo & Mantle Citation2000; Calatayud & Le Rü Citation2006; Fernandes et al. Citation2011). The plant chemical composition is an important determinant of host plant and insect interactions, therefore understanding these relationships is the main objective of modern plant protection (Fernie Citation2007).
It is well known that plant amines and key enzymes of their biosynthesis are involved in plant responses to pathogenic fungi, bacteria, and viruses (Walters Citation2003), but their role in the plant’s defense against herbivorous insects is still not clear. A few published reports suggested that the amino acid decarboxylases state are an important part of insect–plant interactions and mechanism of the phenomenon may be connected with the degradation of amino acids as nutrients for herbivores as well as biosynthesis of plant amines and their hydroxycinnamic acid derivatives (HCAAs). According to Tebayashi et al. (Citation2007) and Bassard et al. (Citation2010), the HCAAs as caffeoyl putrescine, p-coumaroyl putrescine, and dicaffeoyl spermidine were involved in systemic responses of Capsicum annuum (L.) to leaf miner Liriomyza trifolii (Burg.) and Nicotiana attenuata (Torr. ex Wats.) to Manduca sexta (L.) and Spodoptera littoralis (Bois.). Mechanism of HCAAs toxicity to arthropods is connected with paralysis resulting from a binding to quisqualate-type glutamate receptors on exoskeletal muscles and block synaptic transmission (Klose et al. Citation2002). Sempruch, Leszczyński, Kozik, et al. (Citation2010) showed that free PAs such as agmatine, cadaverine, putrescine, spermidine, and spermine, at 0.1% concentration, reduced feeding and survival of grain aphid (Sitobion avenae F.). Putrescine, spermidine, and spermine at a 10 mM level also disturbed the settling behavior of bird cherry-oat aphid (Rhopalosiphum padi L.) on triticale (Sempruch et al. Citation2011). Moreover, free polyamines (PAs) modified the sensitivity of Plutella xylostella (L.) antennae to odors, influencing its behavior (Zhang et al. Citation2008). On the other hand, free tyramine stimulated oviposition of Papilio polyxenes (F.) on Pastinaca sativa (L.) (Carter et al. Citation1998).
Furthermore, the aphid infestation affected the activity of amino acid decarboxylases within plant tissues and the changes were dependent on cultivar (cv.) susceptibility, insect species as well as duration of infestation. In the biosynthesis of PAs within plant tissues, several amino acid decarboxylases participate. For example, ornithine decarboxylase (ODC; EC 4.1.1.17) is a common enzyme catalyzing the transformation of ornithine to putrescine within plant and animal cells (Walters Citation2003; Fariduddin et al. Citation2013). Fabaceae, Poaceae, and Solanaceae plants contain lysine decarboxylase (LDC; EC 4.1.1.18) that participates in cadaverine biosynthesis via lysine decarboxylation (Bagni & Tassoni Citation2001). Moreover, tyrosine decarboxylase (TyDC; EC 4.1.1.25), such as other aromatic amino acid decarboxylases, participate in biosynthesis of some defensive plant compounds (Facchini et al. Citation2000). R. padi feeding reduced LDC activity within triticale seedlings during the first week of the infestation. However, after two weeks an increase in the enzyme activity was proved in more resistant cv. but not in susceptible triticale (Sempruch, Marczuk, Leszczyński, Czerniewicz, et al. Citation2013). The ODC activity was induced in tissues of the susceptible cv. and inhibited in more resistant one after two weeks of infestation. The bird cherry-oat aphid infestations increased TyDC activity after two weeks but only in susceptible cv. Changes in the enzymes activity in R. padi where the triticale was infested resulted in an accumulation of the amines (Sempruch et al. Citation2012). Their levels decreased after one week of the infestation and again after two weeks, but did not in less susceptible plants where the content of cadaverine, spermidine, and tryptamine increased with simultaneous decreases of the putrescine concentration. S. avenae feeding also affected the activity of ODC, LDC, and TyDC within triticale tissues (Sempruch et al. Citation2008, Citation2009; Sempruch, Leszczyński, Wójcicka, et al. Citation2010). The enzymes’ activity was reduced during the first week of grain aphid infestation (with the exception of TyDC) and was induced after two weeks (with the exception of LDC within tissues of susceptible cv.). The pea aphid (Acyrthosiphon pisum Harr.) increased ODC activity in pea tissues after one day and at two weeks of the infestation (Sempruch, Marczuk, Leszczyński, Kozak, et al. Citation2013). TyDC activity was increased during the first week, while LDC activity increased only after the first day and decreased later. An attack by the aphids also affected the enzyme activity in root tissues not directly damaged by the herbivores. The presented data suggest that amino acid decarboxylation may be an important part of local and/or systemic plant responses to feeding of piercing-sucking insects, but previous studies were mainly focused on interactions between different aphid species and their host plants. Since there is no information on the participation of amino acid decarboxylation in a plant’s response to mealybug attack, the present paper deals with the changes in the activity of LDC, TyDC, and ODC in orchid leaves infested by P. longispinus and P. maritimus.
Material and methods
Plants
The orchid (Phalaenopsis × hybridum ‘Innocence’) chosen for the analysis were purchased from JMP Flowers Gardening Enterprise in Stężyca, where they were sold as so-called intermediate products (plants without inflorescence shoots). The experiment was conducted in the laboratory conditions. The plants were grown in plastic transparent pots of a diameter of 12 cm, filled with coarse pine bark bedding, situated in a cultivation chamber on textile subirrigation mats (Polprotex) covered with black agrofabric. The maintenance of the plants only consisted of flooding with tap water once a week. The experiment included orchids (after a 4-week adaptation period) in a phase of seven fully developed leaves, without inflorescence shoots.
Insects
Two species of mealybugs P. maritimus and P. longispinus were reared on Phalaenopsis × hybridum ‘Innocence’ in climatic chamber at 27 ± 1°C, 50% ± 5% RH and photoperiod 16L:8D for 6 months preceding the experiment. Five young females or third instar larvae of each mealybug species (P. longispinus, P. maritimus) were used in the experiments. These developmental stages of Pseudococcidae are less mobile than the younger instars and they remain in one place while feeding.
Plant infestation with insects and sampling
The orchid plants were divided into two groups. Each of them was colonized with one species of scale insects (P. maritimus or P. longispinus). Five young females or third instar larvae of each mealybug species were transferred onto each plant using a bristle brush. The number of mealybug individuals on the orchids did not change for the whole period of the experiment due to the long pre-reproduction period of these species and their low mobility. The sample constituted of three plants, which were artificially infested by the insects two weeks, one week and one day (24 hours) before their sampling to the analyses. After this time the samples were simultaneously taken from each combination and from control plants (without the scale insects). The control plants were similarly grown in another climate chamber under the same conditions. The experiment was conducted in three replications for each combination. The same experimental conditions were applied for all the plants used during this study (temperature 27 ± 1°C; relative humidity 50% ± 5%, photoperiod L:D = 16:8). Freshly collected leaves infested by the insects and control ones were used for chemical analyses. The mealybugs were removed from leaves before analyses.
Enzyme assays
The freshly collected orchid leaves were homogenized with 0.2 M phosphate buffer (pH 8.2) containing of β-mercaptoethanol and ethylenediaminetetraacetic acid (EDTA) for ODC extraction, 0.2 M Tris–HCl buffer (pH 5.6) for LDC extraction, or 0.5 M acetate buffer (pH 5.6) for TyDC extraction. The obtained enzymatic extracts were filtered through two layers of cheesecloth and then centrifuged at 18,000× g at 5°C.
Enzyme activities were assayed with the use of a Hewlett Packard 8453 UV-Visible spectrophotometer according to Ngo et al. (Citation1987) for ODC and Phan et al. (Citation1982, Citation1983) for LDC and TyDC, respectively. Enzyme activity is expressed as μM putrescine (ODC), cadaverine (LDC) or tyramine (TyDC)/1 h enzymatic reaction/1 mg enzymatic protein. The protein quantity in the enzymatic extracts was estimated with the use of the Lowry et al. (Citation1951) method.
Statistics
The entomological and biochemical assays were conducted in three independent repeats. The distribution of obtained data was verified with a chi-square test. The Kruskal–Wallis test, as a non-parametric alternative to ANOVA, was applied after rejection of the normality data (ODC in experiment with P. longispinus; TyDC and ODC in experiment with P. maritimus). A one-way ANOVA was used for data with a normal distribution (LDC and TyDC in experiment with P. longispinus; LDC in experiment with P. maritimus). The significance of differences in enzyme activity between the control and mealybug-infested leaves was checked with the non-parametric Mann–Whitney U-test or Student’s t-test dependent on the data distribution. P ≤ 0.05 was taken to indicate statistical significance.
The arithmetic means with standard errors are presented in the tables and on the figures. Software Statistica for Windows version 9.0 (Statistica StatSoft Inc. Citation2010) was used for all statistical analyses.
Results
Changes in activity of amino acid decarboxylases within orchid leaves
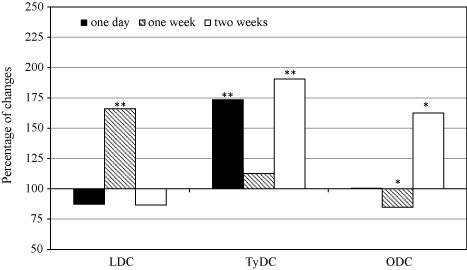
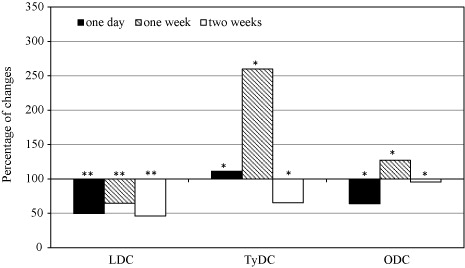
Statistical analyses showed significant differences in the activity of the amino acid decarboxylases in orchid tissues (–). The results of the ANOVA or Kruskal–Wallis test were as follows: F3,8 = 17.76 at p = 6.76 × 10–4 for LDC in experiment with P. longispinus; F3,8 = 20.04 at p = 4.46 × 10–4 for LDC in experiment with P. maritimus; F3,8 = 29.24 at p = 1.16 × 10–5 for TyDC in experiment with P. longispinus; H(3,N = 12) = 10.39 at p = 1.56 × 10–2 for TyDC in the experiment with P. maritimus; H(3,N = 12) = 9.36 at p = 2.49 × 10–2 for ODC in experiment with P. longispinus and H(3,N = 12) = 10.42 at p = 1.53 × 10–2 for ODC in experiment with P. maritimus.
Table 1. Effect of infestation of P. longispinus and P. maritimus on LDC activity (µM cadaverine × mg–1 protein × hour–1) within orchids leaves.
Table 2. Effect of infestation of P. longispinus and P. maritimus on TyDC activity (µM tyrmine × mg–1 protein × hour–1) within orchids leaves.
Table 3. Effect of infestation of P. longispinus and P. maritimus on ODC activity (µM putrescine × mg–1 protein × hour–1) within orchids leaves.
It was stated that LDC activity decreased in orchid leaves after one day and two weeks of P. longispinus infestation and significantly increased after one week (; ). This species also induced an increase in TyDC activity and the changes were statistically confirmed after one day and two weeks (; ). A similar tendency was observed in the case of ODC but only after two weeks. During an earlier period (after one week of the infestation), significant inhibition of the enzyme activity was proved (; ). In the case of interactions between P. maritimus and orchid plants, LDC activity significantly decreased during all experimental periods (; ), and TyDC activity increased at the first week and decreased later (; ). Moreover, a fluctuation of ODC activity in the leaves of P. maritimus-infested orchid was observed. It was induced after one day and two weeks and inhibited after one week (; ).
Discussion
The obtained results suggest that TyDC was strongly involved in the biochemical responses of orchid against the mealybug infestation, since the both studied insect species induced an increase in the enzyme activity. However, the duration of the response in the case of individual insect species was different. The significant changes in TyDC activity were observed after one day and two weeks of P. longispinus infestation and during the first week of P. maritimus feeding. Kmieć et al. (Citation2014) in the earlier studies concerning P. longispinus observed similar changes in physiological parameters (electrolyte outflow, malondialdehyde content and antioxidant enzymes activity) reaching high values during the initial period of orchid’s infestation. In plant tissues, TyDC is involved in the biosynthesis of tyramine and their HCAAs, and such defensive compounds as catecholamines in Solanum tuberosum (L.), hydroxyphenylethanol glycoside – verbascoside in Syringa vulgaris (L.), simple alkaloid phytoalexin – hordinine in Hordeum vulgare (L.), benzylisoquinolines and their derivatives in five plant families (Facchini et al. Citation2000; Świędrych et al. Citation2004; Hagel & Facchini Citation2005; Cordell Citation2013). According to Kanbar et al. (Citation2004) tyramines are toxic compounds produced by coccoid bacteria Melissococcus pluton in honeybee pupae after transmission by the mite Varroa destructor. A lethal dose of tyramine on 4- to 5-day-old larvae was 0.3 mg. The compounds were also characterized by an inhibition of mobility for protozoon Stylonychia lemnae and high membrolitic activity against human red blood cells. Tyramine is good substrate for monoamine oxidase (MAO) and affects the hydroxyl radical formation (Schmit & Fergen Citation2004). However, the phenomenon was still described only in animal tissues. In addition, tyramine is precursor in the biosynthesis of octopamine that functions as a neuromodulator, neurotransmitter, and neurohormone in an insect’s nervous system (Lee et al. Citation2009; Varlinden et al. Citation2010). Thus, free tyramine stimulated the oviposition of P. polyxenes on P. sativa (Carter et al. Citation1998). However, octopamine is a target for toxic essential oils in insects. Such essential oils constituents as eugenol and thymol may block octopamine receptors and potentially stop working through tyramine receptor cascades (Rattan Citation2010). The importance of TyDC for insect–plant interactions is still not clear. Sato et al. (Citation2013) showed that concentrations of tyramine were 4.5-times higher in the soybean strain with a higher resistance against foxglove aphid (Aulacorthum solani Kalt.) than in the susceptible one. Tyramine is located downstream of tyrosine and shikimate, which was present at a higher concentration in the resistant strain before aphid introduction. Grain aphid infestation caused a fluctuation of TyDC activity within tissues of susceptible triticale cv. and a constant increase in the more resistant one (Sempruch et al. Citation2009). The increasing tendency of the enzyme activity was also observed after two weeks of R. padi feeding, but only in the case of susceptible triticale cv. (Sempruch, Marczuk, Leszczyński, Czerniewicz, et al. Citation2013). In addition, TyDC activity was increased within pea tissues during the first day of A. pisum infestation (Sempruch, Marczuk, Leszczyński, Kozak, et al. Citation2013). The presented results suggest that the pattern of the biochemical plant responses to piercing-sucking insect attack is strictly dependent on herbivore species. A biochemical plant defense may be elicited by components of aphids’ saliva and/or wounding of epidermal, mesophyll, and parenchyma cells during their probing behavior (Goggin Citation2007; Giordanengo et al. Citation2010). Mechanical wounding of transgenic tobacco induced activity of TyDC and tryptophan decarboxylase, and increased the tyramine, ferloyltyramine, and 4-coumaroyltyramine levels (Guillet & De Luca Citation2005; Hagel & Facchini Citation2005). Moreover, systemic plant responses induced by aphid and other hemipterous insects may be signalized by jasmonic acid (JA), salicylic acid, ethylene, abscisic acid (ABA), and gibberellic acid (Morkunas et al. Citation2011; Takemoto et al. Citation2013). According to Świędrych et al. (Citation2004) TyDC was activated in potato leaves in response to ABA treatment, and the amount of tyramine increased in the giant knotweed (Fallopia sachalinensis Schm.) treated with methyl jasmonate (MeJA) (Noge & Tamogami Citation2013).
There was an increase of LDC activity after one week of P. longispinus infestation and ODC activity after one week of P. maritimus and two weeks of P. longispinus feeding which has been proved in our works – it may suggest their participation in long-term responses. Sempruch, Leszczyński, Wójcicka, et al. (Citation2010) and Sempruch, Marczuk, Leszczyński, Czerniewicz, et al. (Citation2013) proved that induction of LDC activity was typical for response of less susceptible triticale cvs. towards against cereal aphids were developed after two weeks of the infestation. It might accelerate metabolic transformation caused disturbances in equilibrium between essential amino acid lysine content and cadaverine level, as the product of decarboxylation of this amino acid. Decrease in nutritive value of plant tissues for insects may be resulted from this response. In addition, cadaverine strongly reduced body mass and survival of the grain aphid at 0.1% concentration in in vitro conditions (Sempruch, Leszczyński, Kozik, et al. Citation2010).
According to Xu et al. (Citation2004) exogenous MeJA increased ODC transcript levels in transgenic tobacco. The phytohormone inhibited the expression of arginine decarboxylase (OsAdc1), S-adenosylmethionine decarboxylase (OsSamdc), and spermidine synthase (OsSpds) genes in transgenic rice (Peremarti et al. Citation2010). It also reduced the level of free PAs and elevated concentrations of their conjugates in the roots and shoots of potato plants (Mader Citation1999). In the case of tobacco, MeJA increased the activity of arginine decarboxylase, ODC, and S-adenosylmethionine decarboxylase, accumulation of conjugated PAs derivatives with simultaneous decreased levels of free putrescine, spermidine, and spermine (Biondi et al. Citation2001, Citation2003). Result of our earlier studies (Sempruch et al. Citation2012; Sempruch, Marczuk, Leszczyński, Czerniewicz, et al. Citation2013; Sempruch, Marczuk, Leszczyński, Kozak, et al. Citation2013) suggested that common plant PAs and key enzymes of their biosynthesis may be involved in short-term (local) as well as long-term (systemic) plant responses against the aphid infestation. Moloi and van der Westhuizen (Citation2006) showed that the response of wheat provoked by Russian wheat aphid (Diuraphis noxia Mord.) infestation had a typical local hypersensitive response (HR) nature. Reactive oxygen species were generated during earlier HR events in response to some aphid species (Thompson & Goggin Citation2006; Mishra et al. Citation2012). Cereal aphids S. avenae and R. padi also induced oxidative stress in triticale tissues that was especially strong within less susceptible cultivar (Łukasik et al. Citation2012). PAs participate in a plant’s tolerance to oxidative stresses through the stabilization of proteins, nucleic acid, and phospholipids of cellular membranes. Mandal et al. (Citation2014) showed that application of putrescine to hydroponic medium reduced the generation of protein oxidation as well as moderated activity of guaiacol peroxidase, glutathione reductase, and catalase in Salvinia natans L. treated by hydrogen peroxide. On the other hand, PAs may be oxidized with diamine oxidase and polyamine oxidase, and the transformation generates large quantities of H2O2, inducing the next steps of stress responses (Mohapatra et al. Citation2009). According to Sempruch et al. (Citation2012), Sempruch, Marczuk, Leszczyński, Czerniewicz, et al. (Citation2013) and Sempruch, Marczuk, Leszczyński, Kozak, et al. (Citation2013) plant with high susceptibility to R. padi and A. pisum were characterized by induction of ODC activity and over accumulation of putrescine under the aphids infestation. It might be at last partly connected with degradation of nonprotein ornithine that high concentration in wheat tissues was negatively correlated with S. avenae intrinsic rate of natural increase (Ciepiela & Sempruch Citation1999). On the other hand, putrescine negatively influenced feeding behavior and survival of S. avenae and disturbed seedling of triticale seedlings by R. padi (Sempruch, Leszczyński, Kozik, et al. Citation2010; Sempruch et al. Citation2011). ODC is also involved in biosynthesis of putrescine HCAAs that participate in systemic responses of C. annuum to L. trifolii and N. attenuate to M. sexta and S. littoralis. Thus, we conclude that the phenomenon should be studied in the future.
In conclusion, we can state that TyDC was involved in orchid-mealybugs interactions during shorter and/or a longer term of the infestation. However, LDC and ODC participated rather in long-term responses. The pattern of changes in the amino acid decarboxylases activities was dependent on mealybug species and duration of infestation. It is possible that the differences in the patterns of the changes in activity of studied enzymes induced by particular maealybug species are dependent on their biology, and especially on the feeding behavior. However, this phenomenon needs further, more complex studies.
Funding
The study was financed by Siedlce University of Natural Science and Humanities (Scientific Research Project 245/08/S) and University of Life Sciences in Lublin (Project no. OKE/DS/2 in 2013–2017).
Additional information
Funding
References
- Bagni N, Tassoni A. 2001. Biosynthesis oxidation and conjugation of aliphatic polyamines in higher plants. Amino Acids. 20:301–317. 10.1007/s007260170046
- Bassard J-E, Ullmann P, Bernier F, Werck-Reichhart D. 2010. Phenoloamines: Binding polyamines to the phenolic metabolism. Phytochemistry. 71:1808–1824. 10.1016/j.phytochem.2010.08.003
- Ben-Dov Y, Miller DR, Gibson GAP. 2013. ScaleNet: a database of the scale insects of the world [Internet]. Systematic Entomology Laboratory, United States Department of Agriculture; [revised 2013 Oct 1; cited 2013 Dec 21]. Available from: http://www.sel.barc.usda.gov/scalenet/query.htm
- Biondi S, Scaramagli S, Capitani F, Altamura MM, Torrigiani P. 2001. Methyl jasmonate upregulates biosynthetic gene expression, oxidation and conjugation of polyamines, and inhibits shoot formation in tobacco thin layers. J Exp Bot. 52:231–242. 10.1093/jexbot/52.355.231
- Biondi S, Scoccianti V, Scaramagli S, Ziosi V, Torrigiani P. 2003. Auxin and cytokinin modify methyl jasmonate effects on polyamine metabolism and ethylene biosynthesis in tobacco leaf discs. Plant Sci. 165:95–101. 10.1016/S0168-9452(03)00147-X
- Bogo A, Mantle P. 2000. Oligosaccharides in the honeydew of Coccoidea scale insects: Coccus hesperidum L. and a new Stigmacoccus sp. in Brazil. An Soc Entomol Bras. 29:589–595.
- Calatayud PA, Le Rü B. 2006. Cassava-mealybug interaction. France: Institut de Recherche Pour le Développement (IRD Editions).
- Carter M, Sacdev-Gupta K, Feeny P. 1998. Tyramine from the leaves of wild parsnip: A stimulant and synergist for oviposition by the black swallowtail butterfly. Physiol Entomol. 23:303–312. 10.1046/j.1365-3032.1998.00100.x
- Ciepiela AP, Sempruch C. 1999. Effect of L-3,4-dihydroxyphenylalanine, ornithine and γ-aminobutyric acid on winter wheat resistance to grain aphid. J Appl Entomol. 123:285–288.
- Cordell GA. 2013. Fifty years of alkaloid biosynthesis in phytochemistry. Phytochemistry. 91:29–51. 10.1016/j.phytochem.2012.05.012
- Facchini PJ, Hubner-Allanach KL, Tari LW. 2000. Plant aromatic L-amino acid decarboxylases: evolution, biochemistry, regulation and metabolic engineering applications. Phytochemistry. 54:121–138. 10.1016/S0031-9422(00)00050-9
- Fariduddin Q, Varshney P, Yusuf M, Ahmad A. 2013. Polyamines: potent modulators of plant responses to stress. J Plant Int. 8:1–16.
- Fernandes FL, Picanço MC, Gontijo PC, Fernandes ME, Pereira EJG, Semeão AA. 2011. Induced responses of Coffea arabica to attack of Coccus viridis stimulate locomotion of the herbivore. Entomol Exp Appl. 139:120–127.
- Fernie AR. 2007. The future of metabolic phytochemistry: Large numbers or metabolites, higher resolution, greater understanding. Phytochemistry. 68:2861–2880. 10.1016/j.phytochem.2007.07.010
- Giordanengo P, Brunissen L, Rusterucci C, Vincent C, van Bel A, Dinant S, Girousse C, Faucher M, Bonnemain J-L. 2010. Compatible plant-aphid interactions: How aphids manipulate plant responses. C R Biol. 333:516–523. 10.1016/j.crvi.2010.03.007
- Goggin FL. 2007. Plant-aphid interactions: Molecular and ecological perspectives. Curr Opin Plant Biol. 10:399–408.
- Guillet G, De Luca V. 2005. Wound-inducible biosynthesis of phytoalexin hydroxycinnamic acid amides of tyramine in tryptophan and tyrosine decarboxylase transgenic tobacco lines. Plant Physiol. 137:692–699. 10.1104/pp.104.050294
- Hagel JM, Facchini PJ. 2005. Elevated tyrosine decarboxylase and tyramine hudroxycinnamoltransferase levels increase wound-induced tyramine-derived hydroxycinnamic acid amide accumulation in transgenic tobacco leaves. Planta. 221:904–914. 10.1007/s00425-005-1484-x
- Kanbar G, Engels W, Nicholson GJ, Hartle R, Winkelmann G. 2004. Tyramines functions as a toxin in honey bee larvae during Varroa-transmitted infection by Melissococcus pluton. FEMS Microbiol Lett. 234:149–154.
- Klose MK, Atkinson JK, Mercier AJ. 2002. Effect of hydroxy-cinnamoyl conjugate of spermidine on arthropod neuromuscular junctions. J Comp Physiol. 187:945–952.
- Kmieć K, Kot I, Rubinowska K, Łagowska B, Golan K, Górska-Drabik E. 2014. Physiological reaction of Phalaenopsis x hybridum ‘Innocence’ on Pseudococcus longispinus (Targoni Tozetti) feeding. Acta Sci Pol Hortorum Cultus. 13:85–96.
- Lee K, Kang K, Park M, Park S, Back K. 2009. Enhanced octopamine synthesis through the ectopic expression of tyrosine decarboxylase in rice plants. Plant Sci. 176:46–50. 10.1016/j.plantsci.2008.09.006
- Lowry JOH, Rosebrough NJ, Farr AL, Randal RJ. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem. 193:256–277.
- Łukasik I, Goławska S, Wójcicka A. 2012. Effect of cereal aphid infestation on ascorbate content and ascorbate peroxidase activity in triticale. Pol J Environ Stud. 21:1937–1941.
- Mader JC. 1999. Effects of jasmonic acid, silver nitrate and L-AOPP on the distribution of free and conjugated polyamines in roots and shoots of Solanum tuberosum in vitro. J Plant Physiol. 154:79–88.10.1016/S0176-1617(99)80321-6
- Mandal C, Ghosa V, Dey N, Adak MK. 2014. Effects of putrescine on oxidative stress induced by hydrogen peroxide in Salvinia natans L. J Plant Int. 9:550–558.
- Mishra AK, Sharma K, Misra RS. 2012. Elicitor recognition, signal transduction and induced resistance in plants. J Plant Int. 7:95–120.10.1080/17429145.2011.597517
- Mohapatra S, Minocha R, Long S, Minocha SC. 2009. Putrescine overproduction negatively impacts the oxidative state of poplar cells in culture. Plant Physiol Biochem. 47:262–271.
- Moloi MJ, van der Westhuizen A. 2006. The reactive oxygen species are involved in resistance responses of wheat to the Russian wheat aphid. J Plant Physiol. 163:1118–1125.10.1016/j.jplph.2005.07.014
- Morkunas I, Mai VC, Gabryś B. 2011. Phytohormonal signalling in plants responses on aphid feeding. Acta Physiol Plant. 33:257–2073.
- Ngo TT, Brillhart KL, Davis RH, Womg RC, Bovaird JH, Digangi JJ, Risov JL, Marsh JL, Phan APH, Lenhoff HM. 1987. Spectrophotometric assay for ornithine decarboxylase. Anal Biochem. 160:290–293.
- Noge K, Tamogami S. 2013. Herbivore-induced phenylacetonitrile is biosynthesized from de novo-synthesised L-phenylalanine in the giant knotweed, Fallopia sachalinensis. FEBS Lett. 587:1811–1817. 10.1016/j.febslet.2013.04.038
- Peremarti A, Bassie L, Yuan D, Palacho A, Christou P, Capell T. 2010. Transcriptional regulation of the rice arginine decarboxylase (Adc1) and S-adenosylmethionine decarboxylase (Samdc) genes by methyl jasmonate. Plant Physiol Biochem. 48:553–559.
- Phan APH, Ngo TT, Lenhoff HM. 1982. Spectrophotometric assay for lysine decarboxylase. Anal Biochem. 120:193–197.
- Phan APH, Ngo TT, Lenhoff HM. 1983. Tyrosine decarboxylase. Spectrophotometric assay and application determining pyridoxal-5′-phosphate. Appl Biochem Biotech. 8:127–133.
- Rattan RS. 2010. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 29:913–920. 10.1016/j.cropro.2010.05.008
- Sato D, Akashi A, Sugimoto M, Tomita M, Soga T. 2013. Metabolomic profiling of the response of susceptible and resistant soybean strains to fixglove aphid, Aulacorthum solani Kaltenbach. J. Chromatogr B. 925:95–103.10.1016/j.jchromb.2013.02.036
- Schmit N, Fergen B. 2004. The biogenic trace amine tyramine induces a pronounced hydroxyl radical production via a monoamine oxidase dependent mechanism: an in vivo microdialysis study in mouse striatum. Brain Res. 1012:101–107.10.1016/j.brainres.2004.03.036
- Sempruch C, Horbowicz M, Kosson R, Leszczyński B. 2012. Biochemical interactions between triticale (Triticosecale; Poaceae) amines and bird cherry-oat aphid (Rhopalosiphum padi; Aphididae). Biochem Syst Ecol. 40:162–168.
- Sempruch C, Leszczyński B, Kozik A, Chrzanowski G. 2010. The influence of selected plant polyamines on feeding and survival of grain aphid (Sitobion avenae F.). Pesticides. 1–4:15–20.
- Sempruch C, Leszczyński B, Wójcicka A, Makosz M, Chrzanowski G, Matok H. 2009. Changes in activity of triticale tyrosine decarboxylase caused by grain aphid feeding. Pol J Environ Stud. 18:901–906.
- Sempruch C, Leszczyński B, Wójcicka A, Makosz M, Matok H, Chrzanowski G. 2010. Changes in activity of lysine decarboxylase within winter triticale in response to grain aphid feeding. Acta Biol Hung. 61:512–515.
- Sempruch C, Marczuk W, Leszczyński B, Czerniewicz P. 2013. Participation of amino acid decarboxylases in biochemical interactions between triticale (Triticosecale; Poaceae) and bird cherry-oat aphid (Rhopalosiphum padi; Aphididae). Biochem Syst Ecol. 51:349–356.
- Sempruch C, Marczuk W, Leszczyński B, Kozak A, Zawadzka W, Klewek A, Jankowska J. 2013. Effect of pea aphid infestation on activity of amino acid decarboxylases in pea tissues. Acta Biol Crac Ser Bot. 55:45–50.
- Sempruch C, Osiński P, Leszczyński B. 2011. Influence of selected plant amines on acceptance of winter triticale seedlings by bird cherry-oat aphid. Prog Plant Protect 51:427–430. Polish.
- Sempruch C, Wójcicka A, Makosz M, Leszczyński B. 2008. Changes in activity of ornithine decarboxylase in winter triticale caused by grain aphid feeding. Zesz Probl Post Nauk Rol. 524:401–408.
- Statistica StatSoft Inc. 2010. Data analysis software system version 9.0 [Internet]. Available from: www.statsoft.com.
- Świędrych A, Lorenc-Kukuła K, Skirycz A, Szopa J. 2004. The catecholamine biosynthesis route in potato is affected by stress. Plant Physiol Biochem. 42:593–600.
- Takemoto H, Uefune M, Ozawa R, Arimura GI, Takabayashi J. 2013. Previous infestation of pea aphids Acyrthosiphon pisum on broad bean plants resulted in the increased performance of conspecific nymphs on the plants. J Plant Int. 8:370–374.
- Tebayashi S, Horibata Y, Mikagi E, Kashiwagi T, Mekuria DB, Dekebo A, Ishihara A, Kim C-S. 2007. Induction of resistance against the leafminer, Liriomyza trifolii, by jasmonic acid in sweet pepper. Biosci Biotechnol Biochem. 71:1521–1526.
- Thompson GA, Goggin FL. 2006. Transcriptomics and functional genomics of plant defence induction by phloem-feeding insects. J Exp Bot. 57:755–766.
- Varlinden H, Vleugels R, Marchal E, Badisco L, Pflüger HJ, Blenau W, Broeck JV. 2010. The role octopamine in locusts and other arthropods. J. Insect Physiol. 56:854–867. 10.1016/j.jinsphys.2010.05.018
- Walters DR. 2003. Polyamines and plant diseases. Phytochemistry. 64:97–107. 10.1016/S0031-9422(03)00329-7
- Xu B, Sheehan MJ, Timko MP. 2004. Differential induction of ornithine decarboxylase (ODC) gene family members in transgenic tobacco (Nicotiana tabacum L. cv. Bright yellow 2) cell suspension by methyl-jasmonate treatment. Plant Growth Regul. 44:101–116. 10.1023/B:GROW.0000049419.22779.f5
- Zhang ZC, Wang MQ, Zhang G. 2008. Effects of polyamines and polyamine synthesis inhibitor on antennal electrophysiological responses of diamondback moths, Plutella xylostella. Entomol Exp Appl. 129:18–25.